Abstract
Based on the hypothesis that interferon gamma (IFN-γ) may have stimulating effects on survival of hematopoietic progenitor cells, we examined the effect of IFN-γ on apoptosis of mature erythroid colony-forming cells (ECFCs) derived from human peripheral blood obtained from normal, healthy volunteers. When the cells were cultured in the presence of IFN-γ, even without erythropoietin (EPO), the viability of the cells was maintained for at least 36 hours. When apoptosis of ECFCs was assessed by flow cytometric analysis', using annexin V, IFN-γ reduced the extent of apoptosis of the cells, as well as EPO. DNA fragmentation of ECFCs was also reduced by IFN-γ. In cells cultured with IFN-γ alone, expression of Bcl-x was detected but the level of expression decreased gradually during incubation for 36 hours, and the expression level was lower than incubation with EPO. Fas expression and activation of downstream caspases were assessed by flow cytometric analysis or fluorometric protease assay. IFN-γ induced Fas expression of the cells without the activation of caspase8 or caspase3 during 16 hours of incubation, while deprivation of EPO induced expression of Fas and the activation of both caspase8 and caspase3. We propose that IFN-γ produces a stimulating signal for the survival of mature erythroid progenitor cells by reducing apoptosis through a mechanism other than modulating Fas and one related to the expression of Bcl-x.
Interferon gamma (IFN-γ), produced by activated T cells and by natural killer cells,1 is a potent inhibitor of hematopiesis.2 This cytokine is believed to play a crucial role in the pathophysiology of hematopoietic disorders associated with bone marrow failure such as aplastic anemia3,4 and hemophagocytic syndrome.5 It has been demonstrated that IFN-γ inhibits in vitro colony formation by bone marrow- and blood-derived hematopoietic progenitor cells,6-12 and the inhibitory effects of IFN-γ on hematopoietic cells are apparently due to inhibition of cell cycle progression10 or induction of apoptosis.2,11Apoptosis was facilitated by upmodulation of Fas expression both in CD34+ cells7 and blood-derived immature erythroid colony forming cells (ECFCs).6 It has been reported that IFN-γ reduces receptors for stem cell factor (SCF) and erythropoietin (EPO) in immature ECFCs,8 and these receptors, when activated, normally prevent apoptosis of ECFCs.13 This suggests that IFN-γ inhibits growth of hematopoietic progenitor cells mainly by enhancing apoptosis.
Although numerous studies have focused on the inhibitory effect of IFN-γ on hematopoiesis, IFN-γ also has been reported to stimulate growth of hematopoietic cells.14-16 IFN-γ increased the number of blood CD34+ cells expanded ex vivo when added together with SCF, interleukin-1β (IL-1β), IL-3, IL-6, and EPO,14and IFN-γ enhanced the colony formation induced by IL-3 in purified CD34+ cells.15 These contradictory findings regarding IFN-γ action may depend on the stage of maturation of the hematopoietic progenitors16 and on differences in growth factors added to the cultures.
EPO, the principal growth factor for erythroid progenitor cells, maintains the viability of these cells by allowing them to undergo the process of proliferation and maturation.13,17-21 It has been demonstrated that deprivation of EPO-induced apoptosis of ECFCs occurs through down-regulation of Bcl-x,22 a member of the Bcl-2 family known as an important regulator of apoptosis in various cell systems.23-25 The Fas-Fas ligand system also has a role in apoptosis of erythroid progenitor cells6through deprivation of EPO-induced activation of apopain/caspase3,22 a cystein protease which acts as a downstream signal mediator in the apoptosis induced by Fas activation.26-28
We examined the effects of IFN-γ on apoptosis of mature erythroid progenitor cells (day 7 ECFCs), and found that IFN-γ, as well as EPO, can prevent apoptosis of mature ECFCs through mechanisms other than modulating expression of Fas, and is related to the expression of Bcl-x.
Materials and methods
Reagents
Recombinant human erythropoietin (rhEPO) was kindly provided by Chugai Pharmaceutical Co Ltd (Tokyo, Japan); recombinant human interleukin3 (rhIL-3) and recombinant human stem cell factor (rhSCF) were kindly provided by Kirin-Brewery Co Ltd (Tokyo, Japan); recombinant human interferon gamma 1a (rhIFN-γ) was kindly provided by Shionogi Co Ltd (Osaka, Japan). rhIFN-γ inhibits the proliferation of A-498 cells, which are derived from human renal cell carcinoma, by 50% at a concentration of 2.5 U/mL (data not shown). Anti-IFN-γ antibody (catalog number 955000010) and mouse anti-IgG1 antibody (catalog number 857070000) were purchased from Life Technologies, Inc (Rockville, MD). Rabbit anti Bcl-x antibody (B22 630) was purchased from Transduction Laboratories (Lexington, KY). Rabbit anti-Bax antibody was purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Mouse antiactin antibody (N350) was from Amersham Life Science (Buckinghamshire, England). Horseradish peroxidase (HRP) conjugated antirabbit (NA934) and mouse (NA931) whole Ig secondary antibodies were from Amersham.
Generation of ECFCs
ECFCs were prepared using a modified method described by Sawada et al.17,18 29 Light-density mononuclear cells were obtained from 40 mL of heparinized peripheral blood buffy coat from healthy Japanese volunteers by density centrifugation using lymphocyte separation medium (LSM, density 1.0770-1.0800 g/mL; ICN Biomedicals, Aurora, OH). Red blood cells were lysed by suspending the mononuclear cell pellet in red cell lysis buffer (0.16 mol/L ammonium chloride, 10 mmol/L potassium bicarbonate, 5 mmol/L EDTA). Platelets were removed by cell centrifugation through phosphate-buffered saline (PBS) containing 10% human serum albumin (HSA, kindly provided by the Chemo-Sero-Therapeutic Research Institute, Kumamoto, Japan). Adherent cells were depleted by a 1-hour incubation in a polystyrene tissue-culture flask at 4°C. Nonadherent cells were then collected and 2 cycles of negative selection were performed using anti-CD3, -CD11b, -CD15 and -CD45RA antibodies and immunomagnetic beads with Vario-Macs columns (Miltenyi Biotech, Auburn, CA). The remaining cells were then cultured in Iscove modified Dulbecco medium (IMDM; GIBCO BRL, Grand Island, NY) containing 15% heat-inactivated fetal calf serum (FCS; Commonwealth Serum Laboratories, Melbourne, Australia), 15% pooled human AB serum, 2 U/mL EPO, 20 ng/mL SCF, 10 ng/mL IL-3, 100 U/mL penicillin, and 100 μg/mL streptomycin (GIBCO) at 37°C in a high-humidity, 5% CO2, 95% air incubator (day 0). On day 3, the cells, referred to as day 3 ECFCs, were centrifuged over LSM, then collected and incubated under the same conditions, but without IL-3. The cultured cells were collected on day 7, referred to as day 7 ECFCs, and used in the following experiments. The purity of the day 7 ECFCs, with proerythroblastlike features, was 95% ± 3%, as determined in cytospin preparations. Cell purity was assessed in each experiment.
