Abstract
Human SCID repopulating cells (SRC) are defined based on their functional ability to repopulate the bone marrow of NOD/SCID mice with both myeloid and lymphoid cell populations. The frequency of SRC in umbilical cord blood cells is 1 in 9.3 × 105mononuclear cells. We report that as few as 8 × 104 human cord blood mononuclear cells transplanted into NOD/SCID/B2mnull mice resulted in mutlilineage differentiation in the murine bone marrow, revealing a more than 11-fold higher SRC frequency than in NOD/SCID mice. Moreover, as few as 2 to 5 × 103 CD34+ cells recovered from the bone marrow of primary transplanted NOD/SCID mice were sufficient for engrafting secondary NOD/SCID/B2mnull mice with SRC, suggesting SRC self-renewal. Thus, by using NOD/SCID/B2mnull mice as recipients, we established a functional assay for human stem cells capable of engrafting the bone marrow of primary and secondary transplanted immune-deficient mice with SRC, providing a model that better resembles autologous stem cell transplantation.
Blood-forming hematopoietic stem cells are defined in repopulation assays based on their functional ability to home to the bone marrow microenvironment and to repopulate transplanted recipients durably with both myeloid and lymphoid cell populations.1We and others2-5 developed functional in vivo assays for primitive human cells by transplanting them into sublethally irradiated immune-deficient C.B-17-Prkdcscid (SCID) and, more recently, into NOD/LtSz-Prkdcscid (NOD/SCID) mice, with reduced residual immunity. An alternative approach for reducing innate immunity of the host, used by other investigators,6,7 was to treat these mice with anti-natural killer cell antibodies before transplantation with or without irradiation, resulting in improved engraftment. Quesenberry et al8,9 demonstrated that total body irradiation is not a prerequisite for stem cell engraftment in transplanted murine recipients by multiple daily injections of human or mouse donor cells into nonirradiated hosts. Recently, we demonstrated that total body irradiation of NOD/SCID or normal mice induces SDF-1 secretion in a time-dependent manner, mostly by osteoblast cells in the bone marrow.10 This SDF-1 gradient attracts primitive human CXCR4+ cells, increasing their homing to the bone marrow,11 resulting in higher levels of engraftment compared to nonirradiated recipients.10 The NOD/SCID mouse model is widely used to study many aspects of normal and leukemic human hematopoiesis, such as development,12,13 ex vivo expansion,14-17 gene transfer,3,18mobilization,19 and interactions with the bone marrow stroma.20 More important, this model was used to identify and to characterize the human SCID repopulating cell (SRC) based on its functional ability to repopulate the murine bone marrow with multilineage myeloid and lymphoid cell populations. The cell surface phenotype of SRC was found to be CD34+CD38−5; however, CD34−CD38−, or CD34+CD38+ cells, also have limited engraftment capacities in transplanted NOD/SCID mice.15,21 NOD/SCID mice were also used to compare the frequencies of SRC in cord blood, bone marrow, and mobilized peripheral blood cells evaluating their multilineage repopulating potential.22 The frequency of SRC in cord blood mononuclear cells (MNC) was determined by detecting more than 0.1% human DNA in the bone marrow of transplanted mice with limiting dilution assays, and it was found to be 1 in 9.3 × 105 MNC.22 The frequency of competitive repopulating unit , a primitive human cell population that includes CD34+CD38+ cells and is also capable of engrafting NOD/SCID mice, was found to be 1 in 6 × 105 cord blood MNC.15
The recently developed β2 microglobulin knockout NOD/ LtSz-SCID B2mnull mice (NOD/SCID/B2mnull) have reduced innate immunity because of lack of natural killer (NK) cell activity.23 Recently, we reported that SRC are true stem cells capable of high levels of multilineage repopulation of both primary transplanted NOD/SCID and secondary transplanted NOD/SCID/B2mnull mice.24 The cell surface phenotype of human SRC is CD38−/lowCXCR4+, and their engraftment is dependent on the chemokine SDF-1.24
We report here that NOD/SCID/B2mnullmice are better recipients than NOD/SCID mice for studying human stem cell function because they provide a model that better resembles autologous stem cell transplantation.
