The inability to deliver a therapeutic gene to a sufficient percentage of hematopoietic stem cells is the major obstacle to using gene therapy to treat blood disorders. Providing genetically corrected stem cells with a reversible growth advantage could solve this problem. To this end we have employed small synthetic molecules that can reversibly dimerize and activate fusion proteins which contain a growth factor receptor signaling domain. We have shown that the thrombopoietin receptor (mpl) signaling domain can be used in this system to expand transduced multipotential progenitor cells from mouse bone marrow. In the present study we tested a similar retroviral vector in human CD34-selected cord blood cells. Following transduction, cells cultured in the presence of the dimerizing molecule AP1903 expanded 13.8- to 186-fold relative to cells cultured in the absence of AP1903. The cell type that emerged in suspension culture was erythroid. Contrary to our results in the murine system, cell expansion was transient. Activation of mpl caused the disappearance of BFU-E followed by a transient increase in CFU-E. In contrast, mpl activation had no discernable effect on transduced myeloid progenitor cells. AP1903-mediated expansion was restricted to transduced cells, as demonstrated by immunohistochemical staining. These findings indicate that synthetic dimerizing molecules can be used to expand primary human hematopoietic cells.
Inefficient gene delivery into the human hematopoietic stem cell presents what is arguably the single most formidable obstacle to stem cell gene therapy. One approach to achieving a therapeutically relevant frequency of genetically modified stem cells is to employ selection. Present methods to achieve selection rely on the transfer of a gene that confers resistance to a subsequently administered cytotoxic drug.1 In this setting, selection produces a preferential elimination of unmodified cells.
An alternative approach for achieving selection uses a gene that confers a reversible growth advantage in the presence of a synthetic drug. In this setting, unmodified cells fail to respond to the drug, leading to the preferential expansion of the genetically corrected population. Conditional cell growth can be accomplished through the use of fusion proteins composed of a growth factor receptor signaling domain fused to a binding site for a synthetic drug called a chemical inducer of dimerization (CID).2 3 In order for the effects of selection to persist, it is expected that expansion must occur among genetically modified stem cells. Therefore, in order for this approach to produce a permanent increase in genetically modified cells, signaling molecules that are capable of inducing expansion among stem cells must be identified. A molecule that is a candidate for having this capacity is the thrombopoietin receptor (mpl).
In the context of a viral oncogene, the mpl signaling domain produces a myeloproliferative disorder in mice.4 In addition to the important role of mpl in megakaryocyte growth and differentiation, it has also been demonstrated that mpl plays a role in the maintenance of multipotential progenitors5,6 and stem cells.7,8,9 In previously published studies,10we demonstrated that transfer of a gene encoding a fusion protein which contains the signaling domain of mpl allows for a marked and sustained expansion of multipotential murine progenitor cells in the presence of CID. In the studies presented we evaluated whether CID-mediated activation of the mpl-signaling domain could stimulate expansion of genetically modified primary human CD34-selected cord blood cells.
Materials and methods
Our materials included cytokines human stem cell factor (hSCF), human interleukin-3 (hIL-3), and hIL-6 (Peprotech, Rocky Hill, NJ) and recombinant human erythropoietin (Ortho Biotech, Raritan, NJ). Throughout this report, stated G418 concentrations indicate active drug concentrations.
Retroviral construct
The MFM (MSCVF36Vmpl) retroviral vector is identical to the MSCV-based vector described in our previous report,10with the exception that a point mutation was introduced into the FK506 binding protein (FKBP) domain to produce a substitution at amino acid position 36 from phenylalanine to valine using primer-directed mutagenesis. The mpl signaling domain contained in this vector is derived from murine mpl.
Retroviral producer lines
Retroviral producer lines were generated as previously described.10 In brief, the MFM vector was transfected into the ecotropic packaging cell line PE501. After 48 hours, supernatant was collected and used to transduce the Gibbon ape leukemic virus (GALV)-packaging cell line, PG13.11 We tested G418-resistant clones for genetic stability by Southern analysis testing of KpnI-digested genomic DNA. A clone was obtained with a titer of 5 × 105 colony-forming units/mL. Retroviral supernatant was collected from subconfluent monolayers of MFM/PG13 producer cells after incubation for 48 hours at 33°C.
Isolation of CD34-selected cord blood cells
CD34-selected cells were isolated from normal human umbilical cord blood scheduled for disposal after delivery. Mononuclear cells from total cord blood were separated from red blood cells using density gradient centrifugation (with a density of 1.077) on the cell separation medium (Lymphoprep; Mediatech, Herndon, VA). Adherent cells were removed by incubating the total cell suspension on tissue culture plates for 1 hour at 37°C. Immunomagnetic selection of the CD34+ cells was accomplished using the MACS system (Miltenyi Biotec, Auburn, CA), and the resulting purity of the CD34-selected cord blood cells is shown in Table 1.
