Abstract
We investigated here the changes in von Willebrand factor (vWF) multimers in recurrent, sporadic and familial forms of hemolytic uremic syndrome (HUS)/thrombotic thrombocytopenic purpura (TTP) to see whether they are actually proteolyzed in vivo in these patients. Molecular determinants of fragments in vWF were also characterized to identify possible sites of cleavage of the subunit. Unusually large vWF multimers were found in blood of 8 of 10 patients with recurrent HUS/TTP, both in the acute phase and in remission, but never in familial and sporadic cases. Instead, all of the groups showed evidence of enhanced fragmentation of vWF multimers during the acute phase. Increased fragmentation was also shown by decrease in native 225-kD vWF subunit. In recurrent and sporadic HUS/TTP, enhanced fragmentation normalized at remission, but the abnormality persisted in familial HUS/TTP patients. The latter findings suggest that patients with familial HUS/TTP may have a congenital abnormality in vWF processing. Analysis with specific monoclonal antibodies showed the presence of the normal vWF fragments with apparent molecular mass of 189, 176, and 140 kD in all patients; however, in 6 recurrent and in 5 familial cases, novel fragments that differed in size from normal ones were found. The size of these abnormal fragments differed from one patient to another and none of them was ever found in normal plasma. These results documented, for the first time in HUS/TTP, an abnormal cleavage of the vWF subunit that might account for the increased fragmentation observed in these patients.
HEMOLYTIC UREMIC syndrome (HUS) and thrombotic thrombocytopenic purpura (TTP) are syndromes of hemolytic anemia and thrombocytopenia, which have in common thrombotic occlusion of the microvasculature of various organs1 attributed to platelet consumption and erythrocyte disruption.2 The term HUS better describes a disease of children who mostly have renal insufficiency,3 whereas TTP is often used for adult cases who predominantly, but by no means exclusively, suffer neurological symptoms.2 The two syndromes have different manifestations given the different organ distribution of the lesions, but share the same histopathology, ie, widening of the subendothelial space and intravascular platelet thrombi, which presumably reflect a massive endothelial damage as initiating event.1 Whether the findings given above can be taken as indicative of a similar sequence of pathogenic events is still unclear. The classical form of children HUS, which is mostly sporadic, has remarkably better prognosis3 than forms with tendency to recur that often occur in families.1 Whereas classical children HUS is strongly linked to Escherichia coli infection,1,3the recurrent form may not have a recognizable causative agent and a genetically determined condition has been proposed.1,4-6Evidence that some cases were cured with plasma manipulation suggested a genetic abnormality of plasma component(s) normally modulating endothelial function. After immunohistology studies showing accumulation of von Willebrand factor (vWF) in microvascular thrombi,7 endothelial vWF secretion and handling were extensively evaluated in these conditions. In normals, vWF is formed as large multimers (ultra large [UL] multimers) due to the polymerization in endothelial cells and megakaryocytes of a native subunit with apparent molecular mass of 225 kD8 and is stored as such in Weibel-Palade bodies and platelet α granules. UL multimers do not normally circulate, due to a blood specific protease9 that reduces them into smaller multimers soon after their secretion cleaving the molecule at position 842 Tyr 843 Met of the mature subunit.10 Evidence that proteolytic cleavage is involved in the modification of plasma multimers after secretion has been provided by studies showing that circulating vWF multimers are heterogeneous oligomers of a native 225-kD subunit and of proteolytic fragments with apparent molecular masses of 189, 176, and 140 kD.10-12 In patients with HUS and TTP, in contrast to healthy subjects, UL multimers, similar to the ones stored in endothelial cells and platelets, were occasionally detected in plasma.13-15 In cases who recovered after a single episode, UL multimers were found almost exclusively in the acute phase but no longer in remission, suggesting a massive release from endothelial storage sites8 that possibly overwhelmed transiently the plasma proteolytic capacity.16 By contrast, those cases who had a tendency to recur had circulating UL multimers either in the acute and consistently in the remission phase of the disease, which was initially taken as evidence of a state of persistent endothelial perturbation.13 This has been recently challenged by findings that circulating UL multimers in chronic relapsing TTP were associated with a reduced or totally absent activity of a specific protease that normally cleaves vWF in smaller multimers.17,18 In two recent large studies,19 20 vWF cleaving protease deficiency was described in patients with different forms of HUS/TTP. In nonfamilial cases, an inhibitor of vWF cleaving protease was found, whereas in the familial forms, the defect seems to be caused by a constitutional deficiency of the protease. However, in the studies given above, multimeric analysis has not been performed; thus, no conclusion can be drawn on the relationship between protease activity and UL multimers.
Although the findings listed above may explain some of the abnormalities in vWF in the circulating blood of these patients, circulating UL multimers were found in a patient with recurrent TTP even in the context of a normal vWF processing activity.21On the other hand, we have already documented in previous studies that the most consistent abnormality in the acute phase of plasma resistant HUS22 and recurrent HUS/TTP,23 rather than the presence of UL vWF multimers, is an increase of low molecular weight (Lmw) multimers that would reflect an enhanced proteolytic fragmentation of the molecule. Exactly the same was observed by Furlan et al18 in a patient with recurrent TTP. That vWF undergoes excessive fragmentation in the acute phase of these diseases is remarkably consistent with previous findings of a relative decrease in the native 225-kD vWF subunit that only occurs in the acute phase, accompanied by a relative increase of fragments that can only derive from the cleavage of the native subunit.24 Whether enhanced fragmentation depends on an exuberant activity of the plasma protease(s)25,26 different from that described by Furlan et al9 or is indeed favored by an intrinsic modification of vWF molecule itself is still not fully clear.
We present here the results of a study that formally addressed the changes in multimeric structures of circulating vWF in recurrent, familial, and sporadic forms of HUS/TTP to see whether vWF multimers are or are not actually proteolyzed in vivo in these patients. Finally, we characterized molecular determinants of fragments in vWF immunopurified from patient plasma and reduced to identify possible sites of cleavage of the subunit.
MATERIALS AND METHODS
Patients and Definitions
Ten cases of recurrent HUS/TTP, 15 cases of familial HUS/TTP belonging to 10 families, and 5 cases of sporadic HUS/TTP were recruited from January 1995 to August 1998, through the data base of The Italian Registry of Recurrent and Familial HUS/TTP, a network of 50 Units of Hematology and Nephrology, established in 1995, under the coordination of the Clinical Research Center for Rare Diseases “Aldo & Cele Daccò,” and from patients admitted to the Nephrology Unit of Ospedali Riuniti di Bergamo or referred to the Clinical Research Center for Rare Diseases. All relevant information on clinical history of all the 30 identified cases was recorded through the database of the Registry and by reviewing the patients’ charts. The following criteria were given to guide patient selection.