Materials and methods
Human cell preparation
Human cord blood MNC from full-term deliveries was isolated by standard separation on Ficoll–Hypaque (Pharmacia Biotech, Uppsala, Sweden). Enrichment of CD34+ cells was performed using a MACS separation kit (Miltenyi Biotech, Bergisch Gladbach, Germany) as previously described.17 24 All procedures were approved by the human experimentation and ethics committees of the Weizmann Institute of Science.
Mice
NOD/SCID mice and NOD/SCID β2 microglobulin knockout mice, NOD/SCID/B2mnull,23 were bred and maintained under defined flora conditions at the Weizmann Institute in sterile intraventilated cages (IVC; Techniplast, Buguggiate, Italy). NOD/SCID mice were delivered by cesarean section and foster nursed on pathogen-free foster mice with defined flora. NOD/SCID/B2mnullwere not rederived and carried the opportunistic pathogens, Pasteurella pneumotropica andTrichomonas. These mice received antibiotics (1.4 mg Ciprofloxacin; Bayer, Leverkusen, Germany) per 100 mL acidified autoclaved water during experiments to reduce the mortality from infections. In addition, NOD/SCID/B2mnull mice are prone to development of thymic lymphomas compared with NOD/SCID mice23; however, because young adult (8-week-old) mice were used as recipients, thymic lymphomas, which usually develop later, were rare. Data from rare events of mice having thymic lymphoma during the experiment course were excluded. NOD/SCID/B2mnullmice exhibit increased iron accumulation in the liver23; nevertheless, the increased levels of human SRC engraftment in NOD/SCID/B2mnull mice appears to be a consequence of reduced innate immunity rather than an indirect effect of hematochromatosis. Treatment of NOD/SCID mice with antimouse IL-2 receptor antibody reduced their NK cell activity and increased the engraftment of human peripheral blood mononuclear cells to levels seen in NOD/SCID/B2mnull mice (data not shown). The overall survival of irradiated and transplanted NOD/SCID/B2mnull mice over the course of the experiments was 75% ± 25%. NOD/SCID mice were irradiated with 375 cGy at 67 cGy/min from a 60Co source, whereas NOD/SCID/B2mnull mice were irradiated with 350 cGy because of their increased sensitivity to ionizing radiation.25 Within 24 hours, mice were transplanted intravenously with human cells as previously described17 24and were killed 1 month later. All the experiments were approved by the animal care committee of the Weizmann Institute.
Human cell detection
Progenitor cells were assayed in semisolid cultures selective for human hematopoietic colonies.17,24 Human cells in the marrow of engrafted mice were detected by flow cytometry using human specific monoclonal antibodies (mAb): anti-CD45 fluorescein isothiocyanate (FITC; Immuno Quality Products, Groningen, The Netherlands); anti-CD19, -CD33, -CD38, and -CD56 phycoerythrin (Coulter, Miami FL), and anti-CD34 FITC (Becton Dickinson, San Jose, CA).17,24 For human NK cell induction, bone marrow cells from engrafted mice were cultured for 10 days in RPMI supplemented with 10% fetal calf serum in the presence of human stem cell factor and IL-15 (100 ng/mL; R&D Systems, Minneapolis, MN).17 24