Transduction of CD34-selected cord blood cells
CD34-selected cells were placed in 6-well Costar plates that had been coated with CH296 fibronectin fragment (Retronectin; Takara Biomedicals, Otsu, Japan) according to the manufacturer's instructions. Cells were cultured for 24 hours at 32°C with 7.5% CO2 in Iscove's modified Dulbecco's medium (IMDM) supplemented with fetal bovine serum (FBS), 10%; recombinant human interleukin 6 (rhIL-6), 100 ng/mL; rhIL-3, 50 ng/mL; recombinant human stem cell factor (rhSCF), 50 ng/mL; and protamine sulfate, 4 μg/mL. Retroviral supernatant was added at a multiplicity of infection of approximately 4:1. After 24 hours the supernatant was removed, and fresh viral supernatant was added. After an additional 24-hour incubation, nonadherent cells were removed, counted, and divided among the experimental conditions. Transduction efficiency was determined by performing methylcellulose assays both in the presence and absence of G418 (1.2 mg/mL). In 1 experiment (Table 1), transduction conditions were modified by the addition of a 24-hour preincubation step in which the cells were cultured in DMEM with 16% FBS in the presence of IL-3, IL-6, Flt3L, and SCF (50 ng/mL each).
An additional method for transduction, based on the report of Dao and Nolta,12 was tested as indicated in Table 1. CD34-selected cord blood cells were preincubated for 12 hours (ExVivo 15; Biowhittaker, Walkersville, MD) with hIL-6, hIL-3, hSCF, and Flt3L (50 ng/mL each). Retronectin-coated plates were preloaded with retrovirus by incubating supernatant on the plates 3 times for 15 minutes each incubation. Cells were then placed in wells containing an equivalent volume of retroviral supernatant, which was collected in DMEM with 10% FBS. Anti–transforming growth factor β (anti-TGFβ) antibody (R&D Systems, Minneapolis, MN) was added at a concentration of 5 μg/mL and incubated for 12 hours at 37°C with 5% CO2.
Suspension culture
After retroviral transduction, CD34-selected cord blood cells were washed and cultured (either in the absence or presence of AP1903 [100 nmol/L]) in IMDM containing FBS, 10%; penicillin, 50 units/mL; and streptomycin, 50 μg/mL. Human serum (10%) was also included in the cultures listed in experiments 1 and 2 (Table 1).
Clonogenic assays in semisolid media
Clonogenic assays were performed in triplicate in the presence of methylcellulose, 1.2%; FBS, 30%; bovine serum albumin (BSA), 1%; 2-mercaptoethanol (SIGMA, St. Louis, MO), 5 × 10-4 mol/L; hIL-3, 5 ng/mL; hSCF, 50 ng/mL; and human erythropoietin (h-epo), 5 units/mL (for measurements of erythroid burst-forming units [BFU-Es]). Cells were cultured in a humidified 37°C incubator with 5% CO2. Colonies were counted on day 14.
Erythroid colony-forming unit (CFU-E) and megakaryocyte (CFU-Mk) assays were performed using a plasma clot assay as previously described.13 CD34-selected cells were plated in 10% human plasma, 10% BSA, and 10% bovine citrated plasma in addition to penicillin, 50 units/mL; streptomycin, 50 μg/mL; CaCl2, 2 mmol/L; and thrombin, 0.25 units/mL. Growth factors included erythropoietin (epo), 2 units/mL; IL-3, 5 ng/mL; IL-6, 3.5 ng/mL; SCF, 50 ng/mL; and thrombopoietin conditioned media. CFU-E colonies were analyzed at days 5 to 7, while CFU-Mk colonies were analyzed after 13 days of culture. Individual plasma clots were removed and flattened on a gelatin-treated slide. For CFU-E assays, clots were fixed with glutaraldehyde and stained with hematoxylin and benzidine. CFU-Mk colonies were identified by staining with a biotin-labeled anti-CD41 antibody. This was followed by staining with a streptavidin-labeled alkaline phosphatase (Vector Laboratories, Burlingame, CA) in accordance with the recommendations of the manufacturer.
Flow cytometry
Cells were centrifuged and resuspended in phosphate-buffered saline (PBS)/BSA/Azide, then incubated with directly conjugated primary antibodies phycoerythrin-labeled (PE-labeled) antiglycophorin A and PE-labeled anti-CD33 (Becton Dickinson, Franklin-Lakes, NJ) at 4°C for 30 minutes. The cells were then centrifuged, washed once with PBS/BSA/Azide, resuspended in PBS/BSA/Azide, and analyzed with a flow cytometer (Coulter XL-MCL; Coulter Electronics, Miami, FL). We collected 10 000 gated cellular events during analysis for each specimen. Cellular events were identified on the basis of CD45 expression (PE-Cy5 anti-CD45; Immunotech, Westbrook, ME), and forward/side scatter gating was used to exclude cellular debris and aggregates. Gates were set by comparing each specimen with isotype-matched negative control antibodies.
Immunohistochemical staining
Immunocytochemistry was performed on cytospin preparations of CD34-selected cord blood cells using the monoclonal antibody HA .11 (BAbCO, Richmond, CA). This antibody recognizes the influenza hemaglutinin epitope tag (Figure 1A). After fixation in 95% ethanol, cytospins were incubated overnight at 4°C with HA .11 in PBS. This was followed by secondary staining with a biotinylated goat antimouse antibody (Signet Laboratories, Dedham, MA) at room temperature for 20 minutes. Slides were then incubated with peroxidase-labeled streptavidin (Signet Laboratories) for 20 minutes. The peroxidase reaction was performed by mixing 0.5 mg/mL 3-amino-9-ethylcarbazole (Signet Laboratories) in 0.15 mol/L Tris-HCl with 0.1% hydrogen peroxide. Cells were counterstained with methylene blue (Biochemical Sciences, Swedesboro, NJ). The proportion of positive cells was evaluated under high-power light microscopy. At least 500 cells were scored from each sample.
