Abstract
Induction of transplantation tolerance by means of bone marrow (BM) transplantation could become a reality if it was possible to achieve engraftment of hematopoietic stem cells under nonlethal preparatory cytoreduction of the recipient. To that end, BM facilitating cells, veto cells, or other tolerance-inducing cells, have been extensively studied. In the present study, we show that BM cells within the Sca-1+Lin− cell fraction, previously shown to be enriched for early hematopoietic progenitors, are capable of reducing specifically antidonor CTL-p frequency in vitro and in vivo, and of inducing split chimerism in sublethally 7-Gy–irradiated recipient mice across major histocompatibility complex barriers. The immune tolerance induced by the Sca-1+Lin−cells was also associated with specific tolerance toward donor-type skin grafts. The minimal number of cells required to overcome the host immunity remaining after 7 Gy total body irradiation is very large and, therefore, it may be very difficult to harvest sufficient cells for patients. This challenge was further addressed in our study by demonstrating that non-alloreactive (host × donor)F1 T cells, previously shown to enhance T-cell–depleted BM allografts in lethally irradiated mice, synergize with Sca-1+Lin− cells in their capacity to overcome the major transplantation barrier presented by the sublethal mouse model.
THE INDUCTION of substantial and durable hematopoietic chimerism without graft-versus-host disease (GVHD), following minimal conditioning of the recipients, represents an extremely desirable goal in transplantation biology, because it is generally associated in murine models with a permanent transplantation tolerance.1-4 It has been shown that large doses of T-cell–depleted bone marrow (BM) transplants can permanently paralyze the resistance of host-type T cells remaining after exposure to sublethal 5.5 to 7.5 Gy total body irradiation (TBI).5 Subsequently, similar results were also obtained when the preparation of the recipients was based on treatment with anti–T-cell antibody plus thymic irradiation.6 It was shown in these studies that residual host-type cytotoxic T-lymphocyte precursors (CTL-p), surviving the sublethal conditioning, were markedly abrogated by the megadose BM transplants. This effect could be attributed to several types of accessory cells in the BM, as previously shown in murine models using lethally irradiated recipients7-14 or by in vitro studies measuring veto activity of different mouse15 or monkey16 BM cell subpopulations. A common characteristic of most, although not all, facilitating cells described in the literature is their expression of CD8 molecules on the cell surface. Although different facilitation mechanisms might be associated with different CD8+ cells, studies suggest that the CD8 molecule itself might be directly associated with this effect. Sambhara et al,15 using specific antibodies, showed that the interaction of CD8 molecules on the veto cells with the α3 domain of H-2 class 1 molecules on the effector cells can induce apoptosis specifically in the effector CTL-p, directed against class I antigens of the veto cells. Furthermore, attachment of soluble CD8 to the cell surface of nonveto cells, such as fibroblasts, has endowed these cells with a potent veto activity.17 Altogether, these observations have led to a particular emphasis on BM facilitation mediated by CD8+cells.
Most recently, with the advent of granulocyte colony-stimulating factor (G-CSF) stem cell mobilization in humans, it has been shown that the high rate of graft rejection experienced in the past in leukemic recipients of T-cell–depleted HLA disparate BM transplants can now be overcome by using large doses of stem cells that are collected from the donor’s blood after G-CSF mobilization.18-20 These clinical results indicated that cells within the CD34-enriched transplants are capable of overcoming the host resistance, and very recently Rachamim et al21 22 further supported this hypothesis by demonstrating in vitro that cells within the CD34 cell fraction possess marked veto activity.
Based on these indications, we attempted to investigate, in the mouse model, whether cells within the hematopoietic progenitor BM population are endowed with tolerance-inducing activity and are capable of overcoming the marked resistance, typical of sublethally irradiated allogeneic recipients. To address this question, we purified in the present study different BM cell fractions and we tested their capacity to induce split chimerism as well as to specifically reduce antidonor CTL-p, both in vitro and in vivo. We found that early hematopoietic Sca-1+Lin− cells, depleted of CD8+ cells, exhibit in vitro similar veto activity to that shown for human CD34 cells and, moreover, cells within the Sca-1+Lin− cell fraction are indeed capable of overcoming the marked immunoresistance of the host. However, these studies also indicate that the cell number required for tolerance induction in sublethally irradiated recipients is rather large and it may be difficult to collect a sufficient quantity in humans. Therefore, we explored other cells that might synergize with the Sca-1+Lin− cells in overcoming the challenge of the marked host immunity typical of the sublethal mouse model. To that end, the potential of (host × donor)F1non-alloreactive T cells, previously shown23 to enhance engraftment of T-cell–depleted BM allografts in lethally irradiated recipients, was evaluated.
MATERIALS AND METHODS
Animals.
Mice used were female 6- to 12-week-old C3H/HeJ and 4- to 6-week-old BALB/c, C57BL/6, C57BL/Beige, and (C3H/HeJ×C57BL/6)F1, obtained from the Roscoe B. Jackson Memorial Laboratory (Bar Harbor, ME) or the Weizmann Institute Animal Breeding Center (Rehovot, Israel). All mice were kept in small cages (5 animals in each cage) and fed sterile food and acid water containing cyprofloxacin (20 μg/mL).
Purification of murine stem cells by magnetic sorting.