Human DNA analysis
Frequency analysis
NOD/SCID/B2mnull mice were transplanted with cord blood MNC in a cell dose ranging from 1.25 × 104 to 2.5 × 105 cells per mouse. One month after transplantation, mice were killed and human DNA in the murine bone marrow was determined. A mouse was scored positive if 0.1% or more human DNA was detected by Southern blot. Data were analyzed by applying Poisson statistics to the single-hit model, and the frequency was calculated using the maximum likelihood estimator.22
Results and discussion
Increased frequency of cord blood cells capable of engrafting NOD/SCID/B2mnullcompared to NOD/SCID mice
To evaluate the frequency of cord blood cells capable of engrafting NOD/SCID/B2mnull mice, these mice were transplanted with cord blood MNC in a limiting dilution assay. Mice were scored as positive for engraftment when more than 0.1% human DNA was detected in the murine bone marrow because this method is human specific and very sensitive. Statistical analysis was performed on pooled data from 5 independent experiments with pooled cord blood cells from multiple donors (Table 1), and the frequency of human cells capable of engraftment was calculated as described.22 The frequency of cord blood MNC capable of engrafting NOD/SCID/B2mnull mice, as determined by human DNA measurements, was found to be 1 in 3.6 × 104 MNC (95% CI, 1 in 2 × 104 to 1 in 6.3 × 104) compared to 1 in 9.3 × 105 previously shown for NOD/SCID mice.22
Higher levels of human cell engraftment in NOD/SCID/B2mnullcompared to NOD/SCID mice
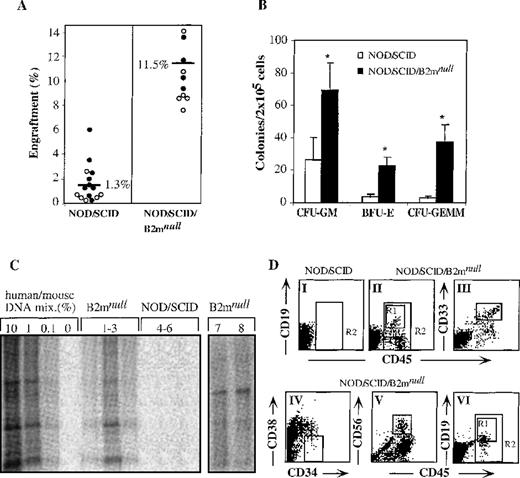
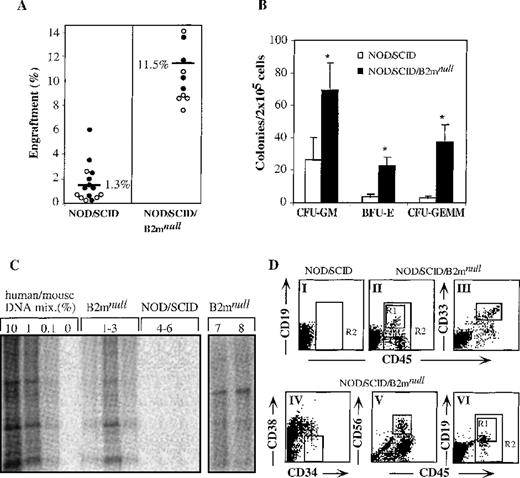
The level of hematopoietic repopulation is a critical parameter in stem cell transplantation. Therefore, we compared the engraftment levels obtained in transplanted mice of both strains. Transplantation of 8 × 104 cord blood CD34+-enriched cells into NOD/SCID mice and 4 × 104 cord blood CD34+ cells into NOD/SCID/B2mnull mice resulted in significantly higher levels of engraftment in NOD/SCID/B2mnull than in NOD/SCID mice (Figure1A). The incidence of immature human progenitor cells in the bone marrow of NOD/SCID/B2mnull mice was also higher than in the bone marrow of NOD/SCID mice (Figure 1B). Differential scoring of colonies revealed more colony-forming units (CFU) of granulocyte–macrophage (CFU-GM, 3-fold), immature erythroid (BFU-E, 6-fold) and the most primitive granulocyte–erythroid–macrophage–megakaryocyte (CFU-GEMM, 12-fold) in NOD/SCID/B2mnull than in NOD/SCID mice (Figure1B). Furthermore, as few as 1 × 103CD34+ cells transplanted into NOD/SCID/B2mnull mice were successfully engrafted, resulting in 0.1% to 1% human DNA in the bone marrow of transplanted mice (Figure 1C, lanes 1-3), whereas NOD/SCID mice could not be engrafted by such low cell doses (Figure 1C, lanes 4-6). Similarly, human bone marrow or mobilized peripheral blood cells engrafted NOD/SCID/B2mnull mice with significantly higher levels than NOD/SCID mice (data not shown).
Higher levels of human cell engraftment in NOD/SCID/B2mnull mice.
