Abstract
Eosinophil accumulation has been associated with the pathogenesis of numerous allergic inflammatory disorders. Despite the great interest in this response, many aspects of eosinophil accumulation remain unknown. This is particularly true with respect to tissue-specific mechanisms that may regulate the accumulation of eosinophils in different organs. This study addressed this issue by investigating and comparing the roles of α4-integrins and vascular cell adhesion molecule 1 (VCAM-1) adhesion pathways in interleukin 4 (IL-4)–induced eosinophil accumulation in 2 different rat models of inflammation, namely pleural and cutaneous inflammation. Similar to our previous findings in studies in rat skin, locally administered IL-4 induced a time- and dose-dependent accumulation of eosinophils in rat pleural cavities, a response that was associated with generation of the chemokine eotaxin. The IL-4–induced eosinophil accumulation in skin and pleural cavities was totally inhibited by an antirat α4-integrins monoclonal antibody (mAb) (TA-2). In contrast, whereas an antirat VCAM-1 mAb (5F10) totally blocked the response in skin, IL-4–induced eosinophil accumulation in rat pleural cavities was not affected by VCAM-1 blockade. A radiolabeled mAb technique demonstrated that endothelial-cell VCAM-1 expression was induced in response to IL-4 in both skin and pleural membrane. The results indicate that although endothelial-cell VCAM-1 is present in skin and pleura, a functional role for it in IL-4–induced eosinophil accumulation was evident only in skin. These findings suggest the existence of tissue-specific adhesive mechanisms in regulating leukocyte migration in vivo and demonstrate a dissociation between VCAM-1 expression and eosinophil accumulation.
Introduction
Interleukin 4 (IL-4), a multifunctional cytokine secreted by T-helper 2 (Th2)–type lymphocytes,1 mast cells,2 basophils,3 and eosinophils,4 is implicated in the pathogenesis of allergic disease states. This cytokine has several important immunomodulatory functions, such as the regulation of IgE synthesis,5-7 the development of Th2-type lymphocytes,8,9 and enhancement of B-cell surface antigens such as the low-affinity receptor for IgE (Fc RII/CD23) and class II major histocompatibility complex molecules.10,11 In addition, through its ability to directly activate venular endothelial cells to express adhesion molecules such as vascular cell adhesion molecule 1 (VCAM-1)12-14 and to induce the release of eosinophil chemoattractants such as eotaxin,15,16 IL-4 is a potent inducer of eosinophil migration in vivo.16,17Indeed, increased expression of IL-4 message or protein was detected in bronchoalveolar lavage or bronchial biopsy specimens from patients with asthma,18-20 a disorder in which eosinophil accumulation is a characteristic feature.
The adhesive mechanisms that mediate IL-4–induced interactions between leukocytes and endothelial cells have largely been investigated in vitro. Early studies demonstrated that cultured endothelial cells stimulated with IL-4 express VCAM-1, with little or no expression of intracellular adhesion molecule (ICAM) 1 or E-selectin.12-14,21 Furthermore, adhesion of eosinophils, basophils, and lymphocytes to IL-4–activated endothelial cells under static conditions was found to depend predominantly on VCAM-1.14,22 In the same studies, neutrophils did not adhere to endothelial cells stimulated with IL-4, results that were explained by the lack of expression of α4-integrins (α4β1 and α4β7), the principal leukocyte ligands for VCAM-1 characterized at that time, on the surface of neutrophils. More recent studies, however, showed that α4-integrins can support neutrophil rolling on VCAM-1 under flow conditions,23-25 indicating the presence of functionally relevant levels of α4-integrins on neutrophils. Two other integrins, αdβ2 and α9β1, which are molecules expressed by neutrophils, were also shown to act as ligands for VCAM-1.26,27 Collectively, these data suggest that under certain inflammatory conditions, IL-4 not only elicits accumulation of eosinophils and mononuclear cells but may induce neutrophil recruitment. Furthermore, Yao et al28 demonstrated a prolonged increase in P-selectin expression on the surface of IL-4–activated endothelial cells, a response that supported neutrophil rolling under flow conditions28 and depended on the P-selectin ligand P-selectin glycoprotein ligand 1.25
In contrast to the increasing number of in vitro studies investigating the adhesive mechanisms involved in IL-4–induced interactions between leukocytes and endothelial cells, few studies have assessed the adhesive mechanisms involved in IL-4–stimulated leukocyte migration in vivo. Although exogenously administered IL-4 induced leukocyte migration in vivo in several investigations17,29-31 (and, in one study, the response was associated with VCAM-1 expression),30 a functional role for VCAM-1 in IL-4–induced leukocyte migration was demonstrated only in a previous study by our group.17 In that study, we found that IL-4–induced eosinophil accumulation in rat skin was blocked by monoclonal antibodies (mAbs) directed against α4- or β2-integrins and VCAM-1 but not by an anti-ICAM-1 mAb.17 More recently, Hickey et al31found that leukocyte rolling in IL-4–activated mouse cremasteric venules depends on α4-integrins, although it is not known whether VCAM-1 supports this α4-integrin–mediated response.