BM cells were prepared as previously described,24 and then subjected to a stem cell purification procedure based on their expression of stem cell antigen-1 (Sca-1)25 and the lack of expression of cell-surface antigens associated with differentiated hematopoietic cell lineages typical for B cells (CD45/B220), myelomonocytic cells (CD11b/mac-1), and T cells (CD4/L3T4, CD8/Ly-2). Sca-1–positive, lineage-negative cells (Sca-1+Lin−) were purified from 6 to 10 × 109 BM cells (obtained from 100 C57BL/6 donors 5 to 9 weeks old, respectively), which were initially enriched for mononuclear cells by separation on Ficoll-Paque Plus (Pharmacia Biotech AB, Uppsala, Sweden). BM cells (50 × 107/20 mL) were layered on Ficoll (30 mL) in 50-mL Falcon tubes (Becton Dickinson, San Jose, CA). The cells were centrifuged at room temperature, at 800g for 30 minutes, and the cells in the Ficoll interface were collected. This cell fraction was magnetically labeled with anti–Sca-1 antibodies conjugated to microbeads using a multi-parameter magnetic cell sorting (MACS) Sca-1 Multisort Kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Sca-1+–labeled cells were then purified by a positive selection column in a magnetic field. The microbeads were then removed from the Sca-1+ cells using Multisort release reagent, to allow subsequent magnetic labeling and separation of Sca-1+cells according to expression of lineage markers. The depletion of Sca-1+ cells expressing lineage markers was performed by positive selection following labeling of Sca-1+ cells with MACS microbeads conjugated to antibodies directed against CD45/B220, CD8/Ly-2, CD4/L3T4, CD11b/mac-1, and anti-NK (DX5), when natural killer (NK) cells were depleted. The negative and positive fractions of this separation, the Sca-1+Lin− and Sca-1+Lin+ cell fractions, were collected. In a typical experiment starting with 6 × 109 BM cells, cell recovery after Ficoll_fractionation and in the Sca-1+, Sca-1+Lin+, or in the Sca-1+Lin− cell fraction was 2 × 109, 1 × 108, 71.5 × 106, and 5 × 106, respectively. Cytofluorimetric analysis of the fractionated cells was performed by double-immunofluorescent staining, using the following directly labeled antibodies (obtained from Pharmingen San Diego, CA): fluorescien isothiocyanate (FITC)-Sca-1/Ly-6A/E (clone D7), R-phycoerythrin (PE)-CD11b (clone M1/70), PE-CD8a/ Ly-2 (clone 53-6.7), PE-CD45/B220 (clone RA3-6B2), PE-CD4/L3T4 (clone RM-4-5), and PE-pan-NK cells (clone DX5) for NK cell detection. Nonspecific staining was analyzed with rat Ig isotype controls: FITC-rat IgG2a, PE-rat IgG2a, and PE-rat IgG2b.
Preparation of non-alloreactive F1 T cells.
Thymocytes from (C3H/HeJ×C57BL/6)F1 mice were separated by differential agglutination with peanut agglutinin into mature and immature fractions as described.26
Irradiation and BM transplantation.
Mice were exposed to a single dose of 7 Gy (sublethal conditioning) or 10 Gy (lethal conditioning) TBI from a Gamma beam 150-A60Co source (produced by the Atomic Energy of Canada, Kanata, Ontario, Canada) with focal skin distance of 75 cm, at a 0.65 Gy/min dose rate. The following day the mice received, intravenously, BM subpopulations as described in Results. In the experiments studying the facilitating effect of non-alloreactive T cells, mice were injected with Sca-1+Lin− cells supplemented with F1 T cells.
Chimerism analysis.
Chimerism was determined 30 days posttransplant by cytofluorimetry. Mice were bled from the retro-orbital vein using heparin-coated glass capillaries. Peripheral blood cells were fractionated on Ficoll-Paque plus, and the isolated mononuclear cells of each mouse were triple-stained by direct immunofluorescence, with FITC anti-H2b monoclonal antibody specific for the donor, PE anti-H2k for host-type major histocompatibility complex (MHC) antigens, and Cy-chrome-anti-CD3 antibody. The following antibodies were obtained from Pharmingen (San Diego, CA): FITC-H-2Dd (clone 34-2-12), PE-H-2Kk (clone 36-7-5), FITC-H-2Kb (clone AF6-88.5), and Cy-Chrome-CD3e (clone: 145-2C11).
Skin grafting.
Skin grafting was performed as previously described,27 with one additional step. Briefly, a circular piece of skin was removed from the recipient mouse and replaced by skin taken from the donor’s trunk after it had been cleaned of fat and connective tissue. The attachment of the skin graft to the recipient was achieved by spraying several thin layers of acrylic plastic spray (Nobecutan Spray; Astra, Sodertalje, Sweden) without any accessories. After the plastic dried (2 minutes), several layers of antibiotics (Rikospray antibiotic; Riker Laboratories, Loughborough, UK) were sprayed on the graft, and the mice were then transferred separately into individual small cages in which they were kept throughout the observation period. The transplanted skin grafts were observed daily and the graft status was recorded, with an acceptance score based on size, color, contraction, and hair growth of the graft. Rejection started with the loss of hair and culminated in necrosis of the graft epithelium that was occasionally associated with ulcer formation. Definite rejection was taken as the time of complete sloughing, or when a dry scab was formed.
Veto activity of Sca-1+Lin− cells.
To determine whether mouse Sca-1+Lin−cells possess veto activity, spleen cells from C3H/HeJ mice (2 × 106/mL) were incubated for 5 days with irradiated (25 Gy) allogeneic spleen cells (1 × 106/mL) from C57BL/6 (stem-cell matched) or BALB/c (third party) mice. C57BL/6 Sca-1+Lin− or Sca-1−cells were added to the primary MLC at a 0.5:1 veto:responder cell ratio. The responder cells were then recultured under limiting dilution conditions for 7 days. The CTL-p activity was determined by51Cr-release assay.