Diagnosis of HUS/TTP.
HUS/TTP was diagnosed in all cases reported to have one or more episodes of microangiopathic hemolytic anemia and thrombocytopenia defined on the basis of hematocrit (Ht) less than 30%, hemoglobin (Hb) less than 10 mg/dL, serum lactate dehydrogenase (LDH) greater than 460 U/L (which is the upper limit of normal range [defined as mean ± 2 SD] of the Laboratories of the Ospedali Riuniti, Azienda Ospedaliera di Bergamo, where all measurements were performed), undetectable haptoglobin, fragmented erythrocytes in the peripheral blood smear, and platelet count less than 150,000/μL. Remission of the microangiopathic process was defined by persistent increase in platelet count greater than 150,000/μL and normalization of the markers of hemolysis (serum lactate dehydrogenase <460 U/L, no fragmented erythrocytes in the peripheral blood smear) for at least 1 week after plasma therapy. In recurrent and familial forms of the disease, a clear distinction between HUS and TTP is often uncertain and controversial. This is well exemplified by the fact that in the same patient the disease can present with the feature of either HUS or TTP in the course of different episodes27-29 and by patients within the same family presenting with clinical manifestation of HUS or TTP.30-33 In this regard, in our series we found 1 patient (R12) who was diagnosed at onset as HUS and thereafter developed a chronic relapsing form of TTP.34 In addition, in 2 families (no. 2 and 19), we had 1 case of HUS and 1 case of TTP each, as diagnosed at onset. For all of these reasons, we felt more appropriate to refer to recurrent and familial cases with the broader term of HUS/TTP.
Diagnosis of recurrent HUS/TTP.
Recurrent HUS/TTP was diagnosed when one or more episodes of the disease occurred in the same subject after complete and persistent (for at least 1 month out off any kind of specific therapy, in particular plasma infusion or exchange) remission of any sign of microangiopathic hemolysis.
Diagnosis of familial HUS/TTP.
HUS/TTP were defined as familial when at least 2 members of the same family were affected by the disease at least 6 months apart and the exposition to a common environmental triggering agent (in particular a verotoxin producing strain of E coli) could be reasonably excluded.
Patients received detailed information of the purposes and design of the project and provided their informed consent according to the Declaration of Helsinki guidelines to enter the study. Patients were therefore referred to the Clinical Research Center for Rare Diseases, where a detailed history was recorded and a blood sample was collected and processed for the laboratory tests. Blood samples from all available healthy relatives of familial cases (n = 53; M/F, 25/28) and from healthy controls (n = 13) were also collected. For in hospital patients, which were most of the patients studied during the acute phase of the disease, blood samples were collected by the referring Clinical Centers. Markers of disease activity, renal dysfunction, and routine biochemicals were evaluated in all cases.
vWF Studies
Five milliliters of blood was drawn as previously described24 to evaluate vWF multimeric composition, native subunit, and fragments. Blood was collected in tubes containing one tenth final volume of 110 mmol/L trisodium citrate as anticoagulant with 5 mmol/L EDTA, 6 mmol/L N-ethylmaleimide, 1 mmol/L leupeptin, and 200 kallikrein inhibitory units (KIU) aprotinin per milliliter to inhibit the in vitro action of calcium-dependent cysteine proteases and serine proteases. In the acute phase, blood was collected before any treatment was started. At remission, blood was taken after at least 2 weeks from the last therapeutic plasma procedure.
Plasma vWF multimeric pattern was analyzed in all subjects by discontinuous sodium dodecyl sulphate (SDS) agarose gel electrophoresis, as described previously,35 with minor modifications.36 Resolving gels of 1.5% high gelling temperature agarose HGT(P) (FMC Corp, Rockland, ME) for high molecular weight (Hmw) multimers resolution were used. After electrophoresis, the gel was fixed, washed, and dried. The dried gel was incubated with125I-rabbit antihuman vWF antibodies (Dakopatts, Glostrup, Denmark), washed, dried, and exposed for autoradiography. Multimers were classified on the basis of their electrophoretic mobility, and samples were applied at the top of the gel and electrophoresed toward the bottom. Lmw multimers were defined as the two fastest bands resolved that positioned at the bottom of the gel, Hmw were the multimers slower than the first 10 bands resolved, and UL multimers were defined as the ultra bands (close to the top of the gel) slower than Hmw multimers found in normal plasma. Densitometric analysis was performed with a computer-based digital image processing. Autoradiographs of the gels were acquired using a digitizing board. Multimers were resolved into a series of peaks and areas under the peaks calculated by specific functions of the software Image 1.60 (National Institutes of Health [NIH], Bethesda, MD). UL multimers area was calculated together with the Hmw area, because it is not possible to resolve extra peaks after the first 10. The corresponding area was computed and expressed as a percentage of the total area for each sample. For each lane, the values were expressed as ratio Hmw/Lmw multimers. Under our conditions, the ratio for control subjects was as follows: controls for recurrent and familial HUS/TTP: 1.04 ± 0.20 (n = 13); controls for sporadic HUS/TTP: 1.05 ± 0.11 (n = 5).
vWF subunit and fragments were analyzed, in the recurrent group, in 4 patients both during an acute episode and in remission, in 1 patient during the acute event only, and in 4 patients only at remission. Subunit and fragments in the familial group were analyzed in 1 patient both during an acute episode and at remission, in 1 patient during the acute event only, and in 12 patients only at remission. One patient with recurrent HUS/TTP and 1 with the familial form were not tested for vWF subunit and fragments, because not enough plasma was available.