Serum-free liquid cultures of ECFC
The cells (day 7 ECFCs, 2 × 105 cells/mL) were incubated in serum-free liquid medium containing 50% IMDM/50% F-12 medium (Sigma Chemical Co, St Louis, MO) with 1% detoxified bovine serum albumin (BSA; Stem Cell Technologies Inc, Vancouver, BC), 300 μg/mL iron-saturated transferrin (652202; Boehringer Mannheim, Mannheim, Germany), lipid suspension (oleic acid, 2.8 μg/mL; L-α-phosphatidylcholine, 4.0 μg/mL; cholesterol, 3.9 μg/mL, Sigma), and prepared as described30 with 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C in a high-humidity, 5% CO2, 95% air incubator. We added rhEPO and rhIFN-γ as indicated.
Determination of cell viability
Viability of the cells was determined by trypan blue exclusion using a hemocytometer.
Plasma clot assay
Erythroid colony-forming capacity of ECFC was determined by the plasma clot method.13 A total of 1 mL medium consisting of IMDM, 20% FCS, 1% BSA, 10 ng/mL SCF, rhEPO as indicated, and 10% pooled citrated human AB plasma containing 600 cells were plated on 3 35-mm culture dishes and incubated at 37°C in a high-humidity, 5% CO2, 95% air incubator for 7 days. The clots were then fixed and stained with 3,3′ dimethoxybenzidine. The colonies of 8 or more hemoglobinized cells were defined as colony-forming unit–erythroid (CFU-E), and aggregates consisting of 2 to 7 hemoglobinized cells were defined as small erythroid clusters. The colonies consisting of 8 to 19 hemoglobinized cells were referred as to medium erythroid colonies, and those consisting of 20 to 49 hemoglobinized cells were referred to as large erythroid colonies.17
Apoptosis assay
Apoptosis was assessed by measuring membrane redistribution of phosphatidilserine31 using an annexin V–fluorescein-5-isothiocyanate (FITC) apoptosis detection kit (Immunotech, Marseille, France) according to the manufacturer's protocol. Briefly, cells were washed twice with PBS and incubated for 30 minutes on ice in 500 μL binding buffer containing FITC-conjugated annexin V antibody (5 μL) and propidium iodide (PI, 5 μL of 250-μg/mL stock). Cells were analyzed on the Epics Elite ESP flow cytometer (Coulter Co, Miami, FL). The annexin V–positive fraction was detected as apoptotic.
Analysis of DNA breakdown
To quantitate breakdown of cellular DNA during apoptosis, the amount of fragmented DNA was measured by a modified method, as previously described.13 Day 7 ECFCs were preincubated in IMDM containing 30% FCS and 0.25% HSA at 37°C for 30 minutes. [3H]thymidine (1.85 × 10-2 MBq/mL; 0.2479 MBq/mmol; New England Nuclear Corp, Boston, MA) was added to 106cells/mL and further incubation at 37°C was carried out for more than 30 minutes. The cells were collected, resuspended in 2 mL IMDM, and carefully layered over 2 mL PBS containing 10% BSA for centrifugation at 1000g, 4° C for 5 minutes. Replicated cells (5 × 105) were then transferred to 1 mL of serum-free medium, as described above. To block further incorporation of [3H]thymidine, thymidine and deoxycytidine (20 μ mol/L; Sigma) were added. IFN-γ was added as indicated. After culture in serum-free medium for 16 hours, the cell replicates were collected and lysed in 1 mL of 50 mmol/L Tris-HCl, pH 8.0, containing 10 mmol/L sodium chloride (NaCl), 20 mmol/L EDTA, 0.5% sodium dodecyl sulfate (SDS), and proteinase K (200 μ g/mL; GIBCO), followed by incubation overnight at 37°C. DNA was extracted with phenol:Chloroform (1:1 vol/vol), precipitated in ethanol, and dissolved in 30 μL of 10 mmol/L Tris-HCl, pH 8.0, containing 1 mmol/L EDTA. The DNA samples were electrophoretically separated on alkaline pH, 0.6% agarose gels. Each lane was cut into sixteen 5 mm fractions, and the radioactivity of each fraction was expressed as a percent of the total radioactivity in each lane. The sum of the radioactivity in fractions 5 through 16 was considered as the amount of fragmented DNA, and was expressed as a percentage of the total radioactivity.
Protein sample preparation and Western blotting
The cells (1 × 106) were lysed in buffer containing 62.5 mmol/L Tris-HCl (pH 6.8), 100 mmol/L dithiothreitol, 2% (wt/vol) SDS, and 10% Glycerol.32 Cellular proteins (80 μg) were separated by 12.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE) and transferred to polyvinylidene difluoride membranes (Immobilon, IPVH00010; Millipore, Bedford, MA). The membranes were blocked in PBS-containing 0.1% Tween 20 (PBST) with 5% skim milk. The membranes were washed 3 times with PBST, then were incubated in PBST containing 5% skim milk with anti-Bcl-x or Bax antibody as a primary antibody at room temperature for 1 hour. The membranes were then washed with PBST and incubated with HRP conjugated secondary antibody (rabbit Ig). Specific signals were detected on X-ray films using an enhanced chemiluminescence detection system (ECL, PRN2106; Amersham). To remove the antibodies, the membranes were incubated in 0.0625 mol/L Tris-HCl (pH 6.8) and 2% SDS at 50°C for 30 minutes and were reblotted first with mouse antiactin antibody and then with HRP mouse Ig as the secondary antibody. Specific signals were detected as described above. K562 cells served as positive controls.
Detection of Fas by flow cytometry
The cells (1 × 106) were washed twice with PBS and suspended in 0.1 mL PBS, then incubated with either FITC-conjugated murine anti-Fas monoclonal antibody (UB2, 1506; Immunotech) at 0.2 μL or FITC-conjugated murine IgG1 (349041 Becton Dickinson, San Jose, CA) on ice for 30 minutes as a control. PBS (400 μL) was added and analysis was performed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
Evaluation of caspase3 activity
Activation of caspase3 was assessed using the PhiPhiLux G1D2 caspase3 activity detection kit (AK304R1G; Oncoimmunin Inc, College Park, MD), according to the manufacturer's instructions. Briefly, 1 × 106 cells were washed twice with PBS, and 50 μL of substrate solution (10 μmol/L; GDEVDGI) was added, followed by incubation for 60 minutes in a 5% CO2, 95% air incubator at 37°C. Five hundred microliters of cold-flow cytometry solution were added to each sample, followed by analysis using a FACScan flow cytometer at 488 nm FL1 channel.
Evaluation of capase8 activity
Activation of caspase8 was evaluated using a FLICE/caspase8 fluorometric protease assay kit (BV-K112; Medical and Biological Laboratories Co, Ltd, Nagoya, Japan) according to the manufacturer's instructions. Briefly, lysates from 1 × 106 cells were incubated with fluorogenic substrate IETDAFC for 60 minutes at 37°C in buffer containing 5 mmol/L dithiothreitol. Samples were then analyzed using an ARVO multilabel counter (Wallac Oy, Turku, Finland) at 535 nm.
Statistical analysis
The t test was used to determine significant differences between the groups.