Direct limiting dilution culture of CTL-p.
Responder cells of the veto activity cultures or splenocytes of C3H/HeJ chimeric mice (irradiated by 7 Gy TBI and transplanted with 2 × 105 Sca-1+Lin− cells from C57BL/6 donors) were fractionated on Ficoll-Paque plus and the isolated mononuclear cells were plated in a limiting dilution culture. Responder cells (40 to 0.16 × 103) were cultured for 7 to 8 days in round-bottomed 96-well plates (Nunc, Roskilde, Denmark) in 16 replicates, in the presence of 106 cells/well of irradiated (20 Gy) allogeneic (C57BL/6, BALB/c) or syngeneic (C3H/HeJ) splenocytes and human recombinant interleukin-2 (10 U/mL; Eurocetus, Milan, Italy) in a final volume of 0.2 mL complete tissue culture medium (CTCM) at 37°C in a 10% CO2 air incubator. CTCM is RPMI 1640 which contains 2 mmol/L L-glutamine, 100 U/mL penicillin, 0.1 mg/mL streptomycin, 2 mmol/L HEPES, 1 mmol/L sodium pyruvate, 0.1 mmol/L nonessential amino acids, and 5 × 10−5 mol/L 2-mercaptoethanol, supplemented with 10% fetal calf serum (Biological Industries, Kibbutz Beit Hemek, Israel).
Estimate of cytotoxic cell frequency.
Splenocytes were harvested from individual limiting dilution culture wells and were assayed for cytotoxic activity by transferring a fixed volume (100 mL) to conical-bottomed 96-well plates (Greiner, Frickenhausen, Germany) containing 5 × 103 51Cr-labeled Concanavalin A (Sigma, St Louis, MO) blasts of C3H/HeJ, C57BL/6, or BALB/c, respectively, as target cells. As described by Ryser and MacDonald,28 microwells were considered positive for cytolytic activity when they exceeded the mean spontaneous release value (determined in a group of parallel wells that contained irradiated stimulating cells and CTCM, but no responding cells) by at least 3 standard deviations of the mean.
RESULTS
Induction of split chimerism by purified Sca-1+Lin− progenitor cells in sublethally irradiated allogeneic mice.
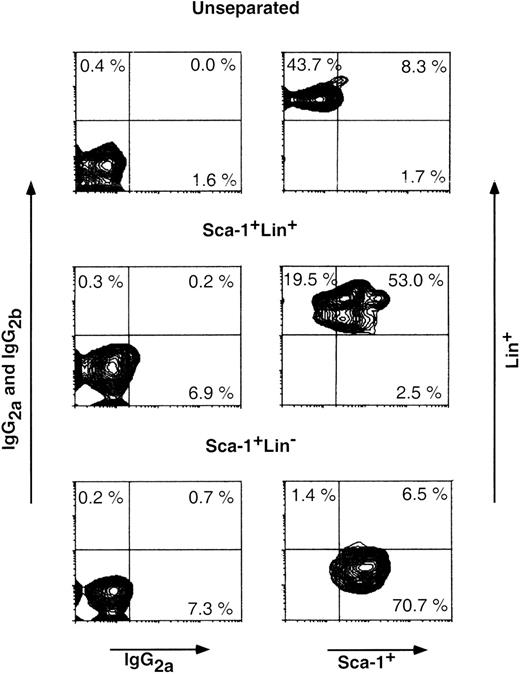
To test the potential of cells within the hematopoietic progenitor cell fraction to overcome host resistance, we attempted to purify (using magnetic beads) Sca-1+Lin− BM cells from C57BL/6 donors, and we tested their capacity to induce chimerism in fully allogeneic C3H/HeJ recipients exposed to sub-lethal 7 Gy TBI. Figure 1 shows an analysis on a fluorescence-activated cell sorter (FACS) of a typical purification of Sca-1+Lin− cells afforded by the MACS double-step procedure. The average frequency of these cells in the initial BM fraction that was applied to the MACS procedure (depleted of red blood cells and neutrophils by Ficoll separation) was 2.7% ± 1.1% (range, 1.8% to 5.1%), and after the 2-step fractionation procedure it was enriched to an average of 68.3% ± 9.8% (range, 53.5% to 84.4%).
Purification of Sca-1+Lin− BM cells. Cells in the different cell fractions obtained by MACS purification (see Materials and Methods) were analyzed by FACS for the presence of Sca-1+Lin+, Sca-1+Lin−, and Sca-1−Lin+ cells. Nonspecific staining was determined by FITC- and PE-conjugated isotype control antibodies. Percentage of each subpopulation is shown in the appropriate area of the dot plot.
Purification of Sca-1+Lin− BM cells. Cells in the different cell fractions obtained by MACS purification (see Materials and Methods) were analyzed by FACS for the presence of Sca-1+Lin+, Sca-1+Lin−, and Sca-1−Lin+ cells. Nonspecific staining was determined by FITC- and PE-conjugated isotype control antibodies. Percentage of each subpopulation is shown in the appropriate area of the dot plot.