Plasma vWF for subunit and fragments analysis was immunoisolated as described37 using anti-vWF monoclonal antibodies (MoAbs) coupled to cyanogen bromide-activated Sepharose 4B beads (Sigma, St Louis, MO). Immunoisolated vWF was reduced in a final concentration of 65 mmol/L dithiothreitol (DTT) for 20 minutes at 56°C and electrophoresed in SDS-5% polyacrylamide gels (SDS-PAGE) as described by Laemmli.38 Transfer of protein from the polyacrylamide gel onto nitrocellulose membrane was performed in 25 mmol/L Tris-HCl, 192 mmol/L glycine, and 20% (vol/vol) methanol buffer, pH 8.3, at a current of 0.25 A at 3°C for 16 hours.37 The nitrocellulose membrane was then reacted with different anti-vWF MoAbs (anti-N terminus, RG7 [aa 185-288] or anti-C terminus, RG5 [aa 1482-1623])-, or with a pool of MoAbs against different epitopes of vWF. The membrane was then incubated with125I-rabbit antimouse IgG and the bands were visualized by autoradiography. In each gel, a lane of pooled normal plasma was run as control. To evaluate the percentage of native subunit and fragments, densitometric analysis was performed with a computer-based digital image processing using specific functions of the software Image 1.60 (NIH).
Linkage Analysis of vWF
Genomic DNA was extracted from peripheral blood according to standard laboratory procedure. Polymorphic microsatellite markers [D12S1685 and D12S1623 (-CA-)n repeats flanking vWF gene, MFD a (-CA-)n repeat located in intron 1 of vWF gene and vWF2 locus, and a (-GATA-)n repeat located in intron 40 of vWF gene] were analyzed using polymerase chain reaction (PCR) and polyacrylamide gel electrophoresis.39
Genetic linkage analysis was performed using the FASTLINK package,40 assuming either an autosomal recessive or autosomal dominant mode of inheritance. The disease frequency was set at 0.00001 and allele frequencies were obtained from the genome database. Heterogeneity tests were performed with the HOMOG program.41
Data Analysis
All data are presented as the mean ± SD. Private, clinical, and laboratory data were recorded in a uniform data extraction form (registration form). Database management was performed using a FileMakerPro software, version 2.1 (Claris Corporation, Santa Clara, CA). Data on vWF multimers and subunit fragmentation were analyzed by one-way ANOVA. Correlation analysis between continuous variables and dichotomous variables was performed using point-biserial correlation coefficient (rpb).42 The level of significance was set at P < .05 (two-tailed).
RESULTS
Patients
Ten patients with recurrent HUS/TTP (9 adults and 1 child) without familial history of the disorder, 15 patients with the familial form of the disease (10 adults and 5 children), and 5 adult patients with sporadic HUS/TTP were studied. For familial cases, the vWF multimeric pattern were also studied in all available healthy relatives to search for possible association between hereditary vWF abnormalities and predisposition to develop HUS/TTP.
At the time of study evaluation, 6 patients of the recurrent group were in an acute phase of the disease, whereas the other 4 patients were in full remission. Five of the 6 patients with active disease were studied again at remission (the sixth patient, R29, died during the acute event). Patient R12 has a chronic relapsing form of the disease with monthly acute episodes and a complete recovery of platelet number between relapses. Among the 15 patients with familial HUS/TTP, 3 were in the acute phase of the disease at time of study evaluation. Two were studied again at remission, whereas the other 1 was lost at follow-up. The other 12 patients had no clinical signs of disease activity. For patients studied at remission (n = 14), the time from the last clinical episode ranged from 5 months to 15 years. Among the 15 patients studied, one or more disease relapses were reported in 11 cases. Nineteen relatives had died of HUS/TTP before coming under our observation, and their plasma samples were not available for investigation.
All patients with sporadic HUS/TTP were studied both during the acute phase of the disease and at remission. The follow-up range of these patients was 1 to 3 years and no relapses occurred in this period in any of the patients; however, the possibility of future late relapses cannot be completely excluded.43
vWF Multimeric Pattern
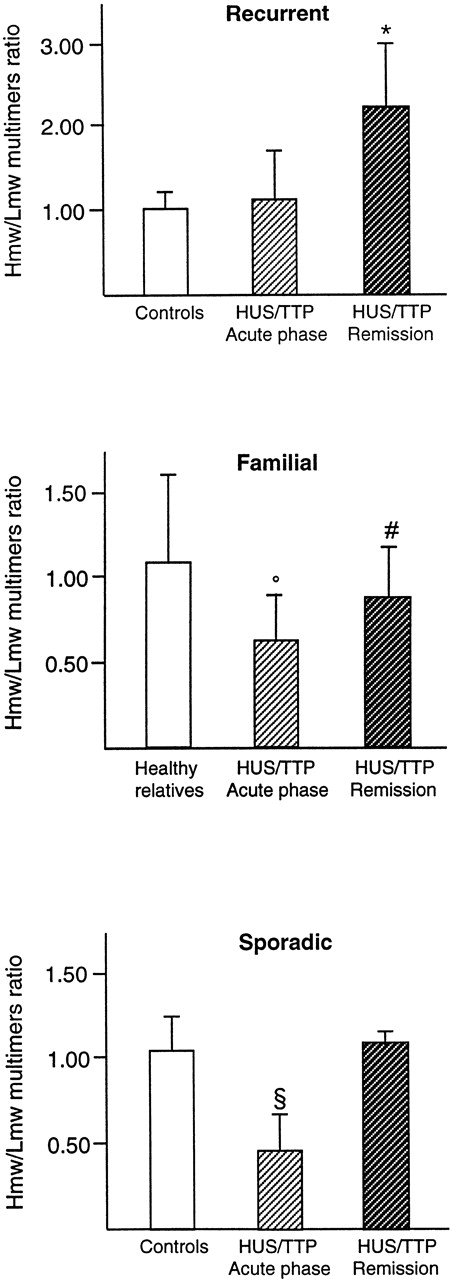
In all but 2 (R7 and R29) patients with recurrent HUS/TTP, there was a characteristic presence of UL vWF multimers during the acute phase of the disease (Table 1 and Fig 1A) that, however, persisted in remission. The presence of UL forms was also evidenced by densitometry with an increase during remission of the Hmw/Lmw multimers ratio as compared with controls (2.28 ± 0.74 [n = 9] v healthy controls, 1.04 ± 0.20 [n = 13]; P < .01; Fig 2). In the same patients, despite the presence of UL multimers, an enhanced fragmentation was found during the acute phase, as shown by lower Hmw/Lmw multimers ratio as compared with remission (1.13 ± 0.63 [n = 6]; P < .01 vremission; Fig 2). This abnormality was no longer evident at remission. At variance with the recurrent patients, no UL multimers were found in any patient with the familial form of the disease either during the acute phase or at remission (Fig 1B) or in any of their healthy relatives (not shown). Increased fragmentation was observed in all patients during the acute phase (Fig 1B and Table 2), which resulted in a decreased Hmw/Lmw multimers ratio (0.61 ± 0.24 [n = 3] v healthy controls, 1.04 ± 0.20 [n = 13]; P < .01; Fig 2). At variance with the recurrent form, in many patients with familial HUS/TTP, this abnormality persisted at remission, as documented by the mean Hmw/Lmw ratio at remission that was significantly lower than controls (0.88 ± 0.27 [n = 14]; P < .05 vhealthy controls; Fig 2), indicating a persistent increased proteolysis of vWF multimers (Table 2 and Fig 1B). Thus, a common characteristic abnormality of vWF multimeric pattern in HUS/TTP is an enhanced fragmentation of the molecule, whereas UL multimers seem to be peculiar to the recurrent form of the disease. This conclusion is further supported by results obtained in a group of patients with the more common, sporadic form of the disease who showed enhanced fragmentation of vWF during the acute phase (Hmw/Lmw multimers ratio: 0.48 ± 0.22 [n = 5]; P < .01 v healthy controls [n = 5]; Figs1C and 2, lower panel), which completely normalized at remission (Hmw/Lmw multimers ratio, 1.11 ± 0.05; P < .01; vacute phase [n = 5]; Fig 2). None of these patients showed any evidence of UL multimers either during the acute phase or at remission.