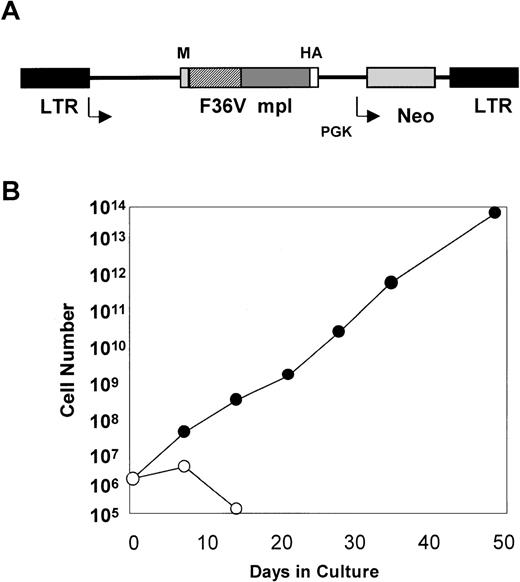
The MFM vector can mediate a dramatic expansion of mouse bone marrow cells in the presence of AP1903.
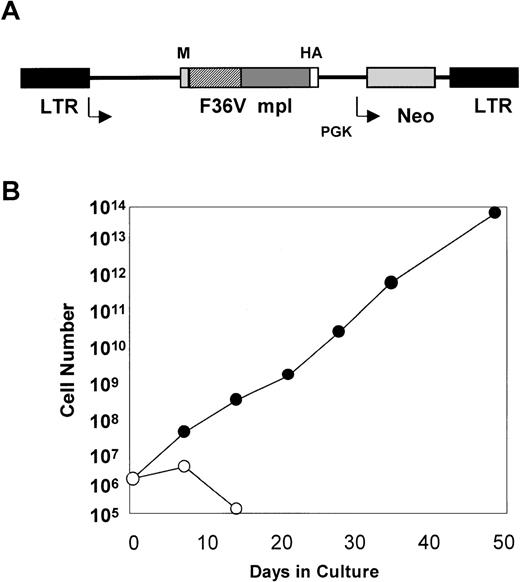
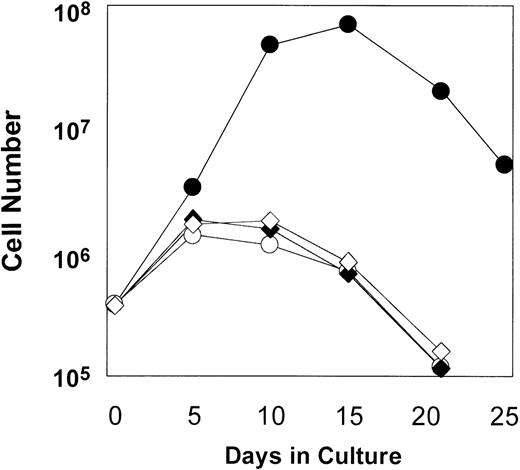
(A) MFM is an MSCV-based retroviral vector. The gene encoding the F36Vmpl fusion protein is transcribed from the long terminal repeat (LTR). The neo gene is transcribed from the phosphoglyceate kinase (PGK) promoter. In Figure 1A, M indicates myristylation domain; HA, epitope tag from influenza hemagglutinin; F36V, the CID-binding domain; and mpl, the intracellular signaling domain of murine mpl. (B) Mouse bone marrow was transduced with the MFM vector and then incubated in the absence (○) or presence (•) of AP1903 (100 nmol/L). The transduced mouse bone marrow cells cultured in the absence of AP1903 died over a 2-week period, while the cells cultured in the presence of AP1903 exhibited a dramatic expansion, similar to our previously published results using FK1012.10
The MFM vector can mediate a dramatic expansion of mouse bone marrow cells in the presence of AP1903.
(A) MFM is an MSCV-based retroviral vector. The gene encoding the F36Vmpl fusion protein is transcribed from the long terminal repeat (LTR). The neo gene is transcribed from the phosphoglyceate kinase (PGK) promoter. In Figure 1A, M indicates myristylation domain; HA, epitope tag from influenza hemagglutinin; F36V, the CID-binding domain; and mpl, the intracellular signaling domain of murine mpl. (B) Mouse bone marrow was transduced with the MFM vector and then incubated in the absence (○) or presence (•) of AP1903 (100 nmol/L). The transduced mouse bone marrow cells cultured in the absence of AP1903 died over a 2-week period, while the cells cultured in the presence of AP1903 exhibited a dramatic expansion, similar to our previously published results using FK1012.10
Results
Experiments were performed to test whether CID-mediated activation of the mpl-signaling domain can function to expand primary human CD34+ cells.