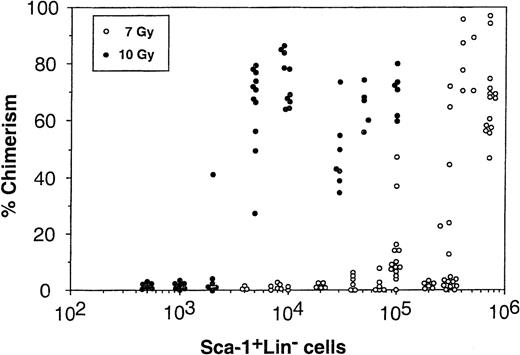
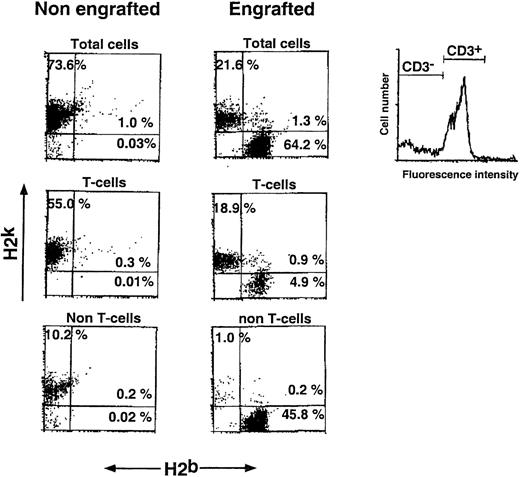
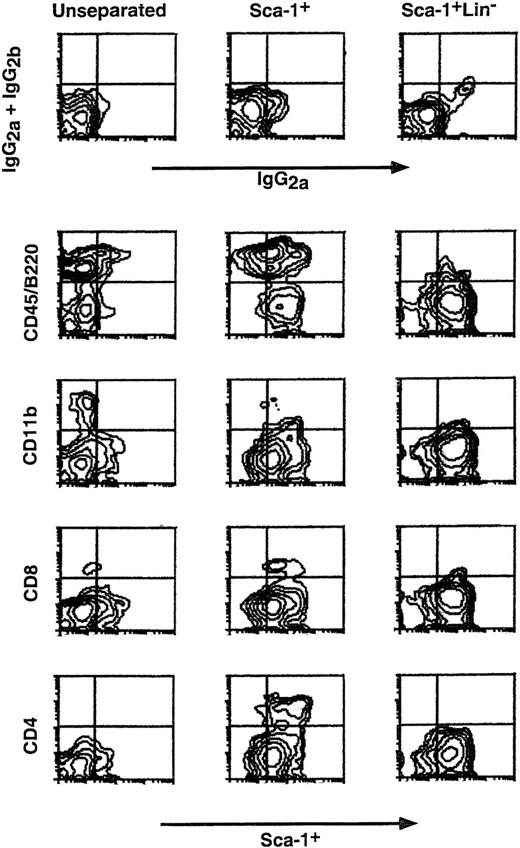
Determination of the different subpopulations within the Lin+ phenotype (Table 1) showed more than 1 log depletion of CD4, CD8, CD11b, and CD45/B220 cells from the Sca-1+Lin− cell fraction compared with that found in the Sca-1+ cell fraction. Determination of donor-type chimerism after infusion of different cell numbers from the Sca-1+Lin− cell fraction showed an exponential dose-response curve similar to those previously described for T-cell–depleted transplants.5 The pooled results of 8 different experiments illustrating the chimerism level induced in each individual mouse exposed to 7 Gy TBI clearly show that substantial chimerism (>20%) was initially noticed upon infusion of 3 × 105 Sca-1+Lin− cells (9 of 20), whereas recipients of 4 × 103 to 2 × 105 Sca-1+Lin− cells very rarely exhibited low levels of donor-type chimerism (2 of 48) (Fig 2). In contrast, when C3H/HeJ recipients of C57BL/6 Sca-1+Lin− BM cells were exposed to 10 Gy lethal TBI, about 75-fold fewer cells (4 × 103 Sca-1+Lin−) were required for the induction of over 50% donor-type chimerism in 10 of 11 recipients (Fig 2). It seems that a large proportion of the cells, required to establish chimerism in the sublethal model, are involved in overcoming the larger number of host immune cells remaining after 7 Gy TBI, compared with the miniscule numbers surviving 10 Gy TBI. Considering that, in C3H/HeJ recipients of C57BL/6 BM, resistance is largely mediated by T-cell–mediated responses while NK-cell–mediated resistance is minimal,29 it should be noted that spleens of C3H/HeJ mice, 1 week after exposure to 7 Gy TBI, contain about 1 log more T cells compared to mice treated with 10 Gy TBI (unpublished results, January 1999). Indeed, FACS analysis of the chimeric mice obtained after exposure to sublethal TBI showed a substantial number of host-type T cells coexisting with donor-type T cells, as well as with non-T cells (Fig 3). No GVHD, as defined by measurement of body weight and the general appearance of the mice, was observed in recipients of Sca1+Lin− cells, in agreement with previous studies, defining the threshold for GVHD in lethally irradiated mice30 by more than an order of magnitude above the numbers that might have been infused in the present study (ie, <1 × 103 T cell/mouse). Furthermore, considering that antihost donor alloreactivity generally manifests itself by eradication of host-type blood cells, our finding of durable long-term substantial mixed chimerism strongly suggests that if there is any T-cell contamination in the infused Sca1+Lin− cell fraction (ie, below detection of our FACS analysis; Table 1), it is not associated with any form of clinical or subclinical GVHD.
Induction of donor type chimerism after transplantation of C57BL/6 Sca-1+Lin− cells into lethally (10 Gy TBI) or sublethally (7 Gy TBI) irradiated C3H/HeJ recipients. The number of Sca-1+Lin− cells infused was calculated based on FACS analysis.