In this study, we extended our investigations into the functional role of α4-integrins–VCAM-1 adhesion pathways in IL-4–induced eosinophil migration in vivo by studying the IL-4–induced response in 2 rat models of inflammation—namely, pleural and cutaneous inflammation—in parallel. We found that although IL-4 can induce expression of VCAM-1 in both skin and pleural membrane, a functional role for VCAM-1 was evident only in IL-4–induced eosinophil accumulation in rat skin. These findings demonstrate a clear dissociation between VCAM-1 expression and a functional role in leukocyte migration, suggesting the existence of an alternative ligand or ligands for α4-integrins in eosinophil accumulation induced by IL-4 in the pleural cavity. Furthermore, the results indicate that leukocyte migration elicited by IL-4 may be mediated by adhesive mechanisms specific to the target tissue.
Materials and methods
Male Sprague-Dawley rats (∼ 220 g) were purchased from Harlan-Olac (Oxfordshire, United Kingdom) for use in this study. Recombinant rat IL-4 was purchased from Serotec (Kidlington, Oxford, United Kingdom) or obtained as a gift from Dr D. Mason (Sir Williams Dunn School of Pathology, University of Oxford, Oxford, United Kingdom). We previously showed that the latter preparation of IL-4 contains less than 1.5 ng/mL of endotoxin as assayed by a limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD).17Furthermore, the IL-4 preparations obtained from the 2 sources had the same bioactivity in vivo. Indium 111 chloride (111InCl3; 0.37 GBq/mL in pyrogen-free 0.04 mol/L hydrochloric acid), technetium 99m sodium pertechnetate (99mTc; 3.33 GBq/mL in pyrogen-free saline), and iodine 125 (125I; 3.76 GBq/mL in dilute sodium hydroxide solution; pH 7-11) were purchased from Amersham International (Amersham, Bucks, United Kingdom). Recombinant soluble VCAM-1 (rsVCAM-1) was from Biogen (Cambridge, MA). Percoll was purchased from Pharmacia Fine Chemicals (Uppsala, Sweden). Giemsa stain, May-Grünwald stain, calcium (Ca2+) ionophore (A23187), and fibronectin (from rat plasma) were purchased from Sigma Chemical (Dorset, United Kingdom). Calcein-AM was purchased from Molecular Probes (Eugene, OR).
mAbs
Antirat VCAM-1 mAb 5F10 (mouse IgG2a)32and the control mAb P1.17 (mouse IgG2a) were gifts from Biogen. Antirat α4-integrins mAb TA-2 (mouse IgG1k)33 and antirat β2-integrins mAb TA-4 (mouse IgG 2a) were purchased from Endogen (Cambridge, MA). The antirat ICAM-1 mAb 1A29 (mouse IgG1)34 was purified from supernatants of hybridoma cell cultures. Control mAb MOPC-21 (mouse myeloma IgG1) was from Sigma Chemical.
Quantification of leukocyte migration into the pleural cavity
Leukocyte migration into the pleural cavities of rats was induced as described previously.35 Briefly, rats were given intrapleural injections of either RPMI (100 μL) or IL-4 (1250-5000 units/100 μL per cavity). Four to 24 hours later, the animals were killed by asphyxiation with carbon dioxide, and the pleural cavity was opened through the sternum and lavaged with 5 mL modified Hanks balanced salt solution (HBSS) containing 0.25% bovine serum albumin (BSA) and heparin (10 U/mL). Total cell counts were obtained after staining of exudate samples with Kimura stain.36 Differential cell analysis was done in exudate smears prepared in a cytocentrifuge (Shandon, Cheshire, United Kingdom) and stained with May-Grünwald and Giemsa stains. For quantification, 500 cells/slide were counted and the results expressed as the number of total leukocytes, mononuclear cells, neutrophils, and eosinophils recovered from each cavity. In some of these rats, IL-4 or RPMI was also injected intradermally (100 μL/site). The skin sites were then punched out as described previously,17 snap frozen in liquid nitrogen, and stored at −80°C for subsequent quantification of eosinophil peroxidase (EPO) content.