CID-mediated expansion of transduced CD34+ cord blood cells
The MFM vector used for our studies (Figure 1) encodes a fusion protein that contains a myristylation domain which targets the molecule to the cell membrane, a CID-binding domain, the cytoplasmic portion of murine mpl, and an epitope tag. The CID-binding domain has been modified to accommodate binding to a new class of synthetic CIDs.14 In contrast to dimerizing agents, such as FK1012, which can bind endogenous FKBPs, a new synthetic dimerizer, AP1903, has been developed to reduce association with naturally occurring FKBPs. The AP1903 dimerizer specifically binds to a mutated FKBP termed F36V, which contains a phenylalanine to valine substitution. This combination of ligand-FKBP has been shown to allow for CID-mediated activation of the Fas signaling pathway.14 Retroviral transfer of the MFM vector into Ba/F3 cells allows for AP1903-mediated cell growth as reported previously for FK1012 (data not shown). In addition, retroviral transfer of the MFM vector into primary mouse bone marrow cells results in dramatic cell growth that is dependent on AP1903 (Figure 1B). This is similar to our previous findings using FK1012.10 The MFM vector was used to generate a PG13-based producer cell line11 for transduction of human CD34-selected cord blood cells.
Transductions of CD34-selected cord blood cells were carried out as described in “Materials and Methods.” The presence of a neo gene in the MFM vector allowed gene transfer rates to be determined by performing colony assays both in the presence and absence of G418. Rates of gene transfer into progenitors ranged between 10% and 66%. A summary of our experiments is shown in Table 1. Following transduction, cells were placed in IMDM with 10% FBS, either in the presence or absence of AP1903, at a concentration previously determined to be optimal for the expansion of transduced mouse bone marrow cells (100 nmol/L). Mock-transduced cells that were incubated either in the presence or absence of AP1903 provided an additional control. The effects of AP1903 were evaluated in the absence of growth factors other than those present in serum.
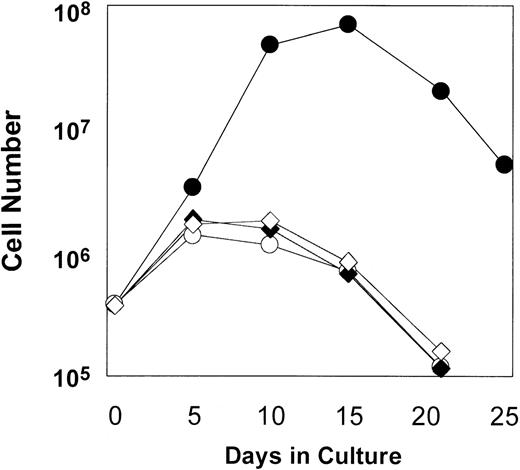
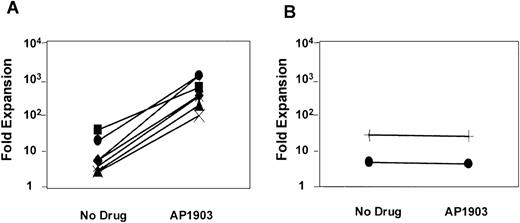
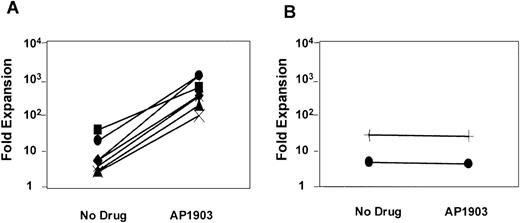
As shown in Figure 2, some degree of cell expansion was observed in each of the conditions tested. Both MFM-transduced cells cultured in the absence of AP1903 and mock-transduced cells displayed a 3-fold increase in cell number, which peaked at days 5-10 of culture and fell thereafter. This relatively modest level of cell growth in the controls may be attributable to the persistent effects of growth factors that were present at the time of the transduction procedure. However, MFM-transduced cells cultured in the presence of AP1903 exhibited a maximal 186-fold increase in cell number by day 15. In contrast to results obtained using primary mouse bone marrow cells,10 sustained growth of transduced human hematopoietic cells was not observed. The addition of Flt3 ligand (50 ng/mL) to AP1903 failed to result in improved cell growth (data not shown). The ability of AP1903 to induce a transient expansion of MFM-transduced cord blood cells was highly reproducible. In each of 7 independent experiments (Figure 3A), MFM-transduced cells cultured in the presence of AP1903 were expanded between 13.8-fold and 186-fold relative to cells cultured in the absence of AP1903. In contrast, AP1903 had no effect on the growth of mock-transduced cells (Figure 3B).
Expansion of transduced cord blood cells in the presence of AP1903.
CD34-selected cord blood cells were transduced using the MFM vector (Table 1, Experiment 3). Following transduction, cells were cultured either in the presence (■) or absence (○) of AP1903. Controls included mock-transduced cells cultured in the presence (♦) or absence (⋄) of AP1903.
Expansion of transduced cord blood cells in the presence of AP1903.
CD34-selected cord blood cells were transduced using the MFM vector (Table 1, Experiment 3). Following transduction, cells were cultured either in the presence (■) or absence (○) of AP1903. Controls included mock-transduced cells cultured in the presence (♦) or absence (⋄) of AP1903.