Results
Effects of IFN-γ on viability of ECFCs
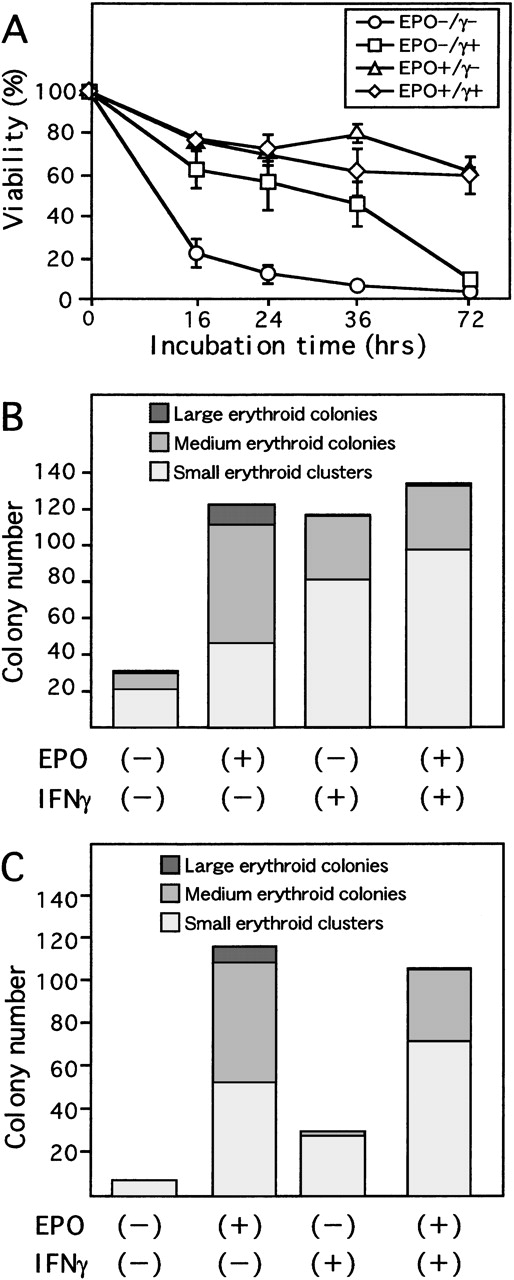
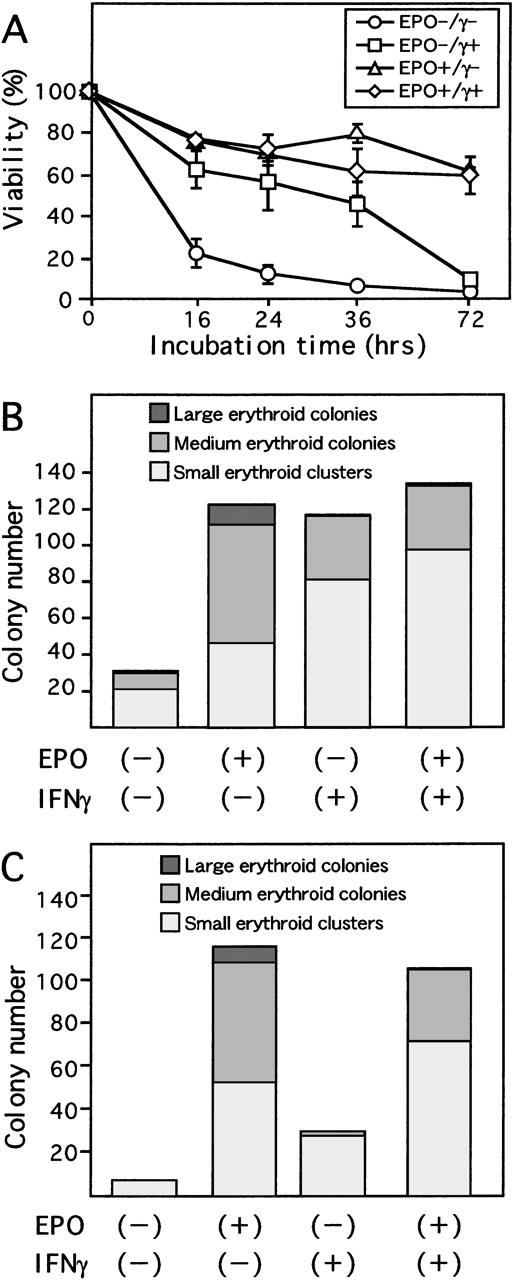
To determine the effect of EPO and IFN-γ on survival of ECFCs, we examined the viability of these cells in serum-free liquid culture in the presence or absence of either rhEPO or rhIFN-γ, or both (Figure 1A). While the viability of ECFCs rapidly decreased without rhEPO and rhIFN-γ (22.9% ± 7.0%, mean ± SD of triplicates at 16 hours), in the presence of rhEPO (10 U/mL), the viability was maintained (61.8% ± 2.1% at 72 hours). When the cells were incubated with rhIFN-γ (1000 U/mL) alone, viability of the cells was significantly greater than that seen without rhEPO for at least 36 hours of culture (45.9% ± 10.8% vs 7.4% ± 1.0%, P < .01 at 36 hours). In the cultures with rhEPO plus rhIFN-γ, viability of the cells was similar to that seen with rhEPO alone (59.5% ± 9.0% vs 61.8% ± 2.1%, P = .70 at 72 hours).
Effect of IFN-γ on day 7 ECFCs.
(A) Day 7 ECFCs were cultured with or without rhEPO (10 U/mL) and/or rhIFN-γ (1000 U/mL) for the indicated time. Viability of the cells was determined by trypan blue exclusion. Each point indicates the mean ± SD of triplicates. (B) Erythroid colony-forming capacity after liquid culture for 16 hours with or without rhEPO (10 U/mL) or rhIFN-γ (1000 U/mL), or both, was determined by plasma clot assay. (C) Colony-forming capacity of day 7 ECFCs. Cells were cultured in plasma clots, in the presence or absence of rhEPO (10 U/mL) or rhIFN-γ (1000 U/mL), or both. Large erythroid colonies include more than 20 hemoglobinized cells per colony; medium erythroid colonies include 8 to 19 cells per colony; and small erythroid clusters include 2 to 7 hemoglobinized cells per aggregate, (B) and (C). Each point indicates the mean of triplicate studies.
Effect of IFN-γ on day 7 ECFCs.
(A) Day 7 ECFCs were cultured with or without rhEPO (10 U/mL) and/or rhIFN-γ (1000 U/mL) for the indicated time. Viability of the cells was determined by trypan blue exclusion. Each point indicates the mean ± SD of triplicates. (B) Erythroid colony-forming capacity after liquid culture for 16 hours with or without rhEPO (10 U/mL) or rhIFN-γ (1000 U/mL), or both, was determined by plasma clot assay. (C) Colony-forming capacity of day 7 ECFCs. Cells were cultured in plasma clots, in the presence or absence of rhEPO (10 U/mL) or rhIFN-γ (1000 U/mL), or both. Large erythroid colonies include more than 20 hemoglobinized cells per colony; medium erythroid colonies include 8 to 19 cells per colony; and small erythroid clusters include 2 to 7 hemoglobinized cells per aggregate, (B) and (C). Each point indicates the mean of triplicate studies.