Quantification of eosinophil accumulation in skin
Frozen skin sites from rats injected with IL-4 or RPMI were chopped during thawing, homogenized in 2 mL phosphate-buffered saline (PBS) containing 0.5% hexadecyltrimethylammonium bromide by using an Ultra-Turrax T-25S7 homogenizer (Janke and Kunkel GmBH and Co, Staufen, Germany), and stored at −80°C. Immediately before the assay of samples for EPO activity, skin homogenates were thawed and then centrifuged at 2800g for 10 minutes followed by 2 rapid spins at 13 000g for 20 minutes. EPO activity was measured by using a modified version of the method described by White et al.37 Homogenates were added to a substrate (100 μL) consisting of 8.6 mmol/L o-phenylenediamine dihydrochloride and 2.9 mmol/L hydrogen peroxide in 0.1 mol/L Tris–hydrochloric acid (pH 8.0) and maintained at room temperature. After 30 minutes, the reaction was stopped by adding 100 μL of 4 mol/L sulfuric acid (H2SO4) and the absorbance was read at 492 nm. Rat EPO standards were prepared from lysed, purified eosinophils as described previously32 and used to express the results as the number of eosinophils per skin site.
Quantification of eotaxin levels by enzyme-linked immunosorbent assay
Rat pleural cavities were washed with HBSS as described above and centrifuged at 2000 rpm for 10 minutes at 4°C. Eotaxin levels in supernatants were measured by an enzyme-linked immunosorbent assay (ELISA) system using a commercially available antimurine eotaxin mAb (R&D Systems, Minneapolis, MN). Briefly, 96-well plates were coated with the primary antibody, left overnight at room temperature, and washed. Then, nonspecific binding sites were blocked by coating the plates with 1% BSA in PBS for 1 hour at room temperature. Samples and standards were added to the plates in duplicate and incubated overnight at room temperature. After washing, biotinylated anti-eotaxin antibody was added to the plates. One hour later, neutravidin–horseradish peroxidase conjugate was added and the plates were allowed to stand for another hour. Plates were developed by using K-blue peroxidase substrate for 30 minutes. The reactions were terminated by adding 0.18 mol/L H2SO4, and the optical density of plates were read at 450 nm. The sample concentrations were determined by reference to the standard curve. The ELISA detected eotaxin concentrations above 10 pg/mL and did not detect the regulated on activation, normal T-cell expressed and secreted (RANTES) cytokine or macrophage inflammatory protein 1α (MIP-1α). Similar ELISA systems were employed to detect RANTES and MIP-1α by using commercially available primary and detector antimurine RANTES and MIP-1α antibodies (R&D Systems), respectively.
Stimulation of purified eosinophils and measurement of EPO activity
Mixed-cell populations recovered from the pleural cavities were layered on a 2-step discontinuous Percoll gradient (56% and 72%) and centrifuged at 3300 rpm (2000g) for 30 minutes. Pure eosinophils (> 90%) were stimulated with the Ca2+ionophore A23187 for 15 minutes at 37°C and the supernatants assayed for the release of EPO, as described previously.37 The amount of enzyme released was quantified as a percentage of the total enzyme in cells lysed with Triton. Cell viability was assessed by the release of lactate dehydrogenase (LDH) as assayed by the rate of oxidation of reduced nicotinamide adenine dinucleotide with pyruvate used as substrate.38
Adhesion assay
Adhesion of fluorochrome-labeled eosinophils to protein-coated plates was quantified by using a modified version of a previously described method.39 Briefly, purified eosinophils suspended in PBS free of Ca2+ and magnesium (Mg2+) were labeled with the fluorochrome Calcein-AM (10 μmol/L, with 30 minutes of incubation at 37°C). The cells were then washed, resuspended in PBS containing Ca2+ and Mg2+, placed in 96-well plates (105/well) previously coated with either fibronectin (3 μg/mL) or rsVCAM-1 (3 μg/mL), and incubated for 30 minutes at 37°C in the dark. The plate fluorescence was read (excitation at 485 nm and emission at 530 nm) before (fluorescence of total cells) and after (fluorescence of adherent cells) the plates were washed. Adherent cells were then calculated as a percentage of total fluorescence after subtraction of the background fluorescence.