MFM-transduced CD34+ cord blood cells consistently expand in the presence of AP1903.
The maximal level of cell expansion of MFM-transduced (A) and mock-transduced (B) cord blood cells was plotted for each of the experiments listed in Table 1. Each line represents one experiment. The addition of AP1903 resulted in a 13.8-fold to 186-fold expansion compared with cells cultured in the absence of AP1903.
MFM-transduced CD34+ cord blood cells consistently expand in the presence of AP1903.
The maximal level of cell expansion of MFM-transduced (A) and mock-transduced (B) cord blood cells was plotted for each of the experiments listed in Table 1. Each line represents one experiment. The addition of AP1903 resulted in a 13.8-fold to 186-fold expansion compared with cells cultured in the absence of AP1903.
Cells expanded in the presence of CID belong primarily to the erythroid lineage
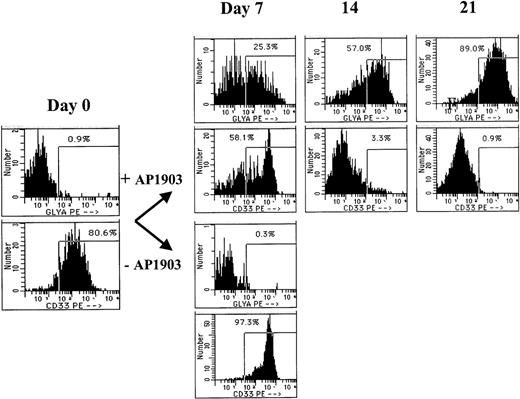
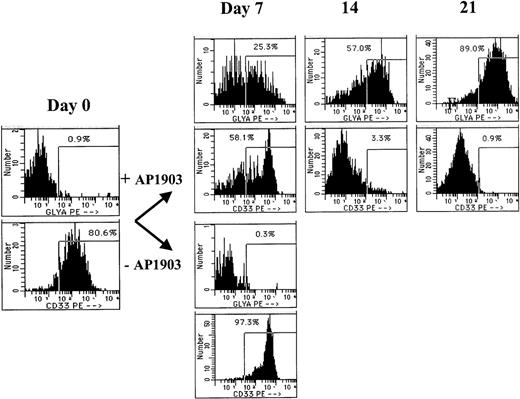
The lineage of cells expanding in response to AP1903 was evaluated by Wright-Giemsa staining and flow cytometry at various time points during the culture. In cultures that contained AP1903, the predominant cell type was erythroid, as confirmed by benzidine staining (data not shown) and flow cytometry using an antibody directed against glycophorin A (Figure 4). Glycophorin A positive cells were evident by day 7 of the culture, and by day 21, 89% of cells cultured in AP1903 were glycophorin A positive, while fewer than 1% of cells displayed the myeloid/monocytic marker CD33. An absence of myeloid cell expansion in response to AP1903 was confirmed by determining absolute neutrophil counts during the course of the culture (data not shown). The absence of myeloid cell expansion occurred despite having achieved transduction rates into granulocyte/macrophage colonies (CFU-GM) of up to 66% (Table 1). In the absence of AP1903, cultures contained a large percentage of dying cells; most surviving cells displayed CD33.
Cells expanded in the presence of AP1903 are predominately erythroid.
CD34-selected cord blood cells were transduced with the MFM vector and incubated in the presence or absence of AP1903. On the days indicated, aliquots of cells were removed from the suspension culture and tested by flow cytometry for binding of antibodies directed against glycophorin A and CD33. The cells incubated without the drug died following day 7 and were therefore not available for analysis.
Cells expanded in the presence of AP1903 are predominately erythroid.
CD34-selected cord blood cells were transduced with the MFM vector and incubated in the presence or absence of AP1903. On the days indicated, aliquots of cells were removed from the suspension culture and tested by flow cytometry for binding of antibodies directed against glycophorin A and CD33. The cells incubated without the drug died following day 7 and were therefore not available for analysis.
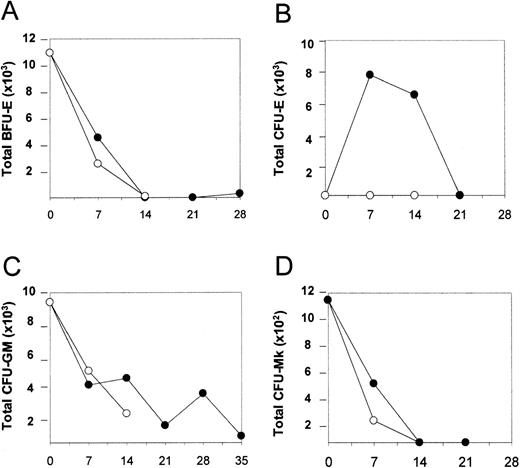
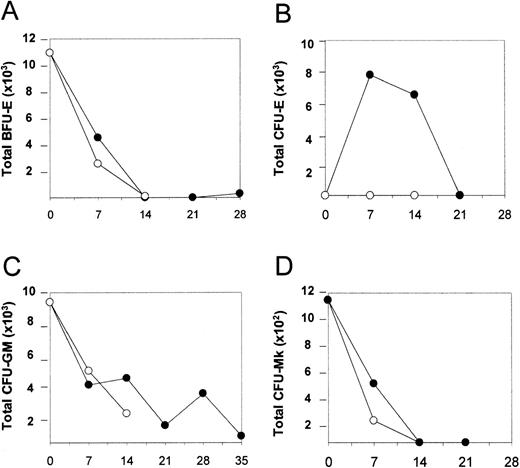
We next investigated whether CID-mediated activation of the mpl-signaling domain could stimulate an expansion of clonogenic progenitor cells. Cells were removed from the culture at various time points and analyzed for progenitor content as previously described. A time-dependent decline in BFU-E, CFU-GM, and CFU-Mk numbers was observed, both in the presence and absence of AP1903 (Figure5, panels A, C, and D respectively). In contrast, a transient expansion of CFU-E in response to AP1903 was observed. This time frame correlated with the disappearance of BFU-E and presaged the emergence of glycophorin A positive cells (Figure 5, panel B). These observations are consistent with CID-mediated differentiation of transduced BFU-E, which results in an orderly progression to CFU-E and then terminal differentiation of erythroid cells.