(A) Summary of engraftment levels in mice transplanted with enriched cord blood CD34+ cells. Similar percentage of human engraftment was detected by either Southern blot with human-specific α satellite probe (open circles) or by flow cytometry using human-specific anti CD45 mAb (filled circles). NOD/SCID mice were transplanted with 8 × 104 cells per mouse and NOD/SCID/B2mnull mice with 4 × 104 cells per mouse, in 5 independent experiments. (B) Bone marrow cells of mice presented in A were assayed for colony formation in semisolid culture selective for the growth of human colonies. Data shown are mean ± SE of 5 experiments. (C) Human DNA in the bone marrow of primary NOD/SCID/B2mnullmice transplanted with 103 CD34+ cells (lanes 1-3) and NOD/SCID mice transplanted with 104CD34+ cells (lanes 4-6). Secondary NOD/SCID/B2mnull recipients were transplanted with 2 to 5 × 103 human CD34+ cells that were recovered from engrafted primary NOD/SCID mice (lanes 7, 8). (D) NOD/SCID mice were transplanted with 12.5 × 104cord blood MNC per mouse (DI) and NOD/SCID/B2mnull mice with 8 × 104or 5 × 104 cord blood MNC per mouse (DII and DVI, respectively). Bone marrow cells from transplanted mice were analyzed 1 month later for the presence of human CD45+cells (R2). Human lineage-specific mAb were used to detect lymphoid CD45+CD19+ (DII, DVI, R1), myeloid CD45+CD33+ (DIII), and immature CD34+CD38−/low (DIV) progenitor cells in the marrow of engrafted NOD/SCID/B2mnull mice. Lymphoid CD45+CD56+ NK cell differentiation was induced in vitro before analysis (DV). A representative mouse out of 9 in 3 different experiments is shown.
Higher levels of human cell engraftment in NOD/SCID/B2mnull mice.
(A) Summary of engraftment levels in mice transplanted with enriched cord blood CD34+ cells. Similar percentage of human engraftment was detected by either Southern blot with human-specific α satellite probe (open circles) or by flow cytometry using human-specific anti CD45 mAb (filled circles). NOD/SCID mice were transplanted with 8 × 104 cells per mouse and NOD/SCID/B2mnull mice with 4 × 104 cells per mouse, in 5 independent experiments. (B) Bone marrow cells of mice presented in A were assayed for colony formation in semisolid culture selective for the growth of human colonies. Data shown are mean ± SE of 5 experiments. (C) Human DNA in the bone marrow of primary NOD/SCID/B2mnullmice transplanted with 103 CD34+ cells (lanes 1-3) and NOD/SCID mice transplanted with 104CD34+ cells (lanes 4-6). Secondary NOD/SCID/B2mnull recipients were transplanted with 2 to 5 × 103 human CD34+ cells that were recovered from engrafted primary NOD/SCID mice (lanes 7, 8). (D) NOD/SCID mice were transplanted with 12.5 × 104cord blood MNC per mouse (DI) and NOD/SCID/B2mnull mice with 8 × 104or 5 × 104 cord blood MNC per mouse (DII and DVI, respectively). Bone marrow cells from transplanted mice were analyzed 1 month later for the presence of human CD45+cells (R2). Human lineage-specific mAb were used to detect lymphoid CD45+CD19+ (DII, DVI, R1), myeloid CD45+CD33+ (DIII), and immature CD34+CD38−/low (DIV) progenitor cells in the marrow of engrafted NOD/SCID/B2mnull mice. Lymphoid CD45+CD56+ NK cell differentiation was induced in vitro before analysis (DV). A representative mouse out of 9 in 3 different experiments is shown.