Quantification of VCAM-1 and ICAM-1 expression using radiolabeled mAbs
The in vivo expression of VCAM-1 and ICAM-1 by endothelial cells was quantified by localization of intravenously administered radiolabeled antibodies (Abs), as described previously.40Briefly, the control IgG2a antibody (P1.17), anti-ICAM-1 mAb (1A29), and anti–VCAM-1 mAb (5F10) were radiolabeled with111In, 99mTc, and 125I, respectively.41 The percentage of total isotope bound to antibody was determined by using instant thin-layer chromatography (Gelman Sciences, Ann Arbor, MI). Expression of VCAM-1 and ICAM-1 in vivo was determined by intravenous administration of radiolabeled mAb. The rats received a mixture of 125I-5F10,99mTc-1A29, and 111In-P1.17 (25 μg each) 5 minutes before they were killed. The animals were anesthetized, the aorta was cannulated, and the inferior vena cava was opened. The circulation was then perfused with PBS to minimize the contribution of blood-pool activity to tissue-specific isotope counts. The pleural membrane or skin sites were removed, weighed, and counted in a γ counter. An aliquot of the injected solution was also counted to allow calculation of the injected dose. After corrections for background spillover between isotopes and decay, the antibody uptake for each sample was expressed as the percentage of injected dose per gram of tissue. The values for the uptake of the radiolabeled anti–VCAM-1 mAb and the radiolabeled anti-ICAM-1 mAb were corrected for the nonspecific uptake of the control mAb before the results were expressed graphically. To assess the specific binding of the radiolabeled anti–VCAM-1 mAb 5F10, some rats were injected with a mixture of the radiolabeled antibodies and a 50-fold excess of unlabeled 5F10 (equivalent to ∼ 4.2 mg/kg).
Statistical analysis
Results are expressed as the means ± SEM for a given number of rats. Data were analyzed by one-way analysis of variance followed by a Newman-Keuls comparison test. For both statistical tests,P < .05 was considered to represent significance.
Results
Characterization of IL-4–induced leukocyte migration into the pleural cavity of rats
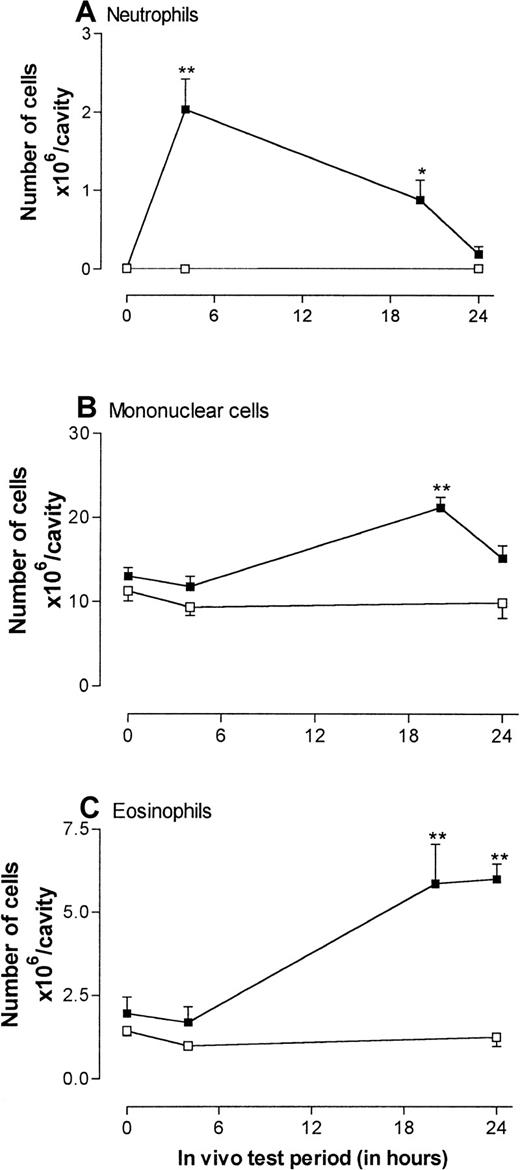
Because IL-4 has not previously been investigated in a pleural model, we first characterized the response to administration of this cytokine into rat pleural cavities. Figure1 shows the time-course profile of IL-4–induced leukocyte migration into the pleural cavities. Based on our previous findings in rat skin,17 IL-4 was injected intrapleurally at a dose of 5000 units/cavity at the beginning of the experiment (time 0), and 4, 20, and 24 hours before the animals were killed. Control rats received RPMI initially (time 0) and 4 and 24 hours before they were killed.
Time course of leukocyte accumulation induced by injection of recombinant rat interleukin 4 (IL-4) into rat pleural cavities.
Rats were injected intrapleurally with IL-4 (5000 units/100 μL per cavity; ▪) or RPMI (100 μL/cavity; ■), and leukocyte migration into the pleural cavity was quantified at different times after the injection. Results are expressed as the number of cells × 106 per cavity and presented as the mean ± SEM value for 3 or 4 animals. A significant difference from levels detected in RPMI-treated animals is indicated by asterisks; 1 asterisk indicates P < .01 and 2 indicateP < .001.
Time course of leukocyte accumulation induced by injection of recombinant rat interleukin 4 (IL-4) into rat pleural cavities.
