Human peripheral blood monocytes differentiate into macrophages when cultured in vitro for a few days. In the present study, we investigated the expression of C-C chemokine and CXCR4 receptors in monocytes at different stages of differentiation. Culturing of monocytes for 7 days resulted in a progressive decrease of the mRNA that encodes for CCR2 and CCR3, whereas the expression of mRNA for other chemokine receptors (CCR1, CCR4, CCR5, and CXCR4) was not substantially affected. The loss of CCR2 mRNA expression in 7-day–cultured macrophages was associated with a strong reduction in the receptor expression at the plasma membrane, as well as in the monocyte chemotactic protein (MCP-1) binding, as compared with freshly isolated monocytes. Furthermore, the biologic response to MCP-1, as measured by intracellular calcium ions increase and chemotactic response, was lost in 7-day–cultured macrophages. Differentiation of monocytes into macrophages also resulted in an increased secretion of MCP-1 that, at least in part, was responsible for the downmodulation of its receptor (CCR2). The loss of CCR2 expression and the parallel increase of MCP-1 secretion triggered by differentiation may represent a feedback mechanism in the regulation of the chemotactic response of monocytes/macrophages.
HUMAN PERIPHERAL BLOOD monocytes mature in different types of tissue histiocytes when they migrate from the bloodstream to various tissues. This differentiation is essential for their functional competence and is probably triggered by environmental signals. In vitro cultivation of blood monocytes results in their adherence to the plastic surface and in the initiation of a series of functional changes that closely resemble their in vivo maturation to macrophages.1-4 Recruitment of monocytes from the blood compartment into tissues is an important process in the inflammatory response and immunity.5 The recruitment of monocytes to inflammatory foci is a 2-step process. The cells first adhere to the vascular endothelium, then they migrate to sites of inflammation in response to specific chemoattractants.
Directional migration of monocytes is regulated by locally produced cell-secreted proteins, bacterial peptides and products of phospholipid metabolism.6,7 In the past few years, a new superfamily of low-molecular-weight chemotactic proteins has been described.5-10 A common feature of these molecules is the presence of 4 conserved cysteine residues.5,8-12 According to the space of the first 2 cysteines, chemokines can be grouped in 2 main subfamilies: the C-X-C (or α) chemokines and the C-C (or β) chemokines.5,7,11-13 New chemokine families have been recently identified, which maintain overall sequence homology, but lack the typical cysteine distribution (C, or γ, and CX3C, or δ, chemokines).8,10 The α chemokines are primarily active on neutrophils, but some activity has also been reported on T lymphocytes.9,10,13 On the other hand, the β chemokines exhibit a wider spectrum of action, in that they are active on multiple leukocyte populations, including monocytes, granulocytes, T and B lymphocytes, and natural killer (NK) and dendritic cells.9,12,13 Lymphotactin, the only γ chemokine described thus far, exerts its action on T lymphocytes and NK cells,10,14 whereas fractalkine is active on T cells and monocytes.8 Chemokines activate their functions through interaction with a family of rhodopsin-like guanosine triphosphate (GTP)-binding protein-coupled 7-transmembrane domain receptors.5,15-18 To date, 8 receptors have been defined for the β chemokines (CCR1 to 8) and 5 for the α chemokines (CXCR1 to 5), together with several putative CC or CXC receptors for which the ligands remain to be determined.5
The CC chemokine monocyte chemotactic protein 1 (MCP-1) was originally described as a potent chemoattractant for monocytes.19MCP-1 is produced by a variety of cell types, including endothelial cells and cells of the monocytic lineage in response to different signals (typically tumor necrosis factor-alpha [TNF-α], interleukin-1 [IL-1], interferon-γ [IFN-γ], bacterial lipopolysaccharide [LPS], platelet-derived growth factor, and oxidized low-density lipoproteins).20-22 Many activities have been subsequently assigned to this protein, including induction of mononuclear phagocytes, basophils, T- and NK-cell migration,12,23 suppression of tumor growth in animal models,24 and neutralization of human immunodeficiency virus type 1 (HIV-1).25-27 MCP-1 exerts its action mostly through the interaction with CCR2, a chemokine receptor that is present in 2 isoforms, named a and b.28-30 The 2 isoforms represent alternatively spliced variants of a single MCP-1 receptor gene that differ only in their carboxyl tails.29,30 The biologic significance of the existence of 2 CCR2 variants has not yet been elucidated.29,30 CCR2 gene expression is highly regulated. In fact, it has been previously described that the expression of CCR2 in primary monocytes can be inhibited by bacterial LPS and other microbial agents.31 Moreover, cytokines have also been shown to modulate either negatively (IFN-γ, TNF-α, and IL-1) or positively (IL-2 and IL-10) the expression of this receptor.32 33
In this study, we investigated the expression of β-chemokine receptors during the differentiation of human peripheral blood monocytes to macrophages. Our results indicate that CCR2, the major receptor for MCP-1, is progressively downmodulated when freshly isolated monocytes are maintained in vitro for a few days. The inhibition of CCR2 mRNA expression is associated with a parallel reduction in the expression of receptors at the plasma membrane, as well as in the binding of and biologic response to MCP-1. These findings may reflect the differences in the chemotactic response of monocytes versus macrophages and provide further evidence that CCR2/MCP-1 interactions represent important events in the functional regulation of these cells.
MATERIALS AND METHODS
Isolation and culture of peripheral blood monocytes.
PBMC were obtained from 18- to 40-year-old healthy men as previously described.4 Monocytes were separated from lymphocytes by Percoll gradient centrifugation.34 Cells were then cultured in endotoxin-free Iscove’s medium containing 15% fetal calf serum (FCS; 0.22 μm filtered) for 24 hours (defined here as day 1 freshly isolated monocytes) or 7 days (7-day–cultured macrophages). Some monocytes were used immediately after isolation to avoid their adherence to plastic (defined here as day 0 monocytes). After different times of culture, both adherent and nonadherent cells were recovered and analyzed as described elsewhere.4 Cytochemical (ie, sodium fluoride–inhibited esterase activity) and surface marker (ie, CD14 antigen) analysis showed that Percoll-purified, as well as adherent cell populations, consisted of greater than 96% monocytes. Monocyte preparations that exhibited less than 95% CD14+ cells have always been discarded.
Chemokines and reagents.
Human recombinant MCP-1, RANTES, and polyclonal antibody to MCP-1 were purchased from Pepro Tech EC (London, UK). Phycoerythrin (PE)-conjugated mouse anti-human monoclonal antibodies to CCR5 (clone 2D7) and CXCR4 (clone 12G5) were purchased from PharMingen (San Diego, CA). Monoclonal anti-human CCR2 antibody (clone 48607.121) was obtained from R&D Systems (Minneapolis, MN). Human recombinant [125I]-MCP-1 and [125I]-RANTES were purchased from NEN Life Science Products (Boston, MA). FMLP was obtained from Nova Biochem (Laufelfingen, Switzerland). Control IgG2a and IgG1k isotypes were obtained from Becton Dickinson (San Jose, CA) and Dako (Glostrup, Denmark), respectively. Antibody to transferrin receptor (CD71) was purchased from Dako. Fluorescein isothiocyanate (FITC) goat anti-mouse IgG antibody was purchased from Sigma (St Louis, MO).
Analysis of chemokine receptors mRNA.
For the RNase protection assay, total RNA was extracted by the method of Chirgwin et al.35 For C-C chemokine receptors mRNA analysis, 5 μg of total RNA was hybridized to a radiolabeled multiprobe produced by an in vitro transcription kit (PharMingen) as indicated in the manufacturer’s instructions. For RNA quantification, the amount of mRNA corresponding to CCR1, CCR2a, CCR2b, and CCR5 present in each sample was normalized to the control glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using a densitometer (UltroScan XL; Pharmacia LKB, Milwaukee, WI). Values are given as chemokine receptor/GAPDH mRNA ratio.
For the RNA–polymerase chain reaction (PCR) assay, total RNA was reverse-transcribed as previously described.4 A 1:10 dilution of the cDNA product was amplified in a 20-μL reaction that contained 0.1 μg of 32P-labeled sense primer and the same amount of antisense primer and 0.5 U of Taq Polymerase (Perkin Elmer, Foster City, CA). PCR amplification was performed for 30 cycles of 45 seconds at 94°C, 1 minute at 60°C, and 2 minutes at 72°C. PCR products were separated on 5% polyacrylamide gel. The sequence of the primers were as follows: porphobilinogen deaminase (PBGD) sense 5′CTGCAACGGCGGAAGAAA; antisense 5′GGCATGTTCAAGCTCCTTGG; CCR1 sense 5′CTTCCCTTCTGGATCGACTAC; antisense 5′AAGAGGTTCAGTTTCAGAGCCT; CCR2 (A + B) sense 5′ATTCACAGGGCTGTATCAC; antisense 5′ GTGGAAAATAAGGGCCACAGAC; CCR3 sense 5′CAGTGCTCTTTACCCAGAGGATAC; antisense 5′TTGGTCCCTCCTCTTTAGG; CCR4 sense 5′ATGGTCAGTGGCTGTGTTCG; antisense 5′TGGATGGCATAGTCCAAGTATC; CCR5 sense 5′ TTCTCTTCTGGGCTCCCTACA; antisense 5′GGAAGAAGACTAAGAGGTAGTT; CXCR4 sense 5′CTATGCAAGGCAGTCCATGT; antisense 5′AGGCAGCCAACAGGCGAAGA.
Flouresence-activated cell sorter analysis of chemokine receptors.
A quantity of 5 × 105 cells was washed with Ca2+-and Mg2+-free phosphate-buffered saline (PBS) that contained 10% human AB serum (Flow Laboratories, McLean, VA). Cells were incubated for 20 minutes at 4°C in Ca2+- and Mg2+-free PBS 10% human AB serum in the presence of an appropriate dilution of PE-conjugated anti-CCR5 (1:20) and anti-CXCR4 (1:20) antibodies or FITC-conjugated anti-CD71 (1:10) or unlabeled anti-CCR2 antibodies (1:20). For direct immunofluorescence studies, cells were then washed with Ca2+- and Mg2+-free PBS 1% human AB serum and fixed in 1% formaldehyde. For indirect fluorescence studies, cells were further incubated for 20 minutes at 4°C with the appropriate secondary antibody and processed as described earlier. To evaluate the level of nonspecific binding, cells were incubated with IgG2a or IgG1k control monoclonal antibodies. Samples were analyzed by fluorescence-activated cell sorting (FACS) on a FACSort (Becton Dickinson) using the Lysis software (Becton Dickinson). For each determination, 10,000 cells were analyzed.
Receptor binding assays.
For MCP-1 and RANTES binding studies, 1 × 106 cells were incubated for 2 hours at 4°C with 0.25 nmol/L125I-MCP-1 or 0.1 nmol/L 125I-RANTES (specific activity, 2,200 Ci/mmol) in the presence of a 200-fold excess of unlabeled chemokines as previously described.36
Migration assay.
Cell migration was evaluated using a chemotaxis microchamber technique37 as previously described.34 A 25-μL quantity of chemoattractant diluted in Iscove’s medium with 1% FCS was loaded in the lower compartment of the chemotaxis chamber (Neuroprobe, Pleasanton, CA). A polycarbonate filter (5-μm pore size; Neuroprobe) was used to separate the 2 compartments. A 50-μL quantity of monocytes suspension (1 × 106) was seeded in the upper chamber. The chamber was incubated at 37°C in air with 5% CO2 for 90 minutes. At the end of incubation, filters were removed, fixed, and stained with Diff-Quick (Baxter S.p.A., Rome, Italy) and 5 high-power oil-immersion fields were counted.
Measurement of intracellular Ca2+ concentration.
Changes in the [Ca2+]i were monitored using the fluorescent probe Indo-1 (Molecular Probes, Eugene OR) in PBS as described elsewhere.34 Samples were analyzed in a spectrophotometer (Perkin Elmer LS-50B) continuously stirred at 37°C.
MCP-1 titration.
MCP-1 released in the culture medium was measured by enzyme-linked immunosorbent assay (ELISA; R&D Systems; detection limit, 5 pg/mL).
Statistical analysis.
Statistical analysis of data was performed by using parametric (analysis of variance) and nonparametric (Kruskall-Wallis) tests. AP value less than .05 was considered significant.
RESULTS
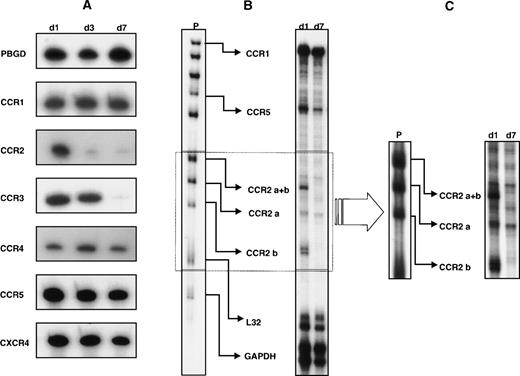
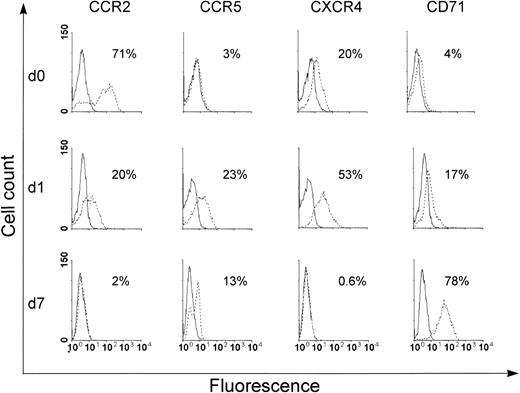
In a first set of experiments, we analyzed the expression of a panel of chemokine receptor mRNA in monocytes at different stages of differentiation by a sensitive radioactive RNA-PCR assay. As shown in Fig 1A, freshly isolated monocytes expressed mRNA for all the receptors investigated. Although the expression of the majority of these transcripts remained constant with time in culture, a significant decrease in the expression of CCR2 mRNA expression was already observed after 3 days with virtually no detectable expression at day 7. Likewise, the accumulation of CCR3 mRNA was also markedly decreased at day 7, even though with a slower kinetics than that observed for CCR2 mRNA. To better quantify differences in the steady-state levels of chemokine receptor mRNA, the expression of these transcripts was analyzed by a multiprobe RNase protection assay system. Although this method allows a more precise quantification of the mRNA level, its detection limit is lower than RNA-PCR, and thus does not allow the detection of low-abundance mRNA. As shown in Fig 1B, by using a multiprobe specific for β-chemokine receptor mRNA, the expression of CCR1, CCR2 (isoform a and b), and CCR5 was clearly detected in RNA samples from day 1 monocytes. In contrast, CCR3 and CCR4 mRNA were not visible under the same conditions, which suggests a low level of expression of these transcripts. In particular, as shown in Fig 1B and C, CCR2 mRNA that corresponded to the a and b isoforms were expressed in day 1 monocytes. An apparent loss in the expression of the b isoform was observed after 7 days of in vitro culture, whereas expression of the a isoform was not significantly modulated. The lack of expression of the CCR2b mRNA, as well as the maintenance of the CCR2a mRNA levels in 7-day–cultured macrophages, is better appreciated in Fig 1C, which shows a longer exposure of the gel in the region that corresponds to the CCR2 isoforms. The expression of other transcripts (CCR1, CCR5, and CXCR4) was generally not affected, even though a slight reduction in the CCR5 and CXCR4 mRNA levels at day 7 was occasionally found in some donors (data not shown). The densitometric analysis of the autoradiography shown in Fig 1B revealed that the steady-state levels of CCR2b mRNA were strongly decreased (CCR2b/GAPDH day 1, 1.33; day 7: no detectable CCR2b signal) during monocyte differentiation. No significant changes were detected in the steady-state levels of mRNA encoding CCR1 (CCR1/GAPDH day 1, 1.55; day 7, 2.5), CCR2a (CCR2a/GAPDH day 1, 0.44; day 7, 0.67) and CCR5 (CCR5/GAPDH day 1, 3.22; day 7, 3.51). To investigate whether the strong reduction in the CCR2 mRNA accumulation observed during monocyte differentiation was associated with a parallel decrease of the corresponding protein, we monitored the expression of this receptor at the cell surface. The expression of CCR2, CCR5, CXCR4, and CD71 was examined by FACS analysis (Fig2). In the same experiments, the expression of CD71 was monitored as control for monocyte differentiation.1-4 As expected, the expression of this differentiation marker was progressively increased with time in culture, thus confirming a time-dependent differentiation of monocytes into macrophages. CCR2 was expressed at the cell surface in day 0 monocytes (ie, before cell seeding). A marked decrease in expression of CCR2 occurred within 24 hours of culture, with undetectable expression at day 7 (Fig 2). CXCR4 was expressed at comparable level in day 0 and day 1 monocytes, but it became undetectable at day 7. In contrast, CCR5 was not expressed in day 0 monocytes, but a marked expression occurred within 24 hours of culture. The expression of this receptor was not maintained with time in culture and a significant reduction was observed at day 7. Similar results were obtained using monocytes/macrophages from 3 different donors.
Expression of chemokine receptors during monocyte differentiation. (A) RNA-PCR assay; 1 μg of total RNA extracted from monocytes at different stages of differentiation (day 1, 3, and 7) was retrotranscribed and amplified as described in Materials and Methods. Porphobilinogen deaminase was used as internal control. Results are representative of 4 independent experiments. (B,C) RNase protection assay; 5 μg of total RNA extracted after 1 and 7 days of monocytes culture was hybridized to the hCR5 multiprobe (P) as described in Materials and Methods. Autoradiographs were exposed for 24 hours (B) or 4 days (C) to better visualize the CCR2 mRNA isoforms. Representative results from 6 independent experiments are shown.
Expression of chemokine receptors during monocyte differentiation. (A) RNA-PCR assay; 1 μg of total RNA extracted from monocytes at different stages of differentiation (day 1, 3, and 7) was retrotranscribed and amplified as described in Materials and Methods. Porphobilinogen deaminase was used as internal control. Results are representative of 4 independent experiments. (B,C) RNase protection assay; 5 μg of total RNA extracted after 1 and 7 days of monocytes culture was hybridized to the hCR5 multiprobe (P) as described in Materials and Methods. Autoradiographs were exposed for 24 hours (B) or 4 days (C) to better visualize the CCR2 mRNA isoforms. Representative results from 6 independent experiments are shown.
Surface expression of CCR2, CCR5, CXCR4, and CD71 in monocytes at different stages of differentiation. Monocytes (day 0, 1, and 7) were directly or indirectly stained with specific antibodies and analyzed by FACS. The immunofluorescence profile obtained for each antibody was compared with that of its corresponding control and the result is shown as an open curve. The level of expression of each surface antigen is calculated by differences between the level of staining with a specific antibody and the baseline of the control antibody. Similar profiles were obtained with 3 different donors.
Surface expression of CCR2, CCR5, CXCR4, and CD71 in monocytes at different stages of differentiation. Monocytes (day 0, 1, and 7) were directly or indirectly stained with specific antibodies and analyzed by FACS. The immunofluorescence profile obtained for each antibody was compared with that of its corresponding control and the result is shown as an open curve. The level of expression of each surface antigen is calculated by differences between the level of staining with a specific antibody and the baseline of the control antibody. Similar profiles were obtained with 3 different donors.
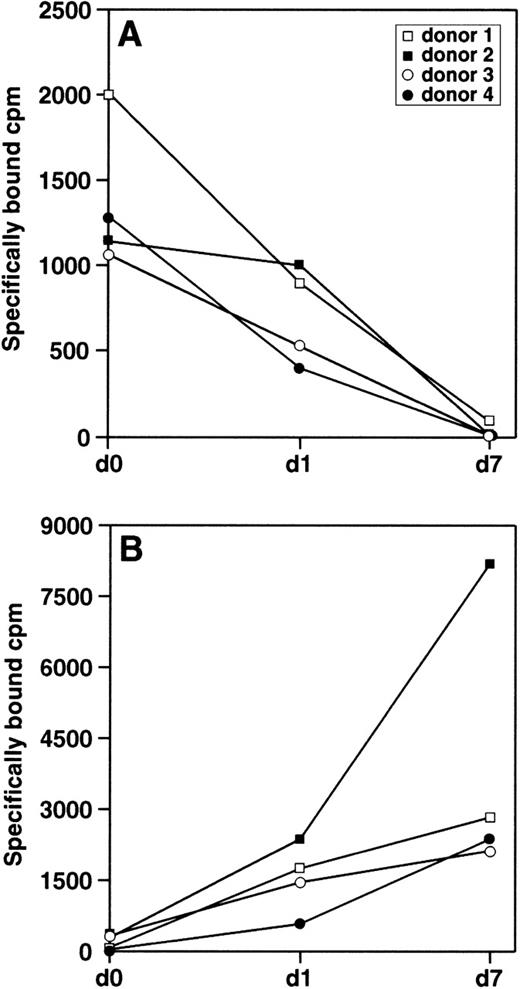
MCP-1 binds CCR2 at high affinity and represents its major ligand in monocyte.28 Therefore, we examined the binding of125I-MCP-1 to freshly isolated monocytes before adherence (day 0) and 24 hours after adherence (day 1), as well as to 7-day–cultured macrophages. As shown in Fig3A, the specific binding of125I-MCP-1 was significantly decreased in 3 of 4 donors after 24 hours of culture as compared with freshly isolated monocytes (day 0), while no specific binding was detected in 7-day–cultured macrophages from all donors. To investigate whether monocyte differentiation was associated with a generalized reduction in the binding of other chemokines, we examined the binding of125I-RANTES to monocytes from the same donors used for MCP-1 binding studies. This chemokine was chosen among others since it binds to a variety of chemokine receptors but not to CCR2. As shown in Fig 3B, the specific binding of 125I-RANTES was significantly increased to a variable extent in 7-day–cultured macrophages as compared with freshly isolated monocytes (day 0 and day 1).
Specific binding of 125I-MCP-1 and125I-RANTES to monocytes/macrophages at different stages of differentiation. Cells from 4 different donors (1 × 106) were incubated with 0.25 nmol/L 125I-MCP-1 (A) or 0.1 nmol/L 125I-RANTES (B). After incubation for 2 hours at 4°C, cell pellets were extensively washed and the radioactivity was measured in a γ counter. Specific binding was defined as the differences between total binding and nonspecific binding in the presence of a 200-fold excess of unlabeled chemokines; nonspecific binding never exceeded 20% of total binding. Each point represents the average of duplicate measurements. Statistical analysis showed that the differences in the binding of MCP-1 and RANTES observed in monocytes at different stages of differentiation were statistically significant (1-way analysis of variance P = .009 for MCP-1 and P= .0397 for RANTES; Kruskall-Wallis test P = .0125 for MCP-1 and P = .0097 for RANTES).
Specific binding of 125I-MCP-1 and125I-RANTES to monocytes/macrophages at different stages of differentiation. Cells from 4 different donors (1 × 106) were incubated with 0.25 nmol/L 125I-MCP-1 (A) or 0.1 nmol/L 125I-RANTES (B). After incubation for 2 hours at 4°C, cell pellets were extensively washed and the radioactivity was measured in a γ counter. Specific binding was defined as the differences between total binding and nonspecific binding in the presence of a 200-fold excess of unlabeled chemokines; nonspecific binding never exceeded 20% of total binding. Each point represents the average of duplicate measurements. Statistical analysis showed that the differences in the binding of MCP-1 and RANTES observed in monocytes at different stages of differentiation were statistically significant (1-way analysis of variance P = .009 for MCP-1 and P= .0397 for RANTES; Kruskall-Wallis test P = .0125 for MCP-1 and P = .0097 for RANTES).
To investigate the functional relevance of the downmodulation of CCR2 receptor expression, we performed experiments aimed to establish whether early or late steps in the responsiveness to MCP-1 were modified during monocyte differentiation. In a first series of experiments, we measured the effect of MCP-1 on the concentration of calcium ions ([Ca2+]i). Thus, monocytes were preloaded with the fluorescent calcium-sensitive probe indo-2, and changes in fluorescence were monitored by fluorimetry. As shown in Fig 4A, the addition of MCP-1 to day 1 monocytes induced a rapid (50 seconds) and transient (1.4 minute) increase in cytosolic [Ca2+]i. In contrast, no induction of intracellular [Ca2+]i was detected in 7-day–cultured macrophages. As shown in Fig 4B, where the average values from 3 independent experiments were illustrated, the maximum [Ca2+]i value measured in day 0 monocytes was 345 ± 36 nmol/L. Twenty-four hours later (day 1 monocytes), this value was already decreased to 159 ± 59 nmol/L and became almost undetectable at day 7 (65 ± 8 nmol/L). However, under the same experimental conditions, FMLP caused a high increase in the [Ca2+]i independently of the differentiation stages of monocytes/macrophages. Notably, 7-day–cultured macrophages responded to RANTES treatment to a greater extent than day 0 monocytes (168 ± 37 nmol/L v 50 ± 12 nmol/L). As expected, 7-day–cultured macrophages exhibited a loss of chemotactic response to MCP-1. In particular, the addition of MCP-1 to freshly isolated monocytes (1 and 24 hours after adherence) resulted in a consistent increase (6.5- to 3.3-fold) in the number of migrating cells, whereas no chemotactic response to MCP-1 was observed in 7-day–cultured macrophages (data not shown). No significant reduction in the response to other chemoattractants, such as FMLP and RANTES, was observed in 7-day–cultured macrophages with respect to day 0 and day 1 monocytes (data not shown).
Effect of MCP-1 on intracellular [Ca2+]i in differentiating monocytes. Cells (5 × 106/mL) were incubated with Indo-1 probe (2 μg/mL final) at 37°C for 30 minutes, washed, and exposed in cuvette (1 × 106/mL) to recombinant MCP-1 (10 ng/mL) or RANTES (10 ng/mL) or FMLP (1 × 10−7 mol/L) in the presence of 1 mmol/L extracellular Ca2+. One representative experiment of 3 is shown in (A). (B) Average values obtained in 3 independent experiments with monocytes from different donors.
Effect of MCP-1 on intracellular [Ca2+]i in differentiating monocytes. Cells (5 × 106/mL) were incubated with Indo-1 probe (2 μg/mL final) at 37°C for 30 minutes, washed, and exposed in cuvette (1 × 106/mL) to recombinant MCP-1 (10 ng/mL) or RANTES (10 ng/mL) or FMLP (1 × 10−7 mol/L) in the presence of 1 mmol/L extracellular Ca2+. One representative experiment of 3 is shown in (A). (B) Average values obtained in 3 independent experiments with monocytes from different donors.
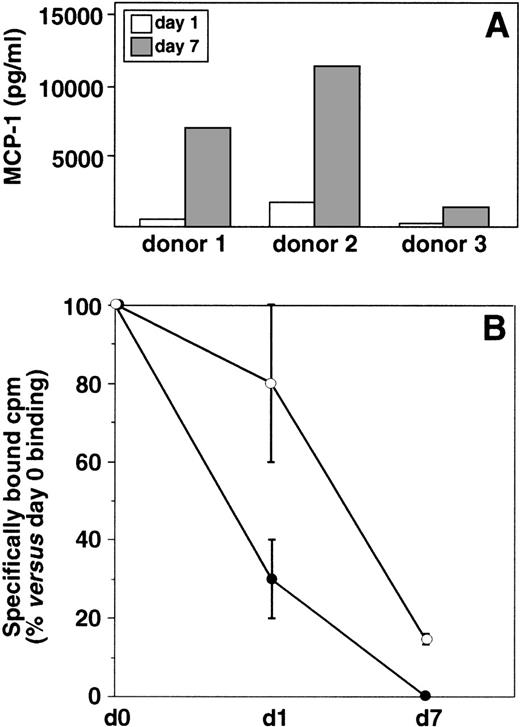
To define the mechanism(s) involved in the downmodulation of CCR2 observed during monocyte differentiation, we performed experiments aimed to clarify the possible role of endogenous MCP-1. In fact, it is well known that this chemokine can be produced by mononuclear phagocytes.34 35 The expression of MCP-1 was analyzed by measuring the amount of protein present in the culture supernatants of day 1 monocytes and 7-day–cultured macrophages. As shown in Fig5A, basal levels of MCP-1 were detected in day 1 monocyte cultures. Despite some variability among donors in the MCP-1 basal expression, a marked increase in the secretion of this chemokine was consistently observed in 7-day–cultured macrophages as compared with day 1 monocytes. No MCP-1 was detected intracellularly in freshly isolated monocytes (day 0) soon after cell seeding (data not shown), which indicates that MCP-1 protein expression was probably induced by adherence. In a second series of experiments, we attempted to clarify the role of this spontaneous release of MCP-1 in the downmodulation of CCR2 expression. The binding of125I-MCP-1 was measured in day 1 monocytes and 7-day–cultured macrophages maintained in the continuous presence of antibody to MCP-1. Figure 5B shows the mean value of results obtained with monocytes from 4 different donors. The results of these experiments indicated that neutralization of endogenous MCP-1 significantly reduced the spontaneous decrease of MCP-1 binding observed in day 1 monocytes. A slight increase in MCP-1 binding was also observed in 7-day–cultured macrophages maintained in the presence of antibody to MCP-1 with respect to control untreated cultures. These results suggest that, at least at early times of culture, endogenous MCP-1 can play a role in the downmodulation of CCR2 expression on the cell membrane.
Secretion of MCP-1 by monocytes/macrophages and its role in the downmodulation of MCP-1–specific binding sites. (A) Secretion of MCP-1 during monocytes differentiation. MCP-1 was measured by ELISA in the culture supernatants of freshly isolated monocytes (day 1) and 7-day–cultured macrophages. Results obtained with 3 different donors are shown. (B) Effect of antibody to MCP-1 on the specific binding of125I-MCP-1 to differentiating monocytes. Cells from 4 different donors were cultured in the presence (○) or in the absence (•) of polyclonal antibody to MCP-1 (5 μg/mL) for 1 or 7 days, extensively washed to remove the unbound antibody, and processed for binding studies as described in Fig 3. The mean percentage values (±SD) versus day 0 MCP-1 specific binding are shown. Statistical analysis showed that the differences in the binding of MCP-1 in monocytes cultured in the presence of antibody to MCP-1 were statistically significant (2-way analysis of variance P = .0194) with respect to untreated control cultures.
Secretion of MCP-1 by monocytes/macrophages and its role in the downmodulation of MCP-1–specific binding sites. (A) Secretion of MCP-1 during monocytes differentiation. MCP-1 was measured by ELISA in the culture supernatants of freshly isolated monocytes (day 1) and 7-day–cultured macrophages. Results obtained with 3 different donors are shown. (B) Effect of antibody to MCP-1 on the specific binding of125I-MCP-1 to differentiating monocytes. Cells from 4 different donors were cultured in the presence (○) or in the absence (•) of polyclonal antibody to MCP-1 (5 μg/mL) for 1 or 7 days, extensively washed to remove the unbound antibody, and processed for binding studies as described in Fig 3. The mean percentage values (±SD) versus day 0 MCP-1 specific binding are shown. Statistical analysis showed that the differences in the binding of MCP-1 in monocytes cultured in the presence of antibody to MCP-1 were statistically significant (2-way analysis of variance P = .0194) with respect to untreated control cultures.
DISCUSSION
The regulated interaction of chemokines with their respective receptors is thought to mediate the controlled recruitment of specific subpopulations required during host defense and inflammation.37,38 The specific biologic functions of chemokines and their receptors have been difficult to predict, since most receptors recognize more than 1 chemokine, and several chemokines bind to more than 1 receptor in vitro. Among the β-chemokine receptors, CCR2 appears to be rather specific for ligands that belong to the MCP family. Even though MCP-1 binds only CCR2 with high affinity, CCR2 also serves as receptor for MCP-1, MCP-3, and MCP-5.39-41 The analysis of CCR2−/−mutant mice has been useful for determining some of its specific physiologic functions. These studies clearly demonstrated that this receptor exhibits a nonredundant function as a major mediator of macrophage recruitment and trafficking and host defense to bacterial infections.42-44 The chemokine ligand MCP-1 is a potent in vitro monocyte activator that is abundantly expressed in a number of pathologic conditions characterized by monocytic infiltration.45 In spite of the existence of many C-C chemokines that attract monocytes in vitro, it has been recently demonstrated that loss of MCP-1 alone by targeted gene disruption is sufficient to impair monocyte trafficking in several inflammation models.46
In this study, we have reported that monocyte differentiation results in a marked downmodulation in the expression of CCR2 and CCR3 mRNA. Some changes in the expression of CCR2 and CCR3 mRNA during the spontaneous differentiation of human peripheral blood monocytes have been reported by Di Marzio et al.47 However, no studies on the possible biologic relevance of this phenomenon have yet been published. In contrast to CCR2 and CCR3 mRNA, the expression of transcripts codifying other chemokine receptors (CCR1, CCR4, CCR5, and CXCR4) was not substantially affected during monocyte differentiation. In this regard, recent results have shown that monocyte differentiation is associated with a differential expression of some chemokine receptors that may contribute to the selectivity of these cells to HIV entry.47-49 In particular, it has been reported that differentiation of monocytes to macrophages results in a significant increase in the number of cells that express CCR5.48 In parallel, a progressive decrease in the expression of CXCR4 at the plasma membrane was also observed.47-49 In agreement with these results, we found that CXCR4 expression at the plasma membrane decreases with time in culture, but we failed to detect any significant increase in the surface expression of CCR5 in 7-day–cultured macrophages with respect to day 1 monocytes. In fact, CCR5 was not expressed in day 0 monocytes, but a marked expression occurred within 24 hours of culture, likely due to adherence activation. However, the expression of this receptor was not maintained with time in culture and a significant reduction was observed after 7 days of culture. In addition, treatment of monocytes with IL-10 resulted in a marked increase in the expression of CCR5 at the plasma membrane (data not shown) in agreement with previously published results,33which indicates that the expression of CCR5 can be up-modulated following an appropriate stimulation. The apparent discrepancy between our results and those published by other groups47-49 can likely be explained by differences in the method of monocyte preparation. In particular, in these studies, monocytes were purified by plastic adherence,47 elutriation,49 or Percoll gradient followed by culture in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF).48 It is well known that the methods used for monocytes/macrophages separation and culture may have variable effects on cell functions and/or result in the isolation of different cell subpopulations.50 Moreover, the method of isolation of monocytes/macrophages has been shown to have effects on the levels of expression of certain cell surface molecules.51 52 Thus, it is not unexpected that the chosen method of separation can influence subsequent results about physiology and biochemistry of monocytes/macrophages.
The most important finding reported in this work is the progressive loss of functional MCP-1 receptors at the cell surface of monocytes differentiating into macrophages. The downmodulation of CCR2 mRNA was associated with a strong reduction in the receptor expression at the plasma membrane and MCP-1–specific binding. Of interest, the biologic response to MCP-1, as measured by intracellular calcium ions increase and MCP-1–induced chemotaxis, was totally lost in differentiated macrophages. Notably, neither RANTES-specific binding nor biologic response to RANTES or FMLP was affected. In this study, we have also provided evidence that monocyte differentiation results in a consistent increase in the secretion of MCP-1 that is likely triggered by adherence. In this regard, it is worth mentioning that, in spite of a consistent expression of MCP-1 mRNA in freshly isolated monocytes before plastic adherence, we failed to detect any intracellular MCP-1 (data not shown). These results would suggest that monocytes are “committed” to produce MCP-1, but an additional signal is required to trigger protein synthesis and secretion. In contrast with our results, Gruss et al reported a reduction in the production of MCP-1 during monocyte differentiation.53 This discrepancy can at least in part be explained by the fact that monocytes were cultured in Teflon (Heraeus, Germany) bags that allows monocyte cultivation under loosely adherent conditions.
Neutralization of MCP-1, spontaneously produced during in vitro culture of monocytes, reduces the initial downmodulation of CCR2 receptors at the plasma membrane in day 1 monocytes, but only marginally in differentiated macrophages, which suggests that, at least at early stages of differentiation, macrophage-derived MCP-1 plays a role in the down-regulation of CCR2 expression at the cell surface. However, in this regard, it is worth noting that the loss of CCR2 mRNA expression in cultured monocytes is likely due to cellular events linked to the macrophage differentiation process itself and cannot be simply explained by a ligand-induced down-regulation of CCR2 receptors. This conclusion is also supported by the finding that the MCP-1 neutralization by specific antibodies does not affect the levels of CCR2 mRNA in monocytes/macrophages cultured for 1 to 7 days in vitro (data not shown).
Taking into account the ensemble of our results, as well as data from the literature, we can envisage the following multistep scenario: (1) peripheral blood circulating monocytes express high levels of CCR2 receptors that are activated by chemoattractant stimuli released at inflammatory sites; (2) monocytes recruitment and subsequent adhesion to vascular endothelium induce an initial downmodulation of CCR2 receptors and a concomitant enhancement of MCP-1 production, while at the same time, adherence also triggers monocyte differentiation; (3) monocyte differentiation further results in a CCR2 downmodulation, which is likely due to both an increased release of MCP-1 (ligand-induced downmodulation of receptor expression) and a differentiation dependent shut-down of CCR2 mRNA; (4) the disappearance of CCR2 in differentiated macrophage results in the unresponsiveness of these cells to MCP-1, which provides an efficient regulatory system for controlling the extent of macrophage recruitment and activation. Thus, the inhibition of CCR2 expression and the parallel increase of MCP-1 secretion triggered by differentiation may represent novel feedback mechanisms in the regulation of the chemotactic response of these cells.
ACKNOWLEDGMENT
We are indebted to Sabrina Tocchio for excellent editorial assistance and to Roberto Gilardi for preparing drawings. We are grateful to Lucia Gabriele, Mauro Biffoni, and Robert Balderas for technical advice and helpful discussion. We thank Alberto Mantovani, Carlo Federico Perno, and Monica Napolitano for helpful discussion and suggestions. We are indebted to Istituto di Ricerca Cesare Serono for technological resources utilization.
Supported by grants from the Italian Ministry of Health (no. 40B/H and 40B/D, The National Research Program on AIDS, 1998). L.F. was the holder of a fellowship on AIDS research from the Italian Ministry of Health.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Sandra Gessani, PhD, Laboratory of Virology, Istituto Superiore di Sanità, Viale Regina Elena, 299-00161 Rome, Italy; e-mail:gessani@virus1.net.iss.it.

![Fig. 4. Effect of MCP-1 on intracellular [Ca2+]i in differentiating monocytes. Cells (5 × 106/mL) were incubated with Indo-1 probe (2 μg/mL final) at 37°C for 30 minutes, washed, and exposed in cuvette (1 × 106/mL) to recombinant MCP-1 (10 ng/mL) or RANTES (10 ng/mL) or FMLP (1 × 10−7 mol/L) in the presence of 1 mmol/L extracellular Ca2+. One representative experiment of 3 is shown in (A). (B) Average values obtained in 3 independent experiments with monocytes from different donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/3/10.1182_blood.v94.3.875.415k28_875_883/5/m_blod41528004x.jpeg?Expires=1766212332&Signature=ZugO2ACIFToCgHXHMbs96KHV39vrN--mpPxd4XCDGhw-gPgXZe5ldFh-Ku9EJjdgwCk2z~hXHKLtg2N8TXNYyApaLtXtaavr4BegNiiyv3SLCE-lly0GPykfV4CqP3DuG~69cDOkggHMA2A-iHaigpPITmip2fCPtbl7WTSgSizNVAI1Wid~Lgiizu3Pud4hBEY1pQyCnRs6lZu-CaSI3mUdBRW3dYZKABn76cy0~kcAp883WVYsO0WoGIpKAl3eUK2LlE2RYmjz1SirvtWG33oekrY-pDb1Ed3trBmkFzwd~XBgZS2evpyG2kPpwFktvtwfE829DQaFxZ4YTdxkjQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)