Induction of donor type chimerism after transplantation of C57BL/6 Sca-1+Lin− cells into lethally (10 Gy TBI) or sublethally (7 Gy TBI) irradiated C3H/HeJ recipients. The number of Sca-1+Lin− cells infused was calculated based on FACS analysis.
Split chimerism after transplantation of C57BL/6 Sca-1+Lin− cells (H2b) into sublethally irradiated (7 Gy) C3H/HeJ recipients (H2k). Percentages of donor and host T cells (determined by gating of CD3 stained cells) and non-T cells are shown in the appropriate FACS dot plots. Peripheral blood chimerism was determined 30 days posttransplant by cytofluorimetry (see Materials and Methods).
Split chimerism after transplantation of C57BL/6 Sca-1+Lin− cells (H2b) into sublethally irradiated (7 Gy) C3H/HeJ recipients (H2k). Percentages of donor and host T cells (determined by gating of CD3 stained cells) and non-T cells are shown in the appropriate FACS dot plots. Peripheral blood chimerism was determined 30 days posttransplant by cytofluorimetry (see Materials and Methods).
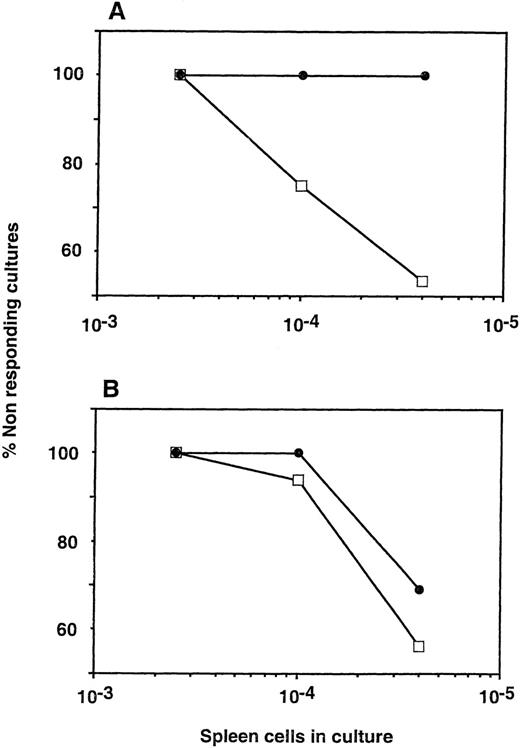
Limit dilution analysis of CTL-p in the spleen of chimeric mice showed no detectable CTL-p against donor type cells (C57BL/6) while the frequency of CTL-p against a third party (BALB/c) was not significantly different from that found in irradiated mice that were not transplanted with Sca-1+Lin−cells (Fig 4). To test the capacity of Sca-1+Lin− cells to affect postthymic T cells that survive the sublethal irradiation and that present a barrier to engraftment during the immediate period posttransplant, we chose to evaluate the level of chimerism at 30 days after transplantation. However, we also tested donor-type chimerism 7 months posttransplant, and it was found to be similar to the level found at the earlier time point (data not shown).
Anti-donor CTL-p in allogeneic chimera (C57BL/6 → C3J/HeJ) generated by transplantation of C57BL/6 Sca-1+Lin− cells. Limiting dilution analysis of CTL-P was performed against donor (A) or third-party (B) splenocytes. (•) Irradiated mice transplanted with Sca-1+Lin− cells. (□) Irradiated mice not receiving a transplant.
Anti-donor CTL-p in allogeneic chimera (C57BL/6 → C3J/HeJ) generated by transplantation of C57BL/6 Sca-1+Lin− cells. Limiting dilution analysis of CTL-P was performed against donor (A) or third-party (B) splenocytes. (•) Irradiated mice transplanted with Sca-1+Lin− cells. (□) Irradiated mice not receiving a transplant.
Therefore, it seems that cells within the purified Sca-1+Lin− cell fraction, or their immediate progeny, can effectively tolerize the marked number of host CTL-p that survive sublethal irradiation. Furthermore, when the established chimera were challenged by skin grafts from donor or third-party origin, only the former were accepted (7 of 7) while the third-party skins were uniformly rejected (7 of 7). Thus, as in radiation chimera following conditioning with lethal TBI, the tolerance induced by the Sca-1+Lin− cells in sublethally irradiated recipients is not limited to hematopoietic antigens.
Enrichment for chimerism-inducing cells is associated both with positive selection of Sca-1+ cells and with the subsequent negative selection for Sca-1+ Lin− cells.