Rats were injected intrapleurally with IL-4 (5000 units/100 μL per cavity; ▪) or RPMI (100 μL/cavity; ■), and leukocyte migration into the pleural cavity was quantified at different times after the injection. Results are expressed as the number of cells × 106 per cavity and presented as the mean ± SEM value for 3 or 4 animals. A significant difference from levels detected in RPMI-treated animals is indicated by asterisks; 1 asterisk indicates P < .01 and 2 indicateP < .001.
As shown in Figure 1, at time 0, pleural exudates consisted entirely of mononuclear leukocytes and eosinophils. However, 4 hours after injection of IL-4, significant numbers of neutrophils were detected in pleural exudates (Figure 1A). The neutrophil migration detected in response to IL-4 could not have been due to endotoxin contamination because the IL-4 preparation used contained less than 1.5 ng/mL endotoxin; ie, less than 0.15 ng would have been injected into each pleural cavity. In this rat model of inflammation, intrapleural injections of endotoxin doses in excess of 50 ng/cavity are required to elicit leukocyte migration.42
The IL-4–induced neutrophil accumulation was transient and declined during the next 24 hours so that, at the 24-hour time point, the numbers of neutrophils observed in pleural washes were not significantly increased. In contrast, the accumulation of mononuclear cells and eosinophils was slower in onset, and significant numbers were not detected until 20 hours after administration of IL-4 (Figure1B-C). As was observed of the neutrophil population, the numbers of mononuclear leukocytes declined toward control levels by 24 hours. In contrast, accumulation of eosinophils was sustained at the 24-hour time point, so that the largest percentage increase in leukocyte migration above control levels was observed in the eosinophil population at this time (Figure 1C). Because the aim of this study was to analyze the adhesive mechanisms involved in IL-4–induced eosinophil accumulation, the 24-hour time point was chosen for all subsequent experiments.
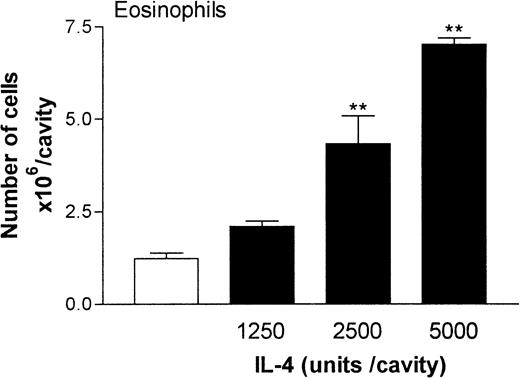
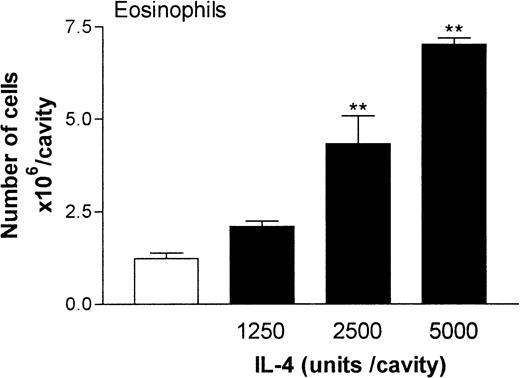
Figure 2 shows the dose-response relation of IL-4–induced eosinophil migration into rat pleural cavities at the 24-hour time point. At doses of 1250 to 5000 units/cavity, IL-4 induced a dose-dependent accumulation of eosinophils compared with results in rats given injections of RPMI. Overall, at the dose of 5000 units/cavity, there was a 3- to 7-fold increase in the number of eosinophils in the pleural cavities. This dose of IL-4 was used in all subsequent experiments.
Dose-response relation of IL-4–induced eosinophil accumulation in rat pleural cavities.
RPMI (100 μL/cavity; ■) or IL-4 (1250, 2500, or 5000 units/100 μL per cavity; ▪) was injected into rat pleural cavities, and eosinophil migration was quantified 24 hours later. Eosinophil accumulation is expressed as the number of cells × 106 per cavity, and the results are presented as the mean ± SEM value for 4 or 5 animals. A significant difference (P < .001) from levels in RPMI-treated rats is indicated by 2 asterisks.
Dose-response relation of IL-4–induced eosinophil accumulation in rat pleural cavities.
RPMI (100 μL/cavity; ■) or IL-4 (1250, 2500, or 5000 units/100 μL per cavity; ▪) was injected into rat pleural cavities, and eosinophil migration was quantified 24 hours later. Eosinophil accumulation is expressed as the number of cells × 106 per cavity, and the results are presented as the mean ± SEM value for 4 or 5 animals. A significant difference (P < .001) from levels in RPMI-treated rats is indicated by 2 asterisks.
Generation of eotaxin in IL-4–stimulated pleural cavities
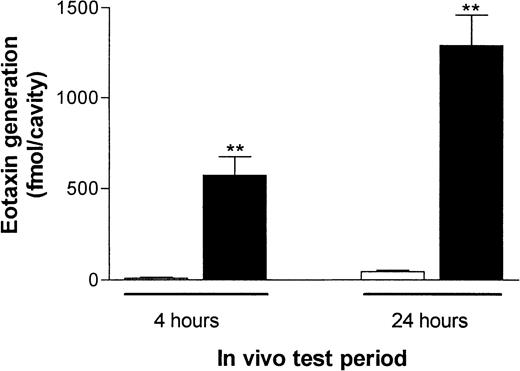
Because we previously found evidence of generation of the chemokine eotaxin in skin sites from rats injected with IL-4,16 we here investigated whether eotaxin was generated in pleural cavities of rats given IL-4 by the intrapleural route. Thus, pleural exudates from RPMI- or IL-4–treated rats obtained 4 and 24 hours after injection were collected and assayed for eotaxin by ELISA. Figure 3 shows that at both time points, significant levels of eotaxin were detected in pleural exudates of rats treated with IL-4 compared with control rats. For comparison, the exudates were also assayed for the presence of 2 other chemokines, RANTES and MIP-1α; no detectable levels of these chemokines were observed (data not shown).
IL-4–induced eotaxin generation in rat pleural cavities.
RPMI (100 μL/cavity; ■) or IL-4 (5000 units/100 μL per cavity; ▪) was injected into rat pleural cavities, and eotaxin levels in pleural exudates were quantified 4 or 24 hours later. Eotaxin generation is expressed in femtomoles per cavity and presented as the mean ± SEM value for 4 or 5 animals. A significant difference (P < .001) from levels in RPMI-treated rats is indicated by 2 asterisks.
IL-4–induced eotaxin generation in rat pleural cavities.
RPMI (100 μL/cavity; ■) or IL-4 (5000 units/100 μL per cavity; ▪) was injected into rat pleural cavities, and eotaxin levels in pleural exudates were quantified 4 or 24 hours later. Eotaxin generation is expressed in femtomoles per cavity and presented as the mean ± SEM value for 4 or 5 animals. A significant difference (P < .001) from levels in RPMI-treated rats is indicated by 2 asterisks.
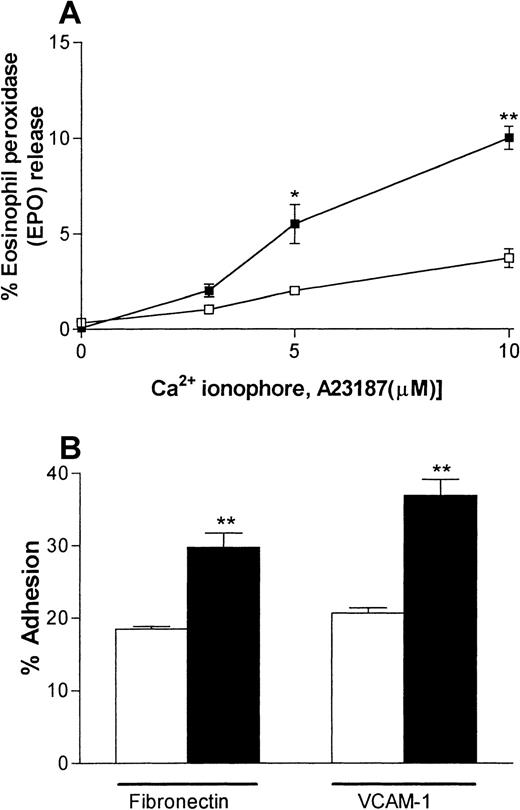
IL-4–elicited pleural eosinophils are more responsive in vitro
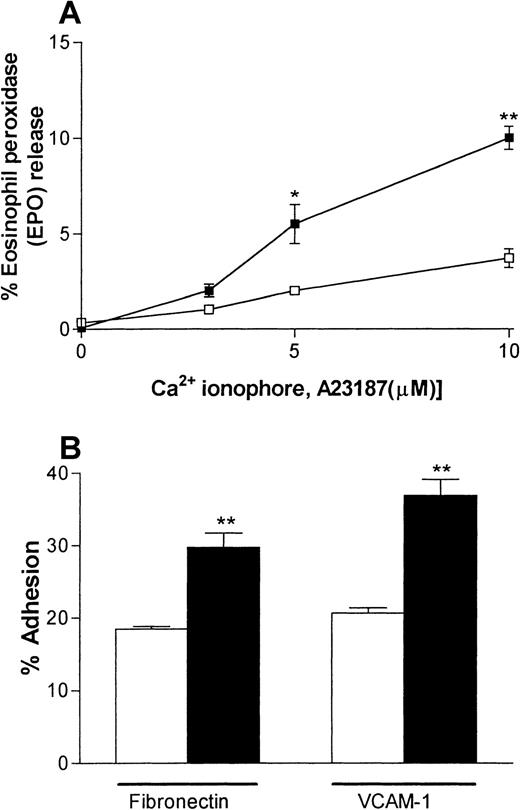
A series of experiments were carried out to investigate the responsiveness of the IL-4–elicited eosinophils in vitro. Specifically, eosinophils recovered from pleural washes were purified and their degranulation and adhesive responses were quantified. Figure4A shows that IL-4–induced pleural eosinophils were more responsive to stimulation by the Ca2+ionophore A23187 with respect to the release of EPO than were eosinophils obtained from pleural exudates of rats injected with RPMI. Assay of the cytoplasmic enzyme LDH demonstrated that A23187 had no effect on cell viability in the dose range used (data not shown). Similarly, eosinophils obtained from rats injected with IL-4 showed a greater level of adhesion to plates coated with fibronectin or rsVCAM-1 than did pleural eosinophils obtained from rats injected with RPMI (Figure 4B).
IL-4–elicited pleural eosinophils show enhanced responsiveness in vitro.
Eosinophils were purified from pleural cavities of rats injected intrapleurally with IL-4 (5000 units/100 μL per cavity, ▪) or RPMI (100 μL/cavity, ■), and their degranulation (A) and adhesive responses (B) were quantified. Panel A shows the ability of rat pleural eosinophils to release eosinophil peroxidase (EPO) in response to the calcium ionophore A23187. Enzyme release is expressed as the percentage of total enzyme in cells lysed with Triton. Panel B shows the adhesion of rat pleural eosinophils to plates coated with fibronectin or recombinant soluble vascular cell adhesion molecule 1 (VCAM-1) (both 3 μg/mL). The results are presented as the mean ± SEM value for 3 animals per group in triplicate. A significant difference from levels in RPMI-treated rats is indicated by asterisks; 1 asterisk indicatesP < .01 and 2 indicate P < .001.
IL-4–elicited pleural eosinophils show enhanced responsiveness in vitro.
Eosinophils were purified from pleural cavities of rats injected intrapleurally with IL-4 (5000 units/100 μL per cavity, ▪) or RPMI (100 μL/cavity, ■), and their degranulation (A) and adhesive responses (B) were quantified. Panel A shows the ability of rat pleural eosinophils to release eosinophil peroxidase (EPO) in response to the calcium ionophore A23187. Enzyme release is expressed as the percentage of total enzyme in cells lysed with Triton. Panel B shows the adhesion of rat pleural eosinophils to plates coated with fibronectin or recombinant soluble vascular cell adhesion molecule 1 (VCAM-1) (both 3 μg/mL). The results are presented as the mean ± SEM value for 3 animals per group in triplicate. A significant difference from levels in RPMI-treated rats is indicated by asterisks; 1 asterisk indicatesP < .01 and 2 indicate P < .001.
An anti-α4-integrin mAb inhibits IL-4–induced eosinophil accumulation in both rat skin and rat pleural cavities: comparison with the effect of an anti–VCAM-1 mAb
The aim of these experiments was to investigate the role of α4-integrins–VCAM-1 adhesion pathways in IL-4–induced eosinophil accumulation in the rat pleural cavities in comparison with the response elicited in skin. The roles of α4-integrins and VCAM-1 were investigated by using the blocking mAbs TA-233 and 5F10,32 respectively. Because we previously found that an anti-β2-integrins mAb suppresses IL-4–induced eosinophil accumulation in rat skin,17 we also assessed the effect of the anti-β2-integrins mAb TA-4 on eosinophil migration into rat pleural cavities.
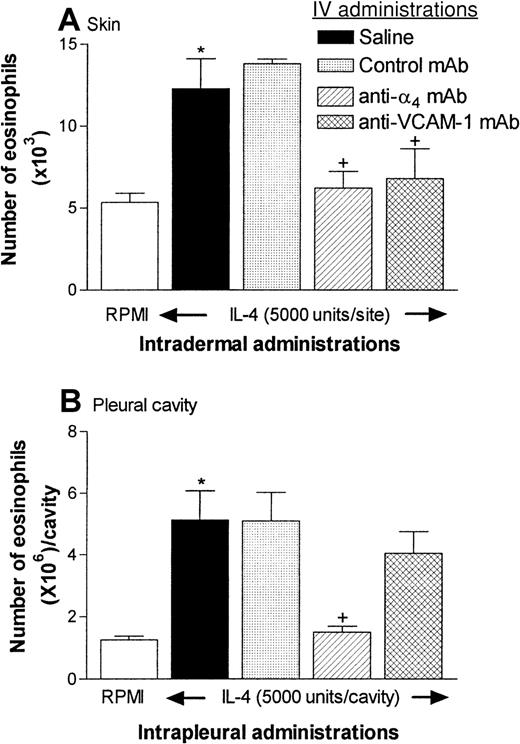
Figure 5A shows that intradermal administration of IL-4 (5000 units/site) induced a significant accumulation of eosinophils in rat skin over a 24-hour period in comparison with the accumulation in skin sites injected with RPMI. In rats treated with an anti-α4-integrins mAb or an anti–VCAM-1 mAb (both 3.5 mg/kg), the IL-4–induced response was totally blocked. These results are in agreement with those of our previous study, in which IL-4–induced eosinophil accumulation in skin was quantified by assessing the local accumulation of intravenously injected 111In-labeled rat peritoneal eosinophils.17
Effect of anti-α4-integrins monoclonal antibody (mAb) (TA-2) and anti–VCAM-1 mAb (5F10) on eosinophil recruitment into rat skin and pleural cavities.
(A) In the experiments with rat skin, 4 groups of rats were given intravenous injections of saline, control mouse IgG (3.5 mg/kg), TA-2 (3.5 mg/kg), or 5F10 (3.5 mg/kg). Fifteen minutes later, the animals were injected intradermally with RPMI (100 μL/site) or IL-4 (5000 units/100 μL per site). Eosinophil accumulation in the skin sites, as quantified by measurement of EPO activity, was determined 24 hours after injection. Because there were no differences between EPO levels in RPMI-injected sites among the 4 different groups tested, these data were pooled (■). Results are expressed as the mean ± SEM value for 3 to 15 pairs of rats. A significant difference (P < .01) from levels in RPMI-treated rats is indicated by one asterisk. Also, significant differences (P < .05) between responses in animals given the control antibody and those given the anti-α4-integrins mAb or the anti–VCAM-1 mAb are indicated by + symbols. (B) The same groups of rats were also used in an experiment in which the animals received intrapleural IL-4 (5000 units/100 μL per cavity) or RPMI (100 μL) 15 minutes after the intravenous injections described above. Eosinophil accumulation in the pleural cavity was determined 24 hours later. The results are expressed as the mean ± SEM value for 6 to 8 animals per group. A significant difference (P < .01) from levels in RPMI-treated rats is indicated by one asterisk. Significant differences (P < .05) between responses in animals given the control antibody and those given the anti-α4-integrins mAb or the anti–VCAM-1 mAb are indicated by + symbols. Data were analyzed with a one-way analysis of variance followed by a Newman-Keuls comparison test.
Effect of anti-α4-integrins monoclonal antibody (mAb) (TA-2) and anti–VCAM-1 mAb (5F10) on eosinophil recruitment into rat skin and pleural cavities.
(A) In the experiments with rat skin, 4 groups of rats were given intravenous injections of saline, control mouse IgG (3.5 mg/kg), TA-2 (3.5 mg/kg), or 5F10 (3.5 mg/kg). Fifteen minutes later, the animals were injected intradermally with RPMI (100 μL/site) or IL-4 (5000 units/100 μL per site). Eosinophil accumulation in the skin sites, as quantified by measurement of EPO activity, was determined 24 hours after injection. Because there were no differences between EPO levels in RPMI-injected sites among the 4 different groups tested, these data were pooled (■). Results are expressed as the mean ± SEM value for 3 to 15 pairs of rats. A significant difference (P < .01) from levels in RPMI-treated rats is indicated by one asterisk. Also, significant differences (P < .05) between responses in animals given the control antibody and those given the anti-α4-integrins mAb or the anti–VCAM-1 mAb are indicated by + symbols. (B) The same groups of rats were also used in an experiment in which the animals received intrapleural IL-4 (5000 units/100 μL per cavity) or RPMI (100 μL) 15 minutes after the intravenous injections described above. Eosinophil accumulation in the pleural cavity was determined 24 hours later. The results are expressed as the mean ± SEM value for 6 to 8 animals per group. A significant difference (P < .01) from levels in RPMI-treated rats is indicated by one asterisk. Significant differences (P < .05) between responses in animals given the control antibody and those given the anti-α4-integrins mAb or the anti–VCAM-1 mAb are indicated by + symbols. Data were analyzed with a one-way analysis of variance followed by a Newman-Keuls comparison test.