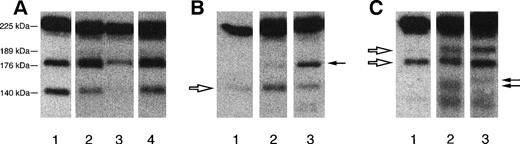
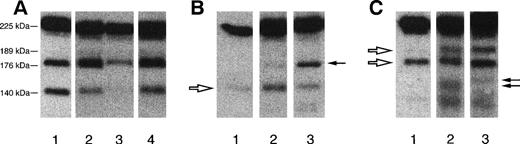
Representative autoradiographic images of Hmw multimers from 1 patient with recurrent TTP (patient R12 [A]), from 1 patient with familial HUS/TTP (patient F39#3 [B]), and from 1 patient with sporadic HUS/TTP (C). The origin of the gel is at the top. (A) Lane 1, plasma from healthy control; lane 2, patient plasma collected during the acute phase; lane 3, patient plasma collected at remission. Brackets indicate the position of UL vWF multimers that are present exclusively in patients with the recurrent form, both during and between relapses. (B) Lane 1, plasma from healthy control; lane 2, patient plasma collected during the acute phase; lane 3, patient plasma collected at remission. Brackets indicate the position of Hmw multimers that are decreased in plasma samples of patients with the familial HUS/TTP both during the acute phase and at remission. (C) Lane 1, patient plasma collected during the acute phase; lane 2, patient plasma collected at remission; lane 3, plasma from healthy control. Brackets indicate the position of Hmw multimers that are decreased in patient plasma during the acute phase of the disease.
Representative autoradiographic images of Hmw multimers from 1 patient with recurrent TTP (patient R12 [A]), from 1 patient with familial HUS/TTP (patient F39#3 [B]), and from 1 patient with sporadic HUS/TTP (C). The origin of the gel is at the top. (A) Lane 1, plasma from healthy control; lane 2, patient plasma collected during the acute phase; lane 3, patient plasma collected at remission. Brackets indicate the position of UL vWF multimers that are present exclusively in patients with the recurrent form, both during and between relapses. (B) Lane 1, plasma from healthy control; lane 2, patient plasma collected during the acute phase; lane 3, patient plasma collected at remission. Brackets indicate the position of Hmw multimers that are decreased in plasma samples of patients with the familial HUS/TTP both during the acute phase and at remission. (C) Lane 1, patient plasma collected during the acute phase; lane 2, patient plasma collected at remission; lane 3, plasma from healthy control. Brackets indicate the position of Hmw multimers that are decreased in patient plasma during the acute phase of the disease.
Hmw/Lmw vWF multimers ratio (calculated by densitometric analysis as described in Materials and Methods) in recurrent HUS/TTP (top panel; controls, n = 13; acute, n = 6; remission, n = 9), in familial HUS/TTP (middle panel; healthy relatives, n = 53; acute, n = 3; remission, n = 13), and in sporadic HUS/TTP (lower panel; controls, n = 5; acute, n = 5; remission, n = 5). *P < .01 versus acute phase and controls; °P < .01, #P < .05 versus healthy relatives and controls; §P < .01 versus controls and versus remission.
Hmw/Lmw vWF multimers ratio (calculated by densitometric analysis as described in Materials and Methods) in recurrent HUS/TTP (top panel; controls, n = 13; acute, n = 6; remission, n = 9), in familial HUS/TTP (middle panel; healthy relatives, n = 53; acute, n = 3; remission, n = 13), and in sporadic HUS/TTP (lower panel; controls, n = 5; acute, n = 5; remission, n = 5). *P < .01 versus acute phase and controls; °P < .01, #P < .05 versus healthy relatives and controls; §P < .01 versus controls and versus remission.
Association Between Increased vWF Fragmentation and Predisposition to HUS/TTP
To investigate for possible relationship between congenital abnormalities leading to increased fragmentation of vWF and predisposition to develop HUS/TTP, we analyzed vWF multimeric pattern in all available healthy relatives of patients with the familial form of the disease. In healthy relatives, the vWF multimeric pattern was normal, as indicated by a mean ratio of Hmw/Lmw multimers that could be superimposed over that of controls (1.06 ± 0.17 [n = 53]; P < .01 v patients in acute phase and at remission; Fig 2). We also calculated the point-biserial correlation coefficient between Hmw/Lmw multimers ratio and the presence of disease and found that, within families, Hmw/Lmw multimers ratio negatively correlated with the disease (rpb = −0.34; P < .01). The same result was obtained when also data from healthy controls were included in the analysis (rpb = −0.32; P < .01). It is noteworthy that, in point-biserial analysis, only patients without clinical signs of disease activity were considered to exclude possible influence of an ongoing microangiopathic process on vWF multimeric distribution. The findings given above strongly suggest a link between increased fragmentation of vWF in circulating blood and the predisposition to develop HUS or TTP.
Two-point linkage analysis indicated exclusion of linkage between vWF locus and the disease either with the recessive hypothesis and the dominant one (data not shown), which excludes the possibility that mutation in vWF molecule may account for the increased fragmentation observed in these patients.