AP1903 produces a transient expansion of CFU-E.
Total BFU-E (A), CFU-E (B), CFU-GM (C), and CFU-Mk (D) values were determined for cord blood transduced with the MFM vector, expanded in the absence and presence of AP1903. Cell expansion occurred in the presence of AP1903, similar to previous experiments (data not shown). BFU-e, CFU-GM, and CFU-Mk values decreased regardless of whether CID was present. CFU-E increased on day 7, then diminished, and was dependent on the presence of AP1903. • = + AP1903; ○ = −AP1903.
AP1903 produces a transient expansion of CFU-E.
Total BFU-E (A), CFU-E (B), CFU-GM (C), and CFU-Mk (D) values were determined for cord blood transduced with the MFM vector, expanded in the absence and presence of AP1903. Cell expansion occurred in the presence of AP1903, similar to previous experiments (data not shown). BFU-e, CFU-GM, and CFU-Mk values decreased regardless of whether CID was present. CFU-E increased on day 7, then diminished, and was dependent on the presence of AP1903. • = + AP1903; ○ = −AP1903.
Cell expansion in response to AP1903 is restricted to the transduced population
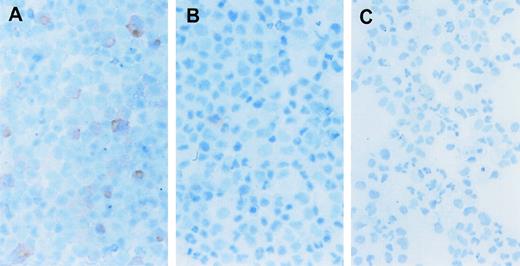
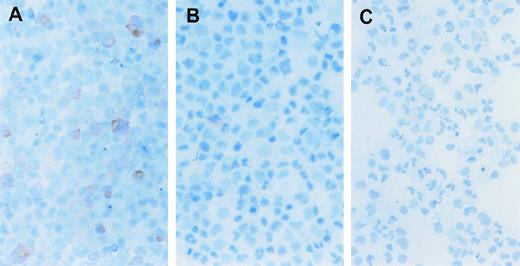
To evaluate the possibility that the erythroid cell expansion was the indirect result of growth factor secretion in response to the CID, we directly examined the expanded cells for the presence of the F36Vmpl fusion protein. Immunohistochemical analysis was performed using an antibody directed against the HA epitope tag present on the fusion protein (Figure 1). Up to 86% of cells expanded in the presence of AP1903 expressed the fusion protein (Figure6A, B, and C; Table2). In contrast, only 9%-14% of cells cultured in the absence of CID expressed the fusion protein. This frequency was similar to the rate of gene transfer into progenitors as assessed by colony assays. The persistence of low-frequency HA positive cells in the absence of CID suggests that transduced cells were neither preferentially expanded nor eliminated from culture in the absence of selection. The restriction of AP1903-mediated expansion to the transduced cell population suggests that AP1903 exerted a direct differentiative effect on genetically modified erythroid progenitor cells.
CD34+ cord blood cells transduced with the MFM vector and expanded with AP1903 express the F36Vmpl transgene. Immunohistochemical staining was performed to identify cells that contain the HA epitope as part of the F36Vmpl transgene. (See “Materials and Methods” for a complete description of the method.) Cord blood cells were transduced with the MFM vector and then incubated in the presence (A) or absence (B) of AP1903 for 14 days prior to staining for the HA epitope. Panel C shows mock transduced cells incubated for 14 days and then stained for the presence of the HA epitope.
CD34+ cord blood cells transduced with the MFM vector and expanded with AP1903 express the F36Vmpl transgene. Immunohistochemical staining was performed to identify cells that contain the HA epitope as part of the F36Vmpl transgene. (See “Materials and Methods” for a complete description of the method.) Cord blood cells were transduced with the MFM vector and then incubated in the presence (A) or absence (B) of AP1903 for 14 days prior to staining for the HA epitope. Panel C shows mock transduced cells incubated for 14 days and then stained for the presence of the HA epitope.