IFN-γ maintains colony-forming capacity of ECFCs
To determine if IFN-γ alone could maintain colony-forming capacity during liquid culture of ECFCs, plasma-clot assays were performed. After 16 hours of incubation, with or without rhIFN-γ or rhEPO or both, in serum-free liquid medium, the cells were transferred to plasma-clot cultures containing rhEPO (2 U/mL), and incubations were carried out for another 7 days (Figure 1B). Addition of rhIFN-γ alone resulted in maintenance of a greater colony-forming capacity than that seen without additives. However, the number of large- and medium-sized colonies formed from the cells incubated with rhIFN-γ alone was significantly smaller than that seen in the culture with rhEPO alone (35.0 ± 10.8 and 76.7 ± 14.6,P < .05, mean colony number from 200 cells ± SD of triplicates, respectively). Addition of rhIFN-γ plus rhEPO resulted in a significant decrease of large- and medium-sized colony-forming capacity (36.6 ± 9.2, P < .05) compared to that seen with rhEPO alone. Instead, addition of rhIFN-γ resulted in a greater increase in the number of small erythroid clusters, both in the presence and absence of rhEPO, than in those without rhIFN-γ. Therefore, no significant difference was present in the sum of the number of large- and medium-sized colonies plus small erythroid clusters among cultures with or without rhIFN-γ, both in the presence and absence of rhEPO.
To determine if IFN-γ could substitute for EPO during erythroid proliferation and maturation, rhIFN-γ was added to plasma clots at the beginning of the cultures of day 7 ECFCs. As can be seen from Figure 1C, addition of rhIFN-γ alone could not support formation of medium- and large-sized erythroid colonies. However, a larger number of small erythroid clusters did form with rhIFN-γ alone than was seen with no additives (no additives, 7.0 ± 10.4; rhIFN-γ alone, 28.3 ± 17.0, P = 0.14). Addition of rhIFN-γ together with rhEPO resulted in a reduced number of large- and medium-sized colonies, but the sum of the number of clusters and colonies was similar to that seen with rhEPO alone (116 ± 9.3 of rhEPO alone and 106 ± 12.8 of both additives, P = 0.31), since the number of small erythroid clusters increased in the presence of rhIFN-γ.
IFN-γ-reduced apoptosis of ECFCs
EPO maintains viability of these cells by reducing apoptosis. Since IFN-γ maintained viability of the cells for up to 36 hours, as did EPO, we wondered if IFN-γ would reduce the apoptosis of ECFCs. Experiments were conducted using serum-free medium to exclude the effect of unknown factors in serum, and apoptosis was measured by flow cytometry using annexin V as described.
Annexin V binds the membrane phospholipid phosphatidylserine (PS), which is externalized from the inner to the outer leaflet of plasma membrane in the early stage of apoptosis. When membrane integrity is lost, as seen in the latter stage of cell death resulting from either the apoptotic or necrotic processes, propidium iodide (PI) staining becomes positive. According to the results of our time-course study, annexin V– and PI–double-positive cells gradually increased during incubation of ECFCs without rhEPO and rhIFN-γ (data not shown). When we determined the ratio of the later stage of apoptosis to the earlier stage of apoptosis, there were no significant differences among various treatments (data not shown). Therefore, it was evident that annexin V–positive and PI-negative cells (earlier stage of apoptosis) and positive cells (later stage of apoptosis) were apoptotic cells.
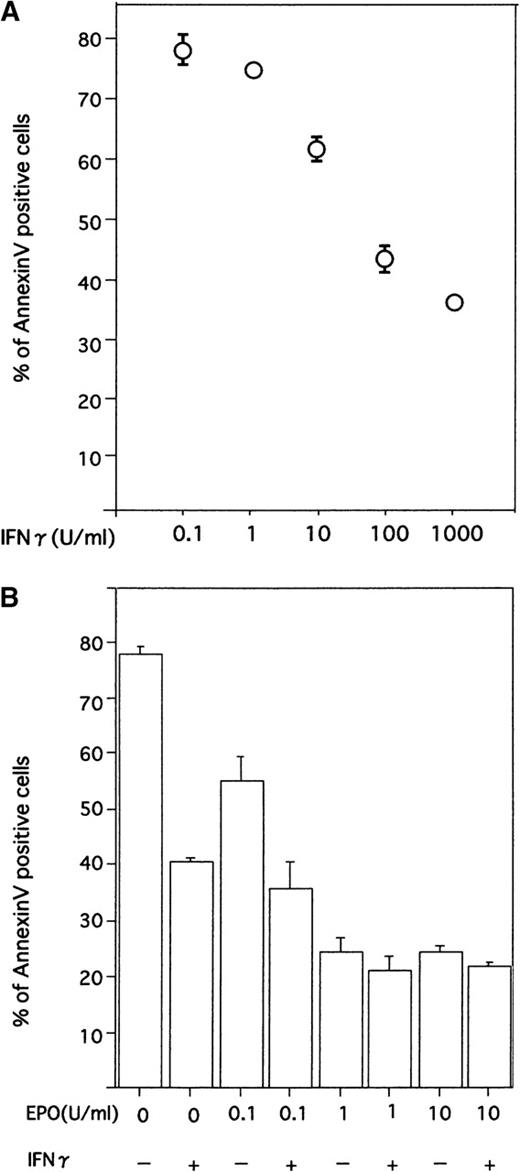
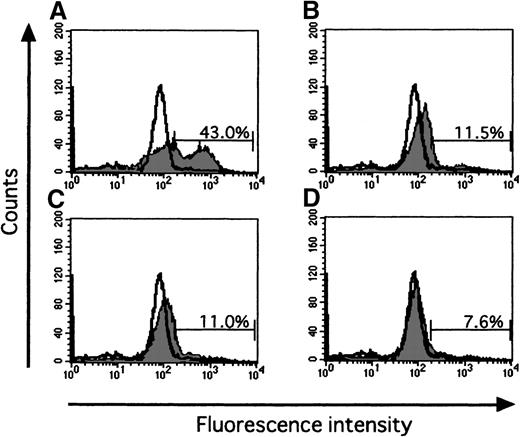
As shown in Figure 2A, when the cells were incubated for 16 hours without rhEPO (10 U/mL) and rhIFN-γ (1000 U/mL), annexin V–positive cells were 66.9% ± 13.2% (mean ± SD of 12 replicates from 4 independent experiments). Addition of rhIFN-γ alone significantly reduced the number of annexin V–positive cells (34.0% ± 8.8%, P < .01), compared to that seen without rhEPO and rhIFN-γ. Addition of rhEPO alone significantly reduced the number of annexin V–positive cells (20.8% ± 4.5%, P < .01), and addition of rhIFN-γ together with rhEPO further significantly reduced the number of annexin V–positive cells (16.7% ± 4.9%,P < .05), compared to that seen with rhEPO alone.
Effect of IFN-γ on apoptosis of ECFCs.