Analysis of the Subunit With Epitope-Specific Antibodies
Recurrent HUS/TTP.
Western blot analysis of vWF immunopurified from plasma and reduced (Fig 3A) was performed using a pool of antibodies that recognize different epitopes of the molecule and showed that the percentage of native subunit in all patients with recurrent HUS/TTP during the acute phase was lower than the mean percentage in controls, although the difference did not reach statistical significance (71% ± 8% [n = 5] v normal plasma pools: 84 ± 14% [n = 6]) and was comparable to controls at remission (83% ± 14% [n = 8]). This confirmed the enhanced in vivo proteolysis of vWF suggested by the results of multimeric analysis.
(A) Autoradiographic image of vWF subunit (225 kD) and fragments (189, 176, and 140 kD) detected with a pool of MoAbs directed against different epitopes of vWF. Lane 1, normal plasma pool; lane 2, fragmentation of the protein in a recurrent patient (R7) during the acute phase; lane 3, the same patient at remission; lane 4, increased fragmentation in a patient with the familial form (F64#5) collected during remission. (B) Autoradiographic image obtained using MoAb anti N terminus (that labels in normal plasma the 140-kD fragment, white arrow). Lane 1, normal plasma pool; lane 2, a recurrent patient (R29, acute phase) showing an abnormal band at the 176-kD position (black arrow); lane 3, a patient with the familial form at remission (F45#2) with a similar abnormal fragment. (C) Representative image obtained using MoAb anti-C terminus (that labels in normal plasma 189- and 176-kD fragments, white arrows). Lane 1, normal plasma pool; lane 2, a recurrent patient (R12) showing both normal fragments and an abnormal fragment as shown by the appearance of a band of 140 kD (black arrow, not detected in normal plasma with this MoAb); lane 3, a patient with the familial form (F108#24 at remission) showing an abnormal band at 150 kD (black arrow) in addition to normal fragments.
(A) Autoradiographic image of vWF subunit (225 kD) and fragments (189, 176, and 140 kD) detected with a pool of MoAbs directed against different epitopes of vWF. Lane 1, normal plasma pool; lane 2, fragmentation of the protein in a recurrent patient (R7) during the acute phase; lane 3, the same patient at remission; lane 4, increased fragmentation in a patient with the familial form (F64#5) collected during remission. (B) Autoradiographic image obtained using MoAb anti N terminus (that labels in normal plasma the 140-kD fragment, white arrow). Lane 1, normal plasma pool; lane 2, a recurrent patient (R29, acute phase) showing an abnormal band at the 176-kD position (black arrow); lane 3, a patient with the familial form at remission (F45#2) with a similar abnormal fragment. (C) Representative image obtained using MoAb anti-C terminus (that labels in normal plasma 189- and 176-kD fragments, white arrows). Lane 1, normal plasma pool; lane 2, a recurrent patient (R12) showing both normal fragments and an abnormal fragment as shown by the appearance of a band of 140 kD (black arrow, not detected in normal plasma with this MoAb); lane 3, a patient with the familial form (F108#24 at remission) showing an abnormal band at 150 kD (black arrow) in addition to normal fragments.
Analysis of fragments with MoAb (RG7) recognizing the N terminus of the molecule showed, in all patients, the normal 140-kD N terminus fragment, indicating that the normal processing activity was present in these patients (Fig 3B). However, in patient R29, the apparent molecular weight of this fragment was slightly greater than 140 kD. Moreover, the autoradiograms disclosed in 4 patients novel proteolytic fragments whose molecular weight was different among patients (Fig 3B and Table 3).
In all patients, the normal C terminus fragments (176 and 189 kD) were present, as evidenced by the MoAb RG5 (Fig 3C); in patient R29, the 176-kD fragment showed a faster mobility, indicating a slightly lower molecular weight. Novel C terminus proteolytic fragments were also found in 5 patients (Table 3 and Fig 3C).
Taken together, the results obtained with the different Abs showed fragment abnormalities in 6 of 9 patients with the recurrent HUS/TTP. In 4 patients, the abnormalities were found during the acute phase (R1, R12, R14, and R29; in patient R12, it persisted at remission); in the other 2 patients, the abnormalities were found at remission. No such fragments were ever identified in normal plasma collected, processed, and stored in the identical manner as HUS/TTP specimens. The generation of fragments different in size from the normal ones indicates that the molecule, at least in some patients, undergoes a proteolytic cleavage that is not commonly observed in normal individuals.
Familial HUS/TTP.
Increased fragmentation was confirmed in patients with the familial form when the subunit and fragments were analyzed by Western blot (Fig3A). A lower proportion of the native subunit was found in these patients during acute phase (acute: 57% and 69% [n = 2]). In most patients (11 of 13) studied in remission, the percentage of native subunit was lower than control mean (67% ± 13% [n = 13]v normal plasma pools: 84% ± 14% [n = 6]; P < .05).
The analysis of fragments with the MoAb directed against the N terminus showed the presence in all patients of the normal 140-kD fragment (Fig3B), indicating that the normal processing activity was present. However, in 1 patient (F45#2), at remission, a novel fragment of apparent molecular weight of approximately 170 kD (Fig 3B) was also found. In all patients at the C terminus, there were the two normal fragments of 176 and 189 kD (Fig 3C); in 5 patients (F45#2, F30#4, F102#19, F106#24, and F29#29a), fragments of abnormal molecular weight were also found (Fig 3C and Table 4). In total, 5 patients showed abnormalities in the cleavage of vWF subunit. All were in the remission phase of the disease at the time of sampling; thus, the generation of the abnormal fragments could not be simply the consequence of the microangiopathic process. Rather, the data shown above indicate that at least some familial patients have a congenital abnormality in vWF proteolytic cleavage.
DISCUSSION
A great number of studies in the past have addressed the nature of vWF multimeric distribution in circulating blood of patients with HUS/TTP and found diverse and complex patterns of abnormalities ranging from UL multimers (that do not circulate in healthy subjects) to lower than normal amount of Hmw multimers.13-15,22,24,44-46 The results of the present study performed in a rather large population of patients with HUS/TTP show a major and consistent abnormality in 8 of 10 recurrent cases: the presence of UL forms in circulating blood both in the acute phase of disease manifestation and in the remission phase. A possible explanation for the presence of UL forms in recurrent patients derives from the recent observation17 18 that patients with recurrent TTP have a deficiency of a vWF specific-cleaving protease in their plasma. We recently analyzed vWF-cleaving protease in our patients with recurrent HUS/TTP; the results are presented in Table 1. We found that all recurrent patients of our series lacked protease activity during the acute phase. Of these recurrent cases, vWF-cleaving protease activity recovered in 3 of 9 cases when patients were studied in the remission phase of the disease (Table 1).