AP1903 can support BFU-E and CFU-Mk development in semisolid culture and can synergize with other cytokines
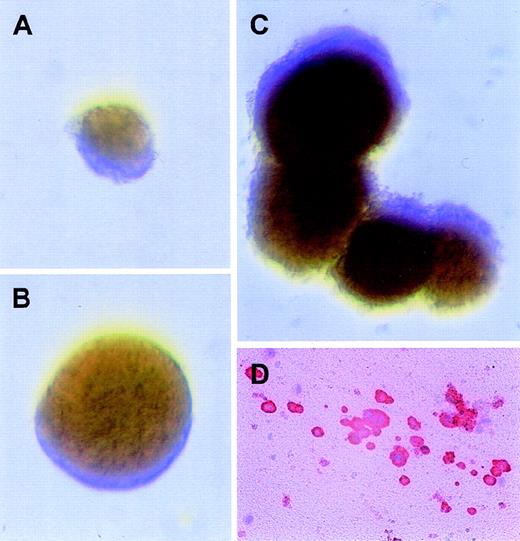
To directly analyze progenitor response to AP1903 in the context of other cytokines, cord blood cells were cultured in semisolid media with AP1903 alone or in combination with SCF and IL-3. To provide positive controls, progenitor assays were performed in the presence of erythropoietin, SCF, and IL-3 (Figure 7, panel C). Colony assays performed in the presence of AP1903 gave rise to BFU-E but failed to generate CFU-GM beyond those observed in the absence of CID (Table 3). The addition of SCF and IL-3 to the culture conditions resulted in an increase in the total number of BFU-E as well as in an increase in the size of the BFU-E colonies (Table 3 and Figure 7, panels A and B), suggesting synergy between the fusion protein and SCF and/or IL-3. The average size of colonies cultured in the presence of AP1903 were 2400 cells/colony; AP1903/SCF/IL-3, 11 400 cells/colony; and epo/SCF/IL-3, 27 600 cells/colony. Plasma clot assays were performed in the presence and absence of AP1903 to determine if CFU-Mk development could be supported in the absence of added cytokines. Figure 7 (panel D) shows a representative megakaryocyte colony that developed in the presence of AP1903. Activation of the mpl signaling domain through CID-mediated dimerization was able to support colony growth from transduced BFU-E and CFU-Mk but was unable to support colony growth from transduced CFU-GM.
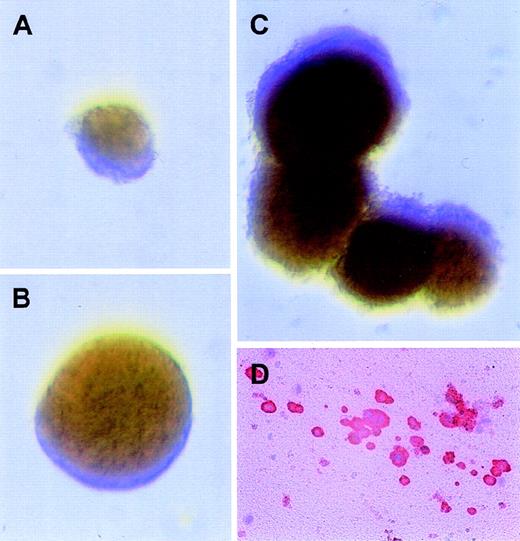
Erythroid and megakaryocytic progenitors transduced with the MFM vector develop into colonies in the presence of AP1903 alone.
Immediately after transduction with the MFM vector, CD34+ cord blood cells were plated in colony assays with different combinations of drug and cytokines. Displayed in panels A-C are representative BFU-E colonies with the following conditions: (A) BFU-E assays with AP1903 alone; (B) BFU-E assays with AP1903, SCF, and IL-3; (C) BFU-E assays with epo, SCF, and IL-3; and (D) a representative megakaryocytic colony from plasma clot assays plated in AP1903 alone. The cells were stained for CD41 expression, as detailed previously.
Erythroid and megakaryocytic progenitors transduced with the MFM vector develop into colonies in the presence of AP1903 alone.
Immediately after transduction with the MFM vector, CD34+ cord blood cells were plated in colony assays with different combinations of drug and cytokines. Displayed in panels A-C are representative BFU-E colonies with the following conditions: (A) BFU-E assays with AP1903 alone; (B) BFU-E assays with AP1903, SCF, and IL-3; (C) BFU-E assays with epo, SCF, and IL-3; and (D) a representative megakaryocytic colony from plasma clot assays plated in AP1903 alone. The cells were stained for CD41 expression, as detailed previously.
Discussion
The inability to deliver genes to more than a small fraction of human stem cells poses a major obstacle to gene therapy for a wide range of hematological disorders. Using selection in vivo has important theoretical advantages. A clinically effective method for selection may allow stem cells to be harvested from a patient, transduced, and reinfused with little or no conditioning. The transduced cell population could then be expanded through in vivo administration of the corresponding selective drug regimen. Two requirements must be met. First, achieving a sustained expansion of the selected cell population requires that selection be imposed among stem cells. Sorrentino and colleagues1 15have recently demonstrated that stem cell selection can be accomplished through transfer of the dihydrofolate reductase gene followed by administration of drugs that preferentially eliminate unmodified progenitors and stem cells. Second, clinical applicability requires that the drugs used for selection be well-tolerated.