Multilineage differentiation by SRC in transplanted NOD/SCID/B2mnullmice
Stem cells are defined by their capability to repopulate the bone marrow of transplanted mice with multilineage differentiation. Therefore, the lymphoid and myeloid differentiation capacity of SRC in the bone marrow of NOD/SCID/B2mnullmice was of interest. When 12.5 × 104 cord blood MNC were transplanted into NOD/SCID mice, we could not detect by flow cytometry any lymphoid, myeloid, or other human cells in the murine bone marrow (Figure 1DI, R2). In contrast, as few as 8 × 104cord blood MNC successfully engrafted NOD/SCID/B2mnullmice in 3 of 3 experiments (Figure 1DII, R2). In these experiments, multilineage differentiation of human SRC was observed in the bone marrow of transplanted NOD/SCID/B2mnullmice, which included lymphoid CD45+CD19+ cells (Figure 1DII R1) and myeloid CD45+CD33+ cells (DIII) as well as primitive CD34+CD38-/low cells (DIV). Cells recovered from the marrow of these mice could also differentiate into lymphoid CD45+CD56+ NK cells (Figure 1DV). These results, obtained with the transplantation of 8 × 104 MNC, indicated more than 11-fold higher frequency of human SRC detected in NOD/SCID/B2mnullmice compared with 1 in 9.3 × 105previously shown for NOD/SCID mice.22 In another set of experiments, 33% of NOD/SCID/B2mnullmice transplanted with as few as 5 × 104 cord blood MNC were also successfully engrafted with SRC, which differentiated into multilineage lymphoid cells (Figure 1DVI) and myeloid cells (data not shown).
Engraftment in secondary transplanted NOD/SCID/B2mnullmice
The ability of hematopoietic stem cells to self-renew, that is, to maintain undifferentiated cells by proliferation and in parallel to produce mature cells by multilineage differentiation, can only be assayed by serial transplantations. In this assay, stem cells that gave rise to multilineage hematopoiesis in primary recipients were also capable of repeating this process in secondary transplanted recipients. Recently, we successfully used NOD/SCID/B2mnull as secondary recipients after primary NOD/SCID mice transplantation with 1 to 2 × 105 cord blood CD34+-enriched cells.24 Each NOD/SCID/B2mnullmouse was transplanted with bone marrow cells recovered from 1 primary transplanted NOD/SCID mouse after in vitro CXCR4 up-regulation by stimulation with stem cell factor and IL-6 for 40 hours. These secondarily transplanted mice had higher levels of multilineage human engraftment, even though only approximately 33% of the total bone marrow cells from a primary transplanted NOD/SCID mouse was transplanted into a secondary NOD/SCID/B2mnullrecipient.24 As few as 2 to 5 × 103human CD34+ cells in the bone marrow recovered from primary transplanted NOD/SCID mice were sufficient to engraft secondarily transplanted NOD/SCID/B2mnullmice (Figure 1C, lanes 7, 8) with multilineage SRC engraftment (data not shown). Taken together, we found that NOD/SCID/B2mnull mice are superior recipients for human stem cell transplantation than NOD/SCID mice because of their reduced innate immunity, resulting in higher levels of engraftment with primitive progenitors and multilineage differentiation. Moreover, by using NOD/SCID/B2mnullmice as recipients, the frequency of human SRC was more than 11-fold higher than with NOD/SCID mice. Finally, as few as 2 to 5 × 103CD34+ cells recovered from the bone marrow of primary transplanted NOD/SCID mice were sufficient for consecutive multilineage engraftment with SRC in secondarily transplanted NOD/SCID/B2mnull mice, suggesting human stem cell self-renewal in the murine bone marrow. We therefore conclude that NOD/SCID/B2mnull mice are better recipients for studying human stem cell function and provide a model that better resembles autologous stem cell transplantation.
Recently, Arevalo et al26 crossed the nude mutation onto the NOD/SCID strain to generate better recipients for human hematopoiesis. The resultant nude athymic NOD/SCID/NUDE mouse has a longer lifespan because of the lack of thymic lymphoma development. Similarly, crossing the B2mnull mutation with the newly developed NOD/LtSz-Rag1nullstrain27 may presumably reduce the radiosensitivity determined by defective DNA repair because of the scidmutation, reduce the incidences of malignant spontaneous thymic lymphomas, and create better recipients for human stem cell transplantation.
Supported in part by grants from the Israel Academy of Science (T.L.), a research grant from the Israel Cancer Research Fund (T.L.), the Godfrey Foundation (A.P), a Germany MINERVA Grant (O.K), the Hood Foundation (D.G.), and National Institutes of Health grants A130 389 (L.S.) and DK57 199 (D.G., L.S.).
Reprints:Tsvee Lapidot, Incumbent of the Pauline Recanati Career Development Chair of Immunology, Department of Immunology, The Weizmann Institute of Science, Rehovot 76,100, Israel; e-mail: litsvee@wicc.weizmann.ac.il.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.