(A) Day 7 ECFCs were incubated in serum-free medium without rhEPO and rhIFN-γ (upper left), with rhEPO (10 U/mL) (upper right), with rhIFN-γ (1000 U/mL) (lower left), and both (lower right). After incubation for 16 hours, apoptosis was measured with PI and annexin V, using a flow cytometer. Data shows typical results of 12 replicates from 4 experiments. In each panel, the right lower quadrant (annexin V–positive and PI negative) indicates early apoptosis, and the right upper quadrant (annexin V– and PI positive) indicates late apoptosis. Both annexin V–positive fractions were assessed as apoptotic cells. (B) The day 7 ECFCs were labeled with [3H]thymidine and cultured in serum-free medium without rhIFN-γ (left), or with rhIFN-γ (1000 U/mL) (right), for 16 hours. Cellular DNA was isolated and analyzed by alkaline pH, 0.6% agarose gel electrophoreses. The sum of the radioactivity of fractionations 1-4 is designated as uncleaved DNA and is expressed as a percentage of the total radioactivity.
Effect of IFN-γ on apoptosis of ECFCs.
(A) Day 7 ECFCs were incubated in serum-free medium without rhEPO and rhIFN-γ (upper left), with rhEPO (10 U/mL) (upper right), with rhIFN-γ (1000 U/mL) (lower left), and both (lower right). After incubation for 16 hours, apoptosis was measured with PI and annexin V, using a flow cytometer. Data shows typical results of 12 replicates from 4 experiments. In each panel, the right lower quadrant (annexin V–positive and PI negative) indicates early apoptosis, and the right upper quadrant (annexin V– and PI positive) indicates late apoptosis. Both annexin V–positive fractions were assessed as apoptotic cells. (B) The day 7 ECFCs were labeled with [3H]thymidine and cultured in serum-free medium without rhIFN-γ (left), or with rhIFN-γ (1000 U/mL) (right), for 16 hours. Cellular DNA was isolated and analyzed by alkaline pH, 0.6% agarose gel electrophoreses. The sum of the radioactivity of fractionations 1-4 is designated as uncleaved DNA and is expressed as a percentage of the total radioactivity.
Apoptosis was qualitatively confirmed by agarose gel electrophoreses of cellular DNA using [3H]thymidine. As shown in Figure 2B, when ECFCs were cultured for 16 hours without rhEPO, high molecular weight DNA was cleaved into small fragments. When cells were cultured with rhIFN-γ, the amount of DNA fragmentation was greatly reduced (71% of uncleaved DNA, sum of fraction 1 to 4, with rhIFN-γ and 19% with no additives).
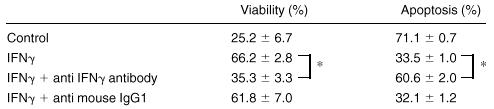
To confirm whether IFN-γ maintains viability of mature ECFCs and prevents them from apoptosis, neutralizing experiments were performed using anti-IFN-γ antibody (Table 1). When neutralizing antibody was added together with IFN-γ, the protective effect of IFN-γ on apoptosis of ECFCs was nil, and both the viability and the number of apoptotic cells were similar to the experiment without IFN-γ.
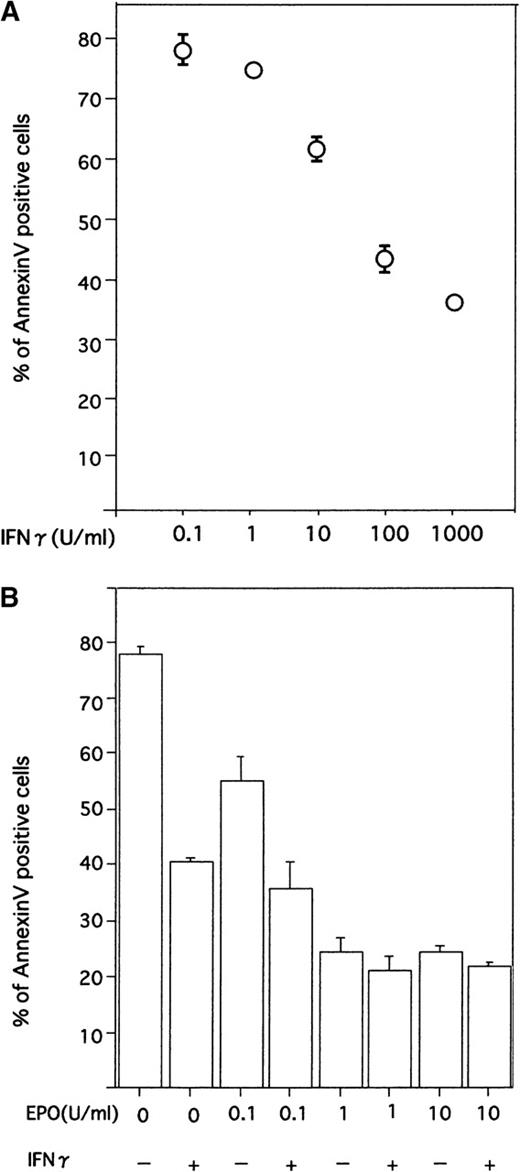
When different concentrations of rhIFN-γ were added to the cultures, a dose-dependent reduction of apoptosis was evident, and significant suppression of apoptosis was obtained with a concentration of 10 U/mL (P < .01, Figure 3A).
Effect of IFN-γ on ECFCs with various concentrations of rhIFN-γ and rhEPO.
(A) Dose-dependent effects of IFN-γ on reducing apoptosis of day 7 ECFCs. Cells were incubated for 16 hours with the indicated concentrations of rhIFN-γ, without rhEPO. (B) Cells were incubated with the indicated concentrations of rhEPO or rhIFN-γ (1000 U/mL), or both. Each point shows the mean ± SD of triplicates. Apoptosis was evaluated by flow cytometry after staining cells with annexin V and PI.
Effect of IFN-γ on ECFCs with various concentrations of rhIFN-γ and rhEPO.
(A) Dose-dependent effects of IFN-γ on reducing apoptosis of day 7 ECFCs. Cells were incubated for 16 hours with the indicated concentrations of rhIFN-γ, without rhEPO. (B) Cells were incubated with the indicated concentrations of rhEPO or rhIFN-γ (1000 U/mL), or both. Each point shows the mean ± SD of triplicates. Apoptosis was evaluated by flow cytometry after staining cells with annexin V and PI.
To determine the effect of IFN-γ under more physiologic conditions, we examined the effect of IFN-γ in the presence of low concentrations of EPO. As shown in Figure 4B, when cells were incubated with rhIFN-γ (1000 U/mL) plus various concentrations of rhEPO, suppression of apoptosis of ECFCs was evident in cultures with a lower concentration of rhEPO.