In the previous literature, the presence in patients with recurrent forms of TTP of circulating UL multimers,13,24,44 which in vitro are capable of supporting platelet aggregation more efficiently than normal multimers,47 was taken as evidence for their pathogenic role in microvascular thrombi.48 However, a direct proof that this was indeed the case was never provided.
Interestingly, the opposite appears true in vivo, because all of the available evidence is against the possibility that UL vWF multimers, when present in the circulation, can even cause intravascular thrombosis. Thus, UL multimers have been found in fetuses and newborns and disappear within the first months of life, indicating that physiologic cleavage of vWF is less efficient at birth and fully develops later in life.49 Moreover, in patients with a rare variant of von Willebrand disease, named Vicenza, UL vWF multimers were constantly found in the circulation.50,51 Nonetheless, these patients never experienced thrombotic episodes; rather, they suffered bleeding tendency.50 51
Here, at variance with our own findings in recurrent forms, either in familial cases or in patients with sporadic HUS/TTP, we never found circulating UL multimers either in the acute phase of the disease or in remission. Finding that 11 of 15 of familial patients actually had episodes of disease recurrence is again another piece of evidence that contradicts the current belief that UL vWF multimers cause microvascular thrombosis in HUS/TTP. Instead, we found here that all patients with HUS/TTP, irrespectively of their classification, showed a remarkably consistent evidence of enhanced fragmentation of vWF during the acute phase of the disease. The presence of increased fragmentation was reflected in more Lmw and fewer Hmw multimers so that their ratio was rather abnormal. The native 225-kD subunit was also decreased in its relative amount in vWF immunopurified and reduced from plasma of both recurrent and familial cases, which can be taken as an additional evidence of enhanced vWF fragmentation. We can reasonably exclude any influence of treatment on vWF fragmentation, because all patients with acute HUS/TTP were studied before any treatment was started, and patients at remission were studied at least 2 weeks after the last plasma therapy (vWF half life is about 18 hours52). That this abnormality is specific for HUS/TTP and not simply the consequence of microangiopathy is supported by previous findings from our laboratory53 showing that, in patients with acute scleroderma, the relative proportion of the intact 225-kD subunit is comparable to that in control plasma. However, we cannot reasonably exclude that other acute microangiopathic disorders might also manifest qualitative abnormalities of vWF.54
Which can be the reason for enhanced vWF fragmentation in these diseases? vWF susceptibility to fragmentation increases in response to rising levels of shear stress,55 which induces protein unfolding and makes vWF proteolytic cleavage site more accessible to a specific plasma protease(s). It is therefore speculated that enhanced shear stress in the severely narrowed damaged microvessels accounts for the abnormal vWF fragmentation observed during the acute phase of HUS/TTP. Evidence of increased capacity of fragmented vWF to bind receptors on activated platelets56 would suggest that shear stress-induced vWF fragmentation may contribute to maintain and further spread microvascular thrombosis. The interpretation given above is supported by data showing that, in plasma-resistant forms of HUS, removal of the kidneys, a major site of vascular bed occlusion and augmented shear stress, was followed by hematologic and clinical remission associated with restoring the vWF fragmentation pathway to normal.22 Consistent with this possibility, we found that, in our patients with recurrent and sporadic HUS/TTP, increased vWF fragmentation normalized after resolution of the microangiophatic process induced by therapy with plasma.
In familial HUS/TTP disease, manifestations are quite severe, with death or permanent neurological and renal sequelae in most cases.4,57 In these forms, a genetically determined condition has been suggested that may predispose to disease development upon exposure to triggering factors. Such interpretation is consistent with the extremely frequent findings of HUS recurrencies after renal transplantation.57 58 That this genetic abnormality involves the vWF pathway is suggested by increased vWF fragmentation, as documented by decreased Hmw/Lmw multimers ratio as well as by relative decrease of intact subunit in familial cases, which was always evident even in the absence of overt signs of disease activity. Evidence that, within families, a low Hmw/Lmw ratio was only found in patients affected by HUS/TTP, whereas it was normal in most of their healthy relatives, further suggests the possibility of a tight (possibly cause and effect) relationship between the vWF abnormality and the disease.
As performed for the recurrent group, we evaluated the activity of the recently described vWF-cleaving protease9 in familial patients and found that it was absent in the single familial case that could be examined during the acute phase of the disease (Table 2). In harmony with findings of Furlan et al,19 in familial cases who had protease studies performed while in the remission phase of the disease, we found no activity in 3 of 13 patients and normal activity in the remaining patients (Table 2). These results are very consistent with those reported in Furlan et al,19 in which our series of patients has been studied within a larger group of familial HUS/TTP patients. It was speculated that the deficiency of vWF cleaving protease activity must have resulted in impaired cleavage of secreted vWF multimers, resulting in the presence of UL multimers into the circulation of these patients.59 However, in none of the previous published studies on protease cleaving have vWF and its multimeric structure been studied simultaneously in recurrent and familial forms in different phases of the disease. On the other hand, in the present study, we found that patients with normal vWF cleaving protease can have UL multimers in the very moment in which protease activity is measured (Table 1, patients R13 and R15), whereas many patients whose protease activity is lacking do not have UL multimers in their circulation (Table 1, R29; and Table 2, F45#2, F48#2, F101#19, and F102#19). In addition, patients without measurable protease activity have an abnormally high proportion of Lmw vWF multimers (Table 1, patients R1, R12, R14, and R29; and Table 2, patients F45#2, F48#2, and F101#19); exactly the same result was observed by Furlan et al18 in a patient with recurrent TTP. How can we reconcile the increased vWF fragmentation in the face of the lack of cleaving protease activity? To address this issue here, we have studied the mode of fragmentation of the 225-kD vWF subunit in vivo in vWF multimers of our patients by using MoAbs specific for N and C terminus epitopes of the subunit. We found that the normal 189-, 176-, and 140-kD fragments are actually present in all recurrent patients and that the amount of fragments formed is even higher than in normal subjects, at least in the acute phase. Also, in familial patients, we found normal fragments whose amount was increased independently of disease activity. Altogether, the findings given above support the concept that the protease described by Furlan et al9 is not the only one that can cleave vWF subunit at position 842 Tyr and 843 Met. We also demonstrated that in some recurrent and familial patients novel fragments of different molecular weight than normal ones were formed that cannot be attributed to the action of the above-mentioned protease. The size of these abnormal fragments differed from one patient to another, and none of them was ever found in normal plasma. These findings could be explained by the presence into the circulation of patients of abnormal proteolytic activity that cleaves the vWF subunit in a position different from the physiologic 842 Tyr 843 Met site. However, the enzyme(s) responsible for this proteolysis remains to be identified. Elastase and other enzymes released from activated polymorphonuclear leukocytes and plasmin, which proteolyze vWF in vitro with resultant loss of Hmw multimers,60,61 are all possible candidates for producing the abnormal vWF cleavage seen in HUS/TTP.62-64 Indeed, some, although not all, of the fragments formed in vitro by the enzymes listed above are very close in dimension to those found in our patients.65 66
In other patients of our series, the pattern of fragments rather resembled those given in vitro by calpain, a cysteine-protease released by activated platelets.65 During acute episodes of TTP, calpain is released in the circulation26 and, interestingly, vWF proteolyzed by calpain in vitro acquires increased capacity to bind to both ADP and calpain-activated platelets.56 The latter finding raises the issue of whether the abnormal fragmentation of vWF in HUS/TTP may render the molecule more active in mediating thrombus formation.