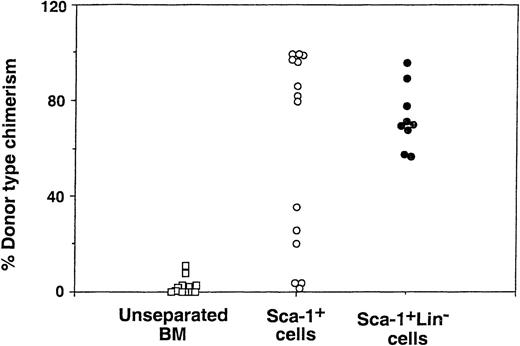
Although the purification procedure afforded over 25-fold enrichment of Sca-1+Lin− cells, the average purity of the Sca-1+Lin− BM cell fraction achieved by this large scale approach was limited to about 68%. Thus, it could be argued that the tolerizing effect exhibited by the infusion of Sca-1+Lin− cells might be mediated in part by other contaminating cells. Therefore, after each fractionation step we evaluated both the enrichment factor of the selected phenotype and the level of chimerism associated with this enrichment. In 2 experiments, no chimeric mice were found upon infusion of 1 × 106 unseparated cells, while following the first step of positive selection of Sca-1+ cells, infusion of 1 × 106 cells of the Sca-1+ fraction led to substantial donor type chimerism (>20%) in 10 of 14 recipients (Fig 5). The second step of negative selection led to further enhancement of engraftment (9 of 9) compared with that observed in the Sca-1+ cell fraction. While the latter enhancement is not statistically significant, there is clearly no reduction of chimerism induction associated with the more than 1 log depletion of Lin+ cells (Table 1), despite the exponential nature of our chimerism induction curves (Fig 2). It seems that, regardless of other cell phenotypes that might possess tolerizing or facilitating activity, the enrichment of Sca-1+Lin− cells, associated with depletion of different cell subpopulations sharing the Sca-1+Lin+ phenotype, is also associated with the capacity to achieve engraftment in the face of marked host immunity remaining after the sublethal conditioning. Because several previous studies have shown that different cell subpopulations bearing CD8 are capable of enhancing BM allografts, we examined the presence of such cells in the different cell fractions. The FACS data of a typical experiment are illustrated in Fig 6, and the level of each cell phenotype (based on the FACS analysis), found in 2 independent experiments, is shown in Table 1. The percentage of CD8+ cells in the unseparated fraction was 0.6% to 0.7%. After purification for Sca-1+ cells, the level of CD8+ cells was enriched to 2.7% and 3.4%, respectively. After depletion of Lin+ cells it was reduced to <0.1% and <0.2%, respectively, in the Sca-1+Lin− cell fraction. Thus, although the enhancement of donor-type chimera in recipients of Sca-1+ cells could be mediated by either Sca-1+CD8+ or Sca-1+Lin− cells, both of which are enriched in this fraction, the high level of chimerism found after the second fractionation step, which is associated with at least a 20-fold depletion of CD8+ cells along with a 12-fold enrichment of Sca-1+Lin− cells, strongly suggests that a CD8-negative cell in the Sca-1+Lin−cell fraction is responsible for the ability of Sca-1+Lin− cells to induce marked donor-type chimerism in face of the substantial host resistance remaining in sublethally irradiated recipients.
Donor type chimerism after transplantation of different BM cell fractions into allogeneic recipients exposed to sublethal (7 Gy) TBI: effect of positive selection for Sca-1+ cells and subsequent depletion of Lin+ cells. The figure shows peripheral blood chimerism at 30 days after transplantation of 1 × 106 cells from each cell fraction.
Donor type chimerism after transplantation of different BM cell fractions into allogeneic recipients exposed to sublethal (7 Gy) TBI: effect of positive selection for Sca-1+ cells and subsequent depletion of Lin+ cells. The figure shows peripheral blood chimerism at 30 days after transplantation of 1 × 106 cells from each cell fraction.
Depletion by MACS of CD8+, CD4+, CD11b+, and CD45/B220+cells after removal of Lin+ cells from the Sca-1+ BM cell fraction. Levels of CD8+, CD4+, CD11b+, and CD45/B220+cells in unseparated, Sca-1+, and Sca-1+Lin− BM cell fractions, obtained by MACS, were analyzed by double staining with antibodies directed against Sca-1 and the above lineage markers. Nonspecific staining was determined by FITC- and PE-conjugated isotype control antibodies.
Depletion by MACS of CD8+, CD4+, CD11b+, and CD45/B220+cells after removal of Lin+ cells from the Sca-1+ BM cell fraction. Levels of CD8+, CD4+, CD11b+, and CD45/B220+cells in unseparated, Sca-1+, and Sca-1+Lin− BM cell fractions, obtained by MACS, were analyzed by double staining with antibodies directed against Sca-1 and the above lineage markers. Nonspecific staining was determined by FITC- and PE-conjugated isotype control antibodies.
Donor NK cells with antihost alloreactivity that might contaminate the Sca-1+Lin− cell fraction or emerge upon engraftment and differentiation of the Sca-1+Lin− cells could also contribute in part to the tolerance observed, by eradicating residual host peripheral T cells. However, addition of anti-NK antibody to the Lin+ depletion step did not abrogate the capacity of Sca-1+Lin− cells to engraft, nor did the use of Sca-1+Lin− cells from NK-deficient C57BL/6-Beige donors (data not shown).
Specific reduction of antidonor CTL-p by Sca-1+Lin− cells.
To evaluate whether the Sca-1+Lin− cell fraction is enriched for cells possessing veto activity, we analyzed different cell fractions for their capacity to specifically reduce antidonor CTL-p in comparison with anti–third-party CTL-p. Mixed lymphocyte reaction (MLR) was set up using spleen cells from C3H/HeJ mice as responder cells and spleen cells from C57BL/6 or BALB/c (third-party) mice as stimulator cells. To evaluate the veto activity of C57BL/6 Sca-1+Lin− cells compared with Sca-1− cells, these cells were added to the MLR at a veto-to-responder cell ratio of 0.5:1, and the CTL-p levels were determined by limiting dilution analysis. As can be seen in Table 2, addition of Sca-1+Lin− cells completely abrogated CTL-p directed against their H-2 antigens (C57BL/6) while substantial cytotoxic activity could be monitored when no cells were added, or upon addition of Sca-1− cells. In contrast, when the same Sca-1+Lin− cells were added to MLR against third-party (BALB/c) stimulators, no reduction of CTL activity was found when compared with the level determined in the absence of added cells. Thus, selection of Sca-1+Lin− cells is associated with enrichment of veto activity.