Effects of IFN-γ on expression of Bcl-2–family proteins
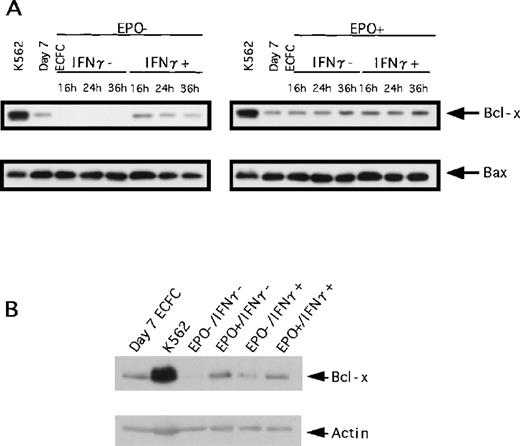
To determine the mechanism by which IFN-γ influences apoptosis of ECFCs, we examined the extent of expression of Bcl-2–family proteins (Bcl-x, Bax, and Bcl-2) using Western blotting (Figure4A). When ECFCs were incubated without rhEPO and rhIFN-γ for the indicated times, Bcl-x was not detected. When the cells were incubated with rhIFN-γ alone, Bcl-x was detected but the level decreased gradually during incubation for 36 hours. As can be seen from Figure 4B, the level of Bcl-x in the cells cultured with rhIFN-γ was lower than that of cells cultured with rhEPO alone or rhEPO and IFN-γ. When cells were cultured with rhEPO alone, expression of Bcl-x was maintained, and addition of rhIFN-γ together with rhEPO did not affect the expression of Bcl-x (Figure4A, right). The expression levels of Bax showed no difference among variables (Figure 4A), and Bcl-2 was not detected in the erythroid progenitors (data not shown).
Effect of IFN-γ on expression of Bcl-x and Bax.
Expression of Bcl-x and Bax was evaluated by Western blot analysis. (A) Cells were incubated with or without rhEPO (10 U/mL) or rhIFN-γ (1000 U/mL), or both, for the indicated time, and the same amount of whole cell lysates was loaded. The upper panel shows the expression of Bcl-x, and the lower panel shows the expression of Bax. (B) Lysates from cells incubated for 16 hours with or without rhEPO or IFN-γ, or both, were loaded.
Effect of IFN-γ on expression of Bcl-x and Bax.
Expression of Bcl-x and Bax was evaluated by Western blot analysis. (A) Cells were incubated with or without rhEPO (10 U/mL) or rhIFN-γ (1000 U/mL), or both, for the indicated time, and the same amount of whole cell lysates was loaded. The upper panel shows the expression of Bcl-x, and the lower panel shows the expression of Bax. (B) Lysates from cells incubated for 16 hours with or without rhEPO or IFN-γ, or both, were loaded.
Effect of IFN-γ on expression of Fas and activation of caspases
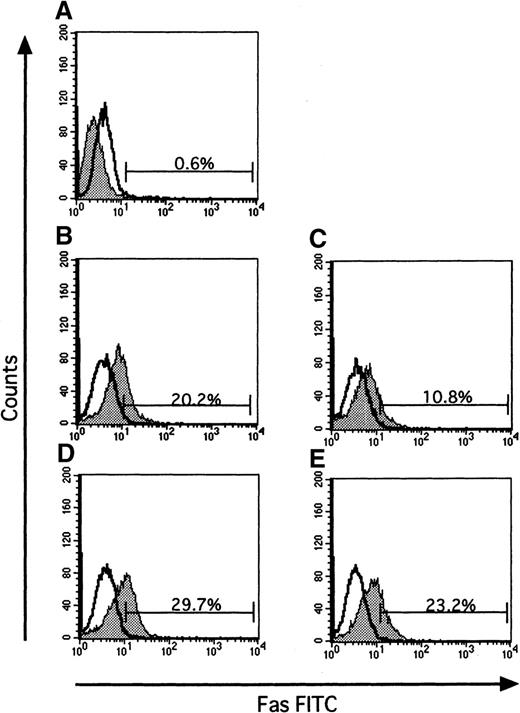
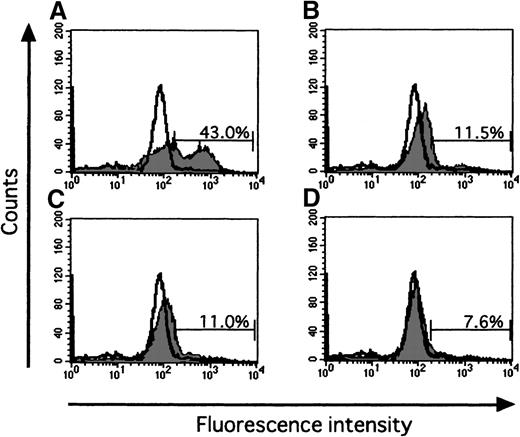
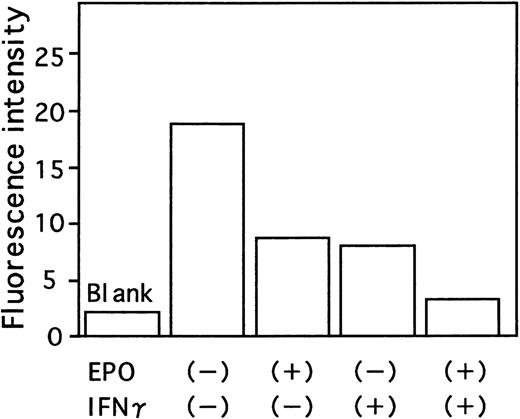
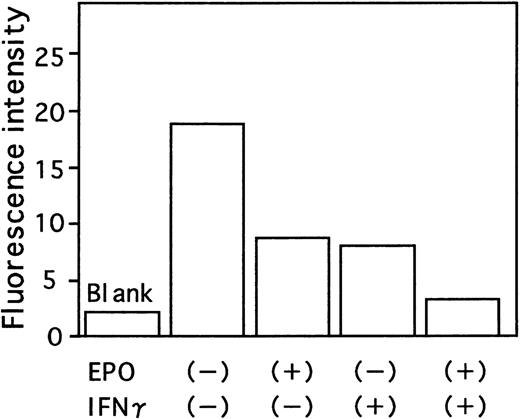
Since the Fas-Fas ligand system mediates the apoptotic signal from various stimuli, we examined the effect of IFN-γ on Fas expression of ECFCs by flow cytometry. When the cells were incubated for 16 hours without rhEPO and rhIFN-γ, Fas was expressed on 20.2% of the cells (Figure 5B), a value exceeding that seen with rhEPO alone (10.8%, Figure 5C). When rhIFN-γ was added to the cultures, the percentage of cells that expressed Fas increased further both in the absence or presence of EPO (29.7% and 23.2% respectively, Figures 5D and E). The activities of caspase3 and caspase8, downstream mediators of apoptotic signaling induced by activation of Fas, were determined by flow cytometry and fluorometric protease assay, respectively (Figures6 and 7). When the cells were incubated with rhEPO alone, the activity of caspase3 was less than that seen without rhEPO and rhIFN-γ (Figure 6A and B). Addition of rhIFN-γ alone for 16 hours reduced the activity of caspase3, as did rhEPO alone(Figure6C), and addition of rhIFN-γ together with rhEPO further reduced the activity of caspase3 at a greater rate than that seen with each additive alone. The response of caspase8 to rhIFN-γ was similar to that seen in caspase3 (Figure 7). IFN-γ as well as EPO reduced the activity of caspase8 at a greater rate than that seen without these additives. Addition of rhIFN-γ plus rhEPO resulted in less caspase8 activity than was seen using each additive alone.