An alternative to preferentially eliminating nontransduced cells is to preferentially expand the transduced cell population. Toward this goal we have adopted a system that allows intracellular signaling to be reversibly controlled using the CID, FK1012.2,16 In previous studies we have used genes that encode fusion proteins containing the epo receptor,3c-kit,17 or c-mpl10 signaling domains to generate FK1012-dependent cell lines. Furthermore, FK1012-mediated activation of the mpl signaling domain produces a dramatic expansion of transduced primary murine bone marrow cells. Identical results have been obtained using the alternative CID, AP1903, and its cognate binding site, the F36V-modified FKBP domain (Figure 1).
Two highly reproducible features of this system merit comment. First, expansion of murine bone marrow cells in the presence of CID is sustained. Murine bone marrow cells can be expanded for longer than 300 days in serum-containing cultures with FK1012 but without the addition of cytokines. Second, cells expanded in response to FK1012 include multipotential progenitor cells and differentiated megakaryocytes. The results that we have obtained using human cells differ significantly from our results in mice. CID-mediated activation of the mpl signaling domain in human CD34-selected cord blood cells produced only a temporary expansion, which was exhausted after 28 days of culture. Transduced human BFU-E, CFU-GM, and CFU-Mk all failed to amplify in response to the CID, while CFU-E rose transiently due to apparent differentiation of transduced BFU-E.
What factors might account for the different responses to mpl activation between mouse and man? There are several possibilities. First, mpl-activated signaling pathways in the human cellular environment may be identical to those in the mouse but incapable of eliciting expansion among human progenitor cells. In particular, human stem cell expansion may require environmental signals in addition to those provided by mpl. In this context it is interesting to note that bcr-abl, an oncogene with a demonstrated capacity for stimulating human stem cell expansion, can produce preferential cell expansion only in the in vivo setting.18,19 This suggests that the selective advantage conferred by this oncogene requires supplemental signals from the environment. Second, there may be a difference in the frequency of the multipotential, expandable progenitors in human cord blood when compared with 5 fluorouracil–treated mouse bone marrow. Third, the lack of human progenitor cell expansion may be attributable to qualitative or quantitative differences in mpl-activated signaling pathways between the murine and human hematopoietic systems. This possibility implies that human progenitor cell expansion might be achievable if the signaling pathways that are activated in the mouse system could be precisely replicated. We are presently testing a retroviral construct that contains the cytoplasmic portion of human mpl. A fourth possibility is that our transduction conditions failed to deliver the vector into the human equivalent of the transduced pluripotent mouse cell that was capable of expanding for over 300 days. Preliminary experiments in the NOD-SCID (non-obese diabetic severe combined immunodeficiency) mouse model indicate that 3% to 5% of SCID repopulating cells have been transduced (data not shown). Nevertheless, these findings do not exclude the possibility that transduction of an even more primitive cell type may be required in order to achieve a sustained proliferative effect. The possibility remains that although the MFM vector may be the proper ‘seed’ to stimulate primitive cell growth, we may have not delivered it into the necessary cellular ‘soil.’20
Our findings demonstrate that mpl signaling in primary human hematopoietic cells is sufficient for full erythroid and megakaryocytic differentiation. The ability of mpl to permit differentiation of erythroid progenitor cells is in agreement with 2 previously published reports.21,22 Thrombopoietin administration was permissive for erythroid differentiation of fetal liver cells derived from the erythropoietin receptor null mouse,22 while ectopic expression of c-mpl in transduced mouse bone marrow cells allowed for thrombopoietin-dependent differentiation of transduced BFU-E.21 In supporting full erythroid maturation, CID-mediated mpl signaling acts in a manner similar to the combination of epo and SCF.23 In our studies, CD34+ cells were exposed to SCF during the transduction procedure, possibly rendering transduced BFU-E competent to respond to mpl activation. In contrast, we did not observe differentiation of transduced CFU-GM when mpl was activated, as has been noted in a previous report.21 These findings indicate that signals emanating from activated cytokine receptors are not strictly interchangeable between progenitors of all lineages.
These studies demonstrate that CIDs can specifically deliver a mitogenic signal to genetically modified primary human hematopoietic cells. Further studies are required to define and manipulate signals that are capable of inducing human stem cell expansion.
Acknowledgments
The authors would like to thank Denise Farrar and Donna Ceniza for expert technical assistance, David W. Emery for technical advice, Mo Dao and Jan Nolta with help developing transduction conditions, and Michael Gilman and Tim Clackson (Ariad Pharmaceuticals) for providing AP1903. We would also like to thank Ortho Biotech of Raritan, NJ, for the generous gift of recombinant human erythropoietin.
Supported by grants from the National Institutes of Health (NIH 1R01 DK5299701, 1R01 DK57525, 5P01 HL53750, 5P30 DK47754), a Junior Faculty Award from the American Society of Hematology, and a grant from the Fanconi Anemic Research Fund.
Reprints:C. Anthony Blau, Mailstop 357710, Health Sciences Building, University of Washington, Seattle, WA 98195; e-mail:tblau@u.washington.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.