Non-alloreactive (host × donor)F1 T cells synergize with Sca-1+Lin− BM cells of donor origin in the induction of donor-type chimerism.
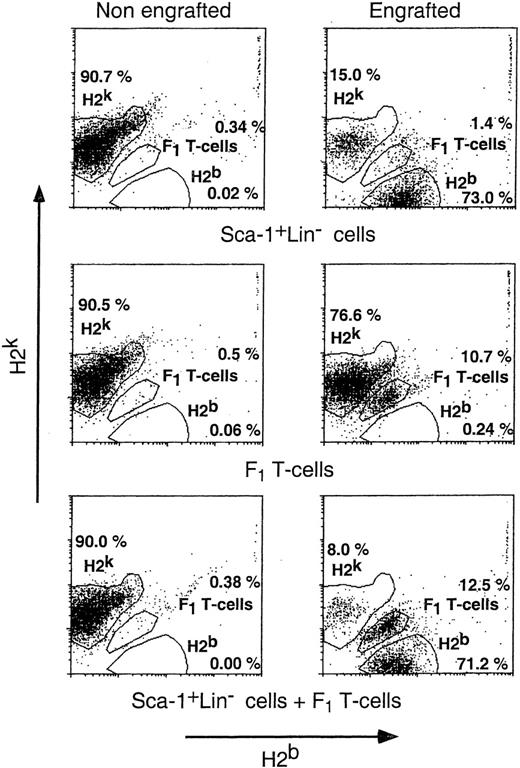
Although we are encouraged by the ability of Sca-1+Lin− cells to overcome the host T cells remaining after sublethal TBI, it is clear that the number of cells required is very high and that it may be quite difficult to collect such large numbers of cells from human donors. For example, in the experiments described above, transplantation into sublethally irradiated recipients required about 75-fold more Sca-1+Lin− cells compared with the number engrafting lethally irradiated mice. To reduce the effective number of Sca-1+Lin− cells needed for overcoming resistance to engraftment, we were interested in studying the potential role of other facilitating or veto cells. Therefore, we attempted to test whether the addition of non-alloreactive (donor × host)F1 T cells, previously shown23 to exhibit marked enhancement of engraftment of T-cell–depleted transplants in mismatched lethally irradiated recipients, could also help to reduce the minimal number of progenitor cells required for chimerism induction in the sublethal model. As can be seen in Fig 7, in 5 experiments monitoring the engraftment of 1 × 107 infused F1 T cells (H2b+H2k+) in sublethally irradiated C3H/HeJ (H2k) recipients by their unique H2b+H2k+ phenotype, we found that only 16.6% of the mice were engrafted, suggesting that infusion of this number of F1 T cells alone could overcome resistance only in a relatively small fraction of the recipients. However, engraftment of F1 T cells was markedly increased to 65% by adding 2.0 × 105 purified Sca-1+Lin− cells. Likewise, engraftment of a suboptimal number of 2.0 × 105 purified (Sca-1+Lin−) cells, monitored by the presence of donor-type cells (stained by anti-H2b and not by anti-H2k), was enhanced, by adding 1 × 107 F1 T cells, from 12.9% of the recipients to 74.0%.
Donor type chimerism after transplantation of Sca-1+Lin− cells: the synergistic effect of non-alloreactive T cells and purified Sca-1+Lin− cells. Different groups of sublethally (7 Gy) irradiated C3H/HeJ recipients (H2k+) were transplanted with 2 × 105 (C57BL/6) Sca-1+Lin− cells (H2b+), 10 × 106 (C3H/HeJ × C57BL/6)F1 T cells (H2k+H2b+), or both. Peripheral blood chimerism was analyzed 30 days posttransplant by flow cytometry, monitoring the engraftment of Sca-1+Lin−cells (C57BL/6) (H2b+) (A) and (C3H/HeJ × C57BL/6)F1 T cells (H2k+H2b+) (B). Data were pooled from 5 independent experiments.
Donor type chimerism after transplantation of Sca-1+Lin− cells: the synergistic effect of non-alloreactive T cells and purified Sca-1+Lin− cells. Different groups of sublethally (7 Gy) irradiated C3H/HeJ recipients (H2k+) were transplanted with 2 × 105 (C57BL/6) Sca-1+Lin− cells (H2b+), 10 × 106 (C3H/HeJ × C57BL/6)F1 T cells (H2k+H2b+), or both. Peripheral blood chimerism was analyzed 30 days posttransplant by flow cytometry, monitoring the engraftment of Sca-1+Lin−cells (C57BL/6) (H2b+) (A) and (C3H/HeJ × C57BL/6)F1 T cells (H2k+H2b+) (B). Data were pooled from 5 independent experiments.
The resulting chimeric mice exhibited stable split chimerism in which significant levels of host (H2k+H2b−) and donor-type T cells (H2b+H2k−) as well as F1 T cells (H2b+H2k+) coexisted with host and donor non-T cells (Fig 8).
A typical profile of split chimerism, exhibited by chimeric mice transplanted with Sca-1+Lin−cells (2 × 105, H2b+), (C3H/HeJ × C57BL/6)F1 T cells (H2k+ + H2b+, 10 × 106), or both. Peripheral blood chimerism was analyzed 30 days posttransplant by flow cytometry, using antibodies directed against host (H2k) and donor (H2b) MHC antigens.
A typical profile of split chimerism, exhibited by chimeric mice transplanted with Sca-1+Lin−cells (2 × 105, H2b+), (C3H/HeJ × C57BL/6)F1 T cells (H2k+ + H2b+, 10 × 106), or both. Peripheral blood chimerism was analyzed 30 days posttransplant by flow cytometry, using antibodies directed against host (H2k) and donor (H2b) MHC antigens.