Effect of IFN-γ on expression of Fas.
Data show typical results of 3 independent experiments. Panel (A) shows the baseline expression of Fas on day 7 ECFCs, prior to serum-free liquid culture. Cells were incubated with or without rhEPO (10 U/mL) or rhIFN-γ (1000 U/mL), or both, for 16 hours. Cells were then incubated on ice with FITC-Fas or FITC-murine IgG1 as control for 30 minutes, and then flow cytometric analysis was performed. The open histogram indicates murine IgG1 as control, and the gray histogram indicates Fas. Each panel shows data on cells incubated without rhEPO and rhIFN-γ (B), with rhEPO alone (C), with rhIFN-γ alone (D), and together with rhEPO plus rhIFN-γ (E).
Effect of IFN-γ on expression of Fas.
Data show typical results of 3 independent experiments. Panel (A) shows the baseline expression of Fas on day 7 ECFCs, prior to serum-free liquid culture. Cells were incubated with or without rhEPO (10 U/mL) or rhIFN-γ (1000 U/mL), or both, for 16 hours. Cells were then incubated on ice with FITC-Fas or FITC-murine IgG1 as control for 30 minutes, and then flow cytometric analysis was performed. The open histogram indicates murine IgG1 as control, and the gray histogram indicates Fas. Each panel shows data on cells incubated without rhEPO and rhIFN-γ (B), with rhEPO alone (C), with rhIFN-γ alone (D), and together with rhEPO plus rhIFN-γ (E).
Effect of IFN-γ on activation of caspase3.
Data show typical results of 3 independent experiments. Cells were incubated with or without rhEPO (10 U/mL) or rhIFN-γ (1000 U/mL), or both, in serum-free medium for 16 hours. Cells were then incubated with caspase3 substrate solution GDEVDGI for 1 hour at 37°C, and flow cytometric analysis was performed. The open histogram shows the fluorescence of day 7 ECFCs as control. Each panel shows data on cells incubated without rhEPO and rhIFN-γ (A), with rhEPO alone (B), with rhIFN-γ alone (C), and together with rhEPO plus rhIFN-γ (D).
Effect of IFN-γ on activation of caspase3.
Data show typical results of 3 independent experiments. Cells were incubated with or without rhEPO (10 U/mL) or rhIFN-γ (1000 U/mL), or both, in serum-free medium for 16 hours. Cells were then incubated with caspase3 substrate solution GDEVDGI for 1 hour at 37°C, and flow cytometric analysis was performed. The open histogram shows the fluorescence of day 7 ECFCs as control. Each panel shows data on cells incubated without rhEPO and rhIFN-γ (A), with rhEPO alone (B), with rhIFN-γ alone (C), and together with rhEPO plus rhIFN-γ (D).
Effect of IFN-γ and EPO on activation of caspase8.
Cells were incubated under the indicated conditions in serum-free medium for 16 hours. Cells were then incubated with fluorogenic caspase8 substrate IETDAFC for 1 hour at 37°C. The fluorescence intensity was then measured using a multilabel counter. The mean of triplicate studies is shown.
Effect of IFN-γ and EPO on activation of caspase8.
Cells were incubated under the indicated conditions in serum-free medium for 16 hours. Cells were then incubated with fluorogenic caspase8 substrate IETDAFC for 1 hour at 37°C. The fluorescence intensity was then measured using a multilabel counter. The mean of triplicate studies is shown.
Discussion
It has been reported that IFN-γ produces apoptosis of hematopoietic progenitor cells, and thus inhibits the growth of hematopoietic cells in vitro and in vivo.2-12However, when we measured the effect produced by IFN-γ during 16 hours of cell culture, this cytokine also significantly reduced apoptosis of day 7 ECFCs, especially in the absence of or with lower concentrations of EPO. This protective effect on the survival of ECFCs was also evident in the erythroid colony assay, though the size of the colonies was reduced by exposure to IFN-γ, thereby indicating the inhibition of cellular proliferation, or production of a later apoptosis, of ECFCs by this cytokine. This early contradictory effect of IFN-γ on apoptosis of erythroid progenitor cells can be reasonably explained as follows: Inhibition of expansion of human erythroid progenitor cells by IFN-γ was described in detail by Dai et al,6,11 33 who used cells purified according to similar methods, but the cells were in an earlier stage of maturation and underwent longer incubation times. In their report, Dai et al clearly indicated that IFN-γ induced apoptosis of erythroid progenitor cells at a relatively restricted stage of maturation through 96 hours of incubation. Mature BFU-E was obtained on days 3-6 in culture, earlier than what was achieved with the progenitor cells that we used in this study. Dai et al did not evaluate the effect of IFN-γ on apoptosis in the absence of EPO, but we did see an apoptotic effect in this report. Therefore, it is not contradictory to see a suppressive effect of IFN-γ on apoptosis instead of apoptotic induction, since cells were in a different stage of maturation and were examined at an earlier time, and thus may show a distinctly different response to the same cytokine.
The number of receptors for IFN-γ gradually decreases during maturation of erythroid progenitor cells, as shown by Taniguchi et al.34 While the amount of expression of the receptor may be altered, intracellular components that mediate the signal induced by IFN-γ could also be reorganized during erythroid maturation and hence would show a different response from that seen in immature cells.
While day 7 ECFCs immediately underwent apoptosis when EPO was excluded from the culture medium, cells cultured for another 3 days (day 10 cells, which are at the poly-ortho chromatic erythroblast stage) are relatively resistant to EPO deprivation (data not shown). This means that erythroid cells probably lose the intracellular “death” mechanism that mediates apoptosis at the terminal stage of erythroid maturation. Again, the report by Dai et al11 showed that 2 to 4 days of contact with IFN-γ is necessary to induce apoptosis of erythroid progenitors. Hence, when IFN-γ was added to day 7 ECFCs, these cells may have undergone the terminal stages of erythroid maturation before IFN-γ initiated the process by which the cells undergo apoptosis. Since day 7 ECFCs are less sensitive to the “death signal” induced by IFN-γ, possibly for the reason mentioned above, this might explain why we observed the enhancing effect of IFN-γ on survival of mature ECFCs, which was not evident during the process of apoptosis as it was in the case of immature erythroid progenitor cells. Alternatively, by looking at cells after 16 hours of incubation without EPO, we may have detected the effects of an early set of genes activated by IFN-γ, the effects of which are not seen during the process of apoptosis.
A stimulating effect of IFN-γ on hematopoietic progenitor cells has also been reported. Brugger et al14 found that IFN-γ increased peripheral blood CD34+ progenitor cell expansion when added to a cocktail of growth factors. Shiohara et al16 reported that the addition of IFN-γ to cultures containing SCF resulted in a synergistic effect on the development of murine hematopoietic progenitors. These data may support the notion that IFN-γ does not always function as a strictly inhibitory factor on hematopoietic progenitor cells. Recently, Baxter et al35reported that tumor necrosis factor-α, which is also an inhibitory cytokine, can stimulate proliferation of mitotically quiescent cells, while it induces apoptosis of mitotically active cells. Interferon alpha (IFN-α) enhances survival of B cells by reducing apoptosis,36 while IFN-α induces apoptosis of malignant plasma cells.37 This observation also supports the notion that an “inhibitory” cytokine can reduce apoptosis of hematopoietic cells under certain conditions and at certain stages of development.