Another possible explanation for the presence of the abnormal fragments could be a mutation in the vWF gene causing amino acid substitution that either generates an additional cleavage site or changes the conformation of the molecule and renders it more susceptible to proteolytic cleavage. However, results of linkage analysis in familial patients do not support this possibility.
The results of this report represent a step forward in clarifying the complex, controversial issue of vWF in the pathogenesis of HUS/TTP. We found that the UL multimers are characteristic of recurrent HUS/TTP and do not occur in familial and sporadic forms. We also documented that the only consistent abnormality during the acute phase of HUS/TTP is an increased fragmentation due to proteolytic cleavage of the molecule. Increased fragmentation was also found in patients who have a deficiency of the recently described vWF-cleaving protease,9 which indicates that another protease(s) could be responsible for vWF cleavage in HUS/TTP. Patients with the familial form of the disease may have a congenital abnormality in vWF multimer processing, as shown by an enhanced proteolytic cleavage present independently of disease activity that, however, seems not to depend on a mutation in the vWF gene. Finally, the analysis of the subunit with epitope-specific antibodies disclosed, for the first time in HUS/TTP, abnormal cleavage of the vWF subunit, both in recurrent and in familial patients, resulting in fragments different from the normal ones.
Further studies are needed to find out the protease(s) involved in the abnormal fragmentation of vWF in HUS/TTP patients.
ACKNOWLEDGMENT
The authors are indebted to Prof Miha Furlan for measurement of vWF-cleaving protease activity. We are indebted to Fabio Sangalli for linkage analysis calculation.
ORGANISATION OF THE ITALIAN REGISTRY FOR RECURRENT AND FAMILIAL HUS/TTP
Coordinators: P. Ruggenenti, MD; M. Noris, Chem.Pharm.D.; G. Remuzzi, MD (Clinical Research for Rare Diseases “Aldo e Cele Daccò,” Ranica, Italy).
Investigators: F. Casucci, MD, F. Cazzato, MD (Division of Nephrology, “Miulli” Hospital, Acquaviva delle Fonti, Bari, Italy); M. Dugo, MD (Division of Nephrology and Dialysis, “S. Giacomo” Hospital, Castelfranco Veneto, Treviso, Italy); C. Cappelletti, MD, C. Ceccarelli, MD (Division of Internal Medicine and Division of Nephrology, “S. Giovanni di Dio” Hospital, Firenze, Italy); G.C. Barbano, MD (Division of Pediatric Nephrology, “G. Gaslini” Institute, Genova, Italy); A. Edefonti, MD (Division of Pediatric Dialysis, “De Marchi” Pediatric Clinic, Milano, Italy); E. Rossi, MD (Blood Transfusion Center, “L. Sacco” Hospital, Milano, Italy); G.B. Haycock, MD (Pediatric Renal Unit, Guy’s Hospital, London, UK); A. Indovina, MD (Bone Marrow Transplantion Unit, “V. Cervello” Hospital, Palermo, Italy); B. Vasile, MD, E. Daina, MD, S. Gamba, Research Nurse, A. Schieppati, MD (Information Center for Rare Diseases, Ranica, Bergamo, Italy); C. Zoccali, MD, F. Malemaci, MD (Division of Nephrology and Dialysis, Hospital of Reggio Calabria, Calabria, Italy), T. Cicchetti, MD, G. Putortı̀, MD (Division of Nephrology and Dialysis, “N. Giannettasio” Hospital, Rossano Calabro, Rossano Calabro, Italy); D. Landau, MD (Pediatric Nephrology, Soroka Medical Center, Beer-Sheba, Israel); O. Amatruda, MD (Division of Nephrology, “Fondazione Macchi” Hospital, Varese, Italy); E. Pogliani, MD, D. Belotti, Biol.Sci.D. (Divison of Hematology and Transfusion Center, “San Gerardo” Hospital, Monza, Italy); M. Sanna, MD (Division of Medical Pathology, Hospital of Sassari, Sassari, Italy); R. Coppo, MD, A. Amore, MD (Division of Nephrology and Dialysis, “Regina Margherita” Pediatric Hospital, Torino, Italy).
Contributing correspondence: R. Bellantuono, MD, T. De Palo, MD (Division of Nephrology and Dialysis, “Giovanni XXIII” Pediatric Hospital, Bari, Italy); T. Barbui, MD, M. Galli, MD (Division of Hematology, “Ospedali Riuniti Azienda Ospedaliera,” Bergamo, Italy); S. Bassi, MD (Division of Nephrology and Dialysis, “Umberto I” Hospital, Brescia, Italy); R. Wens, MD (CHU Brugman, Bruxells, Belgium); M.G. Caletti, MD (Servicio de Nefrologia, Hospital Garrahan, Buenos Aires, Argentina); E. Grandone, MD, F. Aucela, MD (Atherosclerosis and Thrombosis Research Laboratory and Division of Nephrology and Dialysis, “Casa Sollievo della Sofferenza S. Giovanni Rotonbo” Hospital, Foggia, Italy); V. Toschi, MD (Transfusional Center, “San Carlo Borromeo” Hospital, Milano, Italy); P. Prandoni, MD (Division of Medicine, Hospital of Padova, Padova, Italy); R. Marcenó, MD (Bone Marrow Transplantion Unit, “V. Cervello” Hospital, Palermo, Italy); T. Cocchi, MD, M. Sassi, MD (Blood Transfusion Center, Hospital of Parma, Parma, Italy); C. Porta, MD (Division of Medicine, IRCCS Policlinico “San Matteo”, Pavia, Italy); G.F. Rizzoni, MD, A. Gianviti, MD (Division of Nephrology and Dialysis, “Bambino Gesù” Pediatric Hospital, Roma, Italy); A. Pinto, MD (Division of Nephrology and Dialysis, “S. Giovanni di Dio e Ruggi D’Aragona,” Salerno, Italy); A. Khaled, MD (Division of Nephrology, “S. Chiara” Hospital, Trento, Italy); A. Cao, MD (Institute of Clinic and Biology of Evolutive Age, Cagliari, Italy); F. Della Grotta, MD (Division of Nephrology, Hospital of Anzio, Anzio, Italy).
Laboratory analysis: L. Robba, MD, A. Vernocchi, MD, A. Crippa, MD (Division of Laboratory Analysis, “Ospedali Riuniti, Azienda Ospedaliera,” Bergamo, Italy); F. Gaspari, Chem.D. (Clinical Research for Rare Diseases “Aldo e Cele Daccò,” Ranica, Bergamo, Italy).
Biochemical studies: M. Ghilardi, Biol.Sci.D., D. Macconi, Biol. Sci.D., M. Galbusera, Biol.Sci.D., C. Rossi, Chemist, S. Orisio, Biol. Sci.D., J. Caprioli, Biol.Sci.D. (“Mario Negri” Institute for Pharmacological Research, Bergamo, Italy).
Statistical analysis: A. Perna, Stat.Sci. (Clinical Research for Rare Diseases “Aldo e Cele Daccò”, Ranica, Italy).
Supported by Italian Telethon Grant No. E359, by a Baxter Extramural grant, and by a grant from CARIPLO. Z.M.R. was supported by National Institutes of Health Grant No. HL-42846. J.C. is recipient of a fellow of “Associazione per la Ricerca sulle Malattie Rare” (Bergamo, Italy).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Giuseppe Remuzzi, MD, Clinical Research Center for Rare Diseases “Aldo e Cele Daccò,” “Mario Negri” Institute, Via Gavazzeni, 11, 24125 Bergamo, Italy.

![Fig. 1. Representative autoradiographic images of Hmw multimers from 1 patient with recurrent TTP (patient R12 [A]), from 1 patient with familial HUS/TTP (patient F39#3 [B]), and from 1 patient with sporadic HUS/TTP (C). The origin of the gel is at the top. (A) Lane 1, plasma from healthy control; lane 2, patient plasma collected during the acute phase; lane 3, patient plasma collected at remission. Brackets indicate the position of UL vWF multimers that are present exclusively in patients with the recurrent form, both during and between relapses. (B) Lane 1, plasma from healthy control; lane 2, patient plasma collected during the acute phase; lane 3, patient plasma collected at remission. Brackets indicate the position of Hmw multimers that are decreased in plasma samples of patients with the familial HUS/TTP both during the acute phase and at remission. (C) Lane 1, patient plasma collected during the acute phase; lane 2, patient plasma collected at remission; lane 3, plasma from healthy control. Brackets indicate the position of Hmw multimers that are decreased in patient plasma during the acute phase of the disease.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/2/10.1182_blood.v94.2.610/5/m_blod41422001w.jpeg?Expires=1766060007&Signature=SjpxXf88PZHAwCmusVc2iPgvwcRgopfHiUm7UMISmXYhAjYjNVyzgkL14Yiqu~-gkRpj3i67n9bJEkkrsNdf1VPPCnBEuolKdIp7JHWGWTAY8vMrObkIUfn1yXtwyS2O6tJ560xftS3imWVJNNKQ~xO8oHJjPMVzV~bL0L4qlYCV53YZHHcSeLdzTyKR5Tzzfn7aeJLqRmNpofGezvHaOKYoouee~XfeskchAKi7gRyswZ65XgvKTZIPV3YLTMal9AH7I3mNjGMoqPv-70mFlcac~cSSOCVF0y5mfV5vdCgW4lbR94t9lXM6P-ij1NVIOxX3io6DqnAHc0RWazlSLA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 1. Representative autoradiographic images of Hmw multimers from 1 patient with recurrent TTP (patient R12 [A]), from 1 patient with familial HUS/TTP (patient F39#3 [B]), and from 1 patient with sporadic HUS/TTP (C). The origin of the gel is at the top. (A) Lane 1, plasma from healthy control; lane 2, patient plasma collected during the acute phase; lane 3, patient plasma collected at remission. Brackets indicate the position of UL vWF multimers that are present exclusively in patients with the recurrent form, both during and between relapses. (B) Lane 1, plasma from healthy control; lane 2, patient plasma collected during the acute phase; lane 3, patient plasma collected at remission. Brackets indicate the position of Hmw multimers that are decreased in plasma samples of patients with the familial HUS/TTP both during the acute phase and at remission. (C) Lane 1, patient plasma collected during the acute phase; lane 2, patient plasma collected at remission; lane 3, plasma from healthy control. Brackets indicate the position of Hmw multimers that are decreased in patient plasma during the acute phase of the disease.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/2/10.1182_blood.v94.2.610/5/m_blod41422001w.jpeg?Expires=1766538819&Signature=QLA6mEMxfriX0ONioZTwlNyNfUS12xqrdMzMUOBg08j0H-QeQQlpFVDIb3iZkIKoouZtyFjZYxL01Z4j6owLvI98GdV6inUAJhrG8FH23~~jIMTLM9z-bfIlMaRPEATmzZc36AjBrjfb4a2Mw5MU5t7HZjAAiA9G0UQL2n3flS5ZjtyQMHQWJ784umGWv9e2onzYgTmtulA6sRlycPgNKm9KNn77-6mxI9BC-9spca5JoXfvJa2ImYMiWOlqlk6HTzeoyfjcBAMBO9dCmfGVWaj80ZeIiy4nEIFWY1uWRuXYjHvfc-L2ChWzeFv0g0Acb~tHIg1bsE2BEWX-JTFDtg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)