DISCUSSION
Our results demonstrate that cells within the BM Sca-1+Lin− subpopulation, previously shown to comprise the pluripotential hematopoietic stem cells as well as more differentiated progenitors, possess a marked capacity to overcome the substantial host resistance found in recipients conditioned by sublethal 7 Gy TBI. Considering that it is virtually impossible to achieve homogeneous purity by any methodology of BM cell fractionation, we resorted in our analysis to evaluation of specific activities before and after each of the purification steps. We found that the initial step of positive selection of Sca-1+ cells contributes significantly to the enhancement of donor-type chimerism and that over 1 log depletion of Lin+ cells from the Sca-1+ cell fraction does not retract from the engraftment potency of the resulting Sca-1+Lin− cell fraction but, rather, further enhances donor-type chimerism.
In particular, our demonstration that the chimerism-inducing activity is enhanced upon purification of Sca-1+Lin− cells from the Sca-1+ cell fraction, while the frequency of CD8+ cells is reduced by about 20-fold, suggests that the ability of Sca-1+Lin− cells to overcome the marked host immunity is probably mediated by a non-CD8–mediated mechanism. Likewise, the use of NK-deficient “beige” donors or NK-depleted Sca-1+Lin− cells rule out the possibility that the latter cells are critical for the effect exerted by cells in the Sca-1+Lin− cell fraction.
It could be argued that chimerism induction associated with purification of Sca-1+Lin− cells might involve not only the capacity to overcome the host immune system, but it may also be mediated by their capacity to compete with host hematopoietic stem cells.31,32 This inherent duality of Sca-1+Lin− cells was addressed by our demonstration that Sca-1+Lin− cells facilitate not only their own engraftment, which could be reflecting, in part, donor-versus-host stem cell competition, but also the engraftment of (donor × host)F1 T cells, as well as acceptance of donor-type but not third-party skin. Thus, we were able to separate in vivo the potent capacity to overcome host immunity associated with the purified Sca-1+Lin−cells from their hematopoietic repopulating capacity, which might also contribute to donor-type chimerism induction. We have shown recently that escalation of human CD34 progenitor cell transplants enables overcoming major HLA barriers in the treatment of heavily conditioned leukemia patients.18-20 The capacity of the “mega dose” transplants to neutralize residual host antidonor CTL-p was attributed in part to the veto activity shown in the hematopoietic progenitor CD34 cell compartment.21 22
Our present study, finding great similarity between the Sca-1+Lin− and the human CD34 cell fraction, suggests that further insight into the mechanism of tolerance induction by hematopoietic progenitor cells might be facilitated by using different natural or genetically engineered mutant strains of mice.
Our demonstration that resistance to fully allogeneic stem cell transplants found in sublethally irradiated recipients can be overcome by escalation of cell dose is in accordance with the recent study of Uchida et al,33 which showed that lethally irradiated allogeneic recipients transplanted with highly purified c-Kit+Thy-1.1lo-Lin−/loSca-1+stem cells require about 10-fold more cells compared with congenic recipients. However, the comparison between the allogeneic and the congenic groups in that study was made using animals pretreated by a different TBI dose. Moreover, there are no data regarding recipients treated with sublethal TBI. Thus, further studies are required to define whether the c-Kit+Thy-1.1lo-Lin−/loSca-1+cells within the Sac-1+Line− cell fraction will exhibit a similar capacity to overcome BM allograft rejection to that exhibited in the present study by the Sca-1+Line− cells in the sublethal model.
The characterization of new cell phenotypes that are capable of enhancing acceptance of T-cell–depleted BM allografts is of great relevance to the continuing efforts to achieve HLA disparate hematopoietic engraftment in humans. Our present results in the mouse model, although demonstrating that major transplantation barriers can be overcome by megadoses of BM progenitors, also suggest that it might be difficult to harvest sufficient cells in humans. We found that the minimal number of Sca-1+Lin− cells required to achieve donor type chimerism in 7 Gy TBI treated mice was about 75-fold higher than that needed in lethally irradiated (10 Gy TBI) recipients.
One approach to overcome this quantitative problem might be afforded if it were possible to expand the veto cells within the CD34+progenitor cell fraction. Thus, while it is still very difficult to expand ex vivo the most primitive pluripotential hematopoietic stem cells, it may be possible to expand in vitro the CD34+cells possessing veto activity and use them together with a small number of pluripotential cells for transplantation. Further studies to elucidate the cell subpopulations that are most relevant for the induction of tolerance, in the human CD34 or in the mouse Sca-1+Lin− subpopulation, might help to find simple means to expand these valuable cells in vitro, and could help develop strategies for the elucidation of the mechanism by which their effect is mediated.
An alternative approach to achieving engraftment in the face of marked residual host immunity is based on the use of CD34 cells, together with other veto cells or facilitating cells. One example for such cells is shown in the present study by using (host × donor)F1non-alloreactive T cells, previously shown to synergize with suboptimal doses of T-cell–depleted BM allografts in the lethal mouse model.30 In humans, non-alloreactive T cells can be generated by purging interleukin-2 receptor (CD25) MLR reactive T cells34 or by anergy induction upon incubation with CTLA-4.35 36
Supported in part by a grant from the Israel Academy of Science, the Leukemia Society of America, and the Rich Foundation. Y.R. is the incumbent of the Henry Drake Professorial Chair.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Yair Reisner, Department of Immunology, The Weizmann Institute of Science, Rehovot 76100, Israel.