While IFN-γ increased Fas expression of mature ECFCs, this expression did not directly induce apoptosis in these cells. Deprivation of EPO induced Fas expression plus activation of caspase8 and caspase3, which are known to be activated during Fas-mediated apoptosis prior to DNA fragmentation.26,28,38 Since the Fas ligand (FasL) is present on the surface of ECFCs,6 Fas-FasL interaction may play an important role in the apoptosis of ECFCs induced by EPO deprivation. The Fas-FasL system appears to play an important role in erythroid homeostasis through its induction of apoptosis in immature erythroblasts.39 In contrast, while IFN-γ also induced Fas expression on mature ECFCs, activation of caspase8 and caspase3 was not increased over 16 hours, and apoptosis of the cells was reduced despite the absence of EPO. These data indicate that IFN-γ induces expression of Fas throughout different stages of erythroid maturation, but influences downstream caspases in a differential manner according to the stage of erythroid maturation and the time of incubation. IFN-γ induces Fas expression but might block the Fas-mediated apoptosis of mature ECFCs through intracellular components such as FLIPs (FLICE- [Fas-associated death-domain–like IL-1β–converting enzyme] inhibitory protein)40 that block activation of caspase8 and downstream caspases in the T cells resistant to Fas-FasL-induced apoptosis or other mechanisms.
Bcl-x plays an important role in protecting cells from apoptosis by blocking the release of cytochrome c from mitochondria, a critical step in the activation of the caspase protease cascade.41 Gregoli and Bondurant22 demonstrated that deprivation of EPO reduced expression of Bcl-x to induce apoptosis of ECFCs via activation of caspase3. They also showed that expression of Bcl-x was highly EPO-dependent and that it increased greatly during the terminal differentiation of ECFCs. When mature ECFCs were cultured in the presence of IFN-γ without EPO, an increased amount of Bcl-x was detected compared to findings in cells without EPO and IFN-γ. However, the level of expression was less than in cultures with EPO. This suggests that IFN-γ may at least partially protect ECFCs from apoptosis through a pathway independent of expression of Bcl-x, which still plays an important role in the maintenance and survival of the cells in the presence of IFN-γ.
The complete significance of suppression of apoptosis shown in this report is not fully understood. IFN-γ may allow mature erythroid progenitor cells to maintain erythropoiesis, while immature progenitors are impaired during inflammatory stress.
Acknowledgments
We express our deep appreciation to S. Aoki and S. Isewaki for excellent technical assistance.
Supported in part by National Institutes of Health grant DK-15 555 of S.B.K.).
Reprints:Koichiro Muta, Department of Medicine and Bioregulatory Science, Graduate School of Medical Science, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan; e-mail: mmmmm@intmed3.med.kyushu-u.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 2. Effect of IFN-γ on apoptosis of ECFCs. / (A) Day 7 ECFCs were incubated in serum-free medium without rhEPO and rhIFN-γ (upper left), with rhEPO (10 U/mL) (upper right), with rhIFN-γ (1000 U/mL) (lower left), and both (lower right). After incubation for 16 hours, apoptosis was measured with PI and annexin V, using a flow cytometer. Data shows typical results of 12 replicates from 4 experiments. In each panel, the right lower quadrant (annexin V–positive and PI negative) indicates early apoptosis, and the right upper quadrant (annexin V– and PI positive) indicates late apoptosis. Both annexin V–positive fractions were assessed as apoptotic cells. (B) The day 7 ECFCs were labeled with [3H]thymidine and cultured in serum-free medium without rhIFN-γ (left), or with rhIFN-γ (1000 U/mL) (right), for 16 hours. Cellular DNA was isolated and analyzed by alkaline pH, 0.6% agarose gel electrophoreses. The sum of the radioactivity of fractionations 1-4 is designated as uncleaved DNA and is expressed as a percentage of the total radioactivity.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/12/10.1182_blood.v95.12.3742/8/m_bloo01209002x.jpeg?Expires=1766246362&Signature=anNjF90sQeBsENXsbRfgKi-CCfbJXzpVfSRBCs7VELQvkqImn9MDPYT6IciYF1O4P4y9opVzE8wEuT8s4cd2R3~KwKyg49eAi~dwe2mJyNTJfJM0CXzrbfoAh01jWMcHyidiGeYcbECPuX~6Hn2SIQ2D7F9kIn~oivQdFjUmB-4CpfxuRrb~I9gg6kbFB7iD2eJhTrSjMA4WXtgmYZDiM9x~3t6TYyWbFLj-7GEA0cCnjOfN4KSmA7iDiHJ~LAYXqdk3Ojz1pmVx0M2vzcdZ8d3P~vGHTPiDM~VTATs9V~1vsGLgkwuhYvs3WG-5ypRKPUi0IVTJvaWcl7Pw4FOyOw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 2. Effect of IFN-γ on apoptosis of ECFCs. / (A) Day 7 ECFCs were incubated in serum-free medium without rhEPO and rhIFN-γ (upper left), with rhEPO (10 U/mL) (upper right), with rhIFN-γ (1000 U/mL) (lower left), and both (lower right). After incubation for 16 hours, apoptosis was measured with PI and annexin V, using a flow cytometer. Data shows typical results of 12 replicates from 4 experiments. In each panel, the right lower quadrant (annexin V–positive and PI negative) indicates early apoptosis, and the right upper quadrant (annexin V– and PI positive) indicates late apoptosis. Both annexin V–positive fractions were assessed as apoptotic cells. (B) The day 7 ECFCs were labeled with [3H]thymidine and cultured in serum-free medium without rhIFN-γ (left), or with rhIFN-γ (1000 U/mL) (right), for 16 hours. Cellular DNA was isolated and analyzed by alkaline pH, 0.6% agarose gel electrophoreses. The sum of the radioactivity of fractionations 1-4 is designated as uncleaved DNA and is expressed as a percentage of the total radioactivity.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/12/10.1182_blood.v95.12.3742/8/m_bloo01209002x.jpeg?Expires=1766246363&Signature=Kmx4wBMkMT52zyQL~26TpTfrpeVKfX9MwBgjAHYqYD9lq3PJ7x~564ZKGn2xDhEH0FqukQdd3lK6qKSSbO1p9k-SaTX9MZpxNAgVfPkykwTBwjz90w0k2A~Q5Z3GfVS~LxwaVfbqY~7p2Z14BndbnMtS-LPMc5pm7yOeNtl80-AK-G6~07~NmzwrEuWxVUvOvj9VbdTQrqtnJ8mfcDIsP9oWli3W62KBqXtCsAK5iNxaWiyfFdGGrl8Jm6qViFNJ6yppfKT65CZeyB-ATUCgPniRpiQI0F4fYsCnxMb1RziLWGi6JIDzIsmwvUfiGCOYHo6c3xo85eRLntGxsm5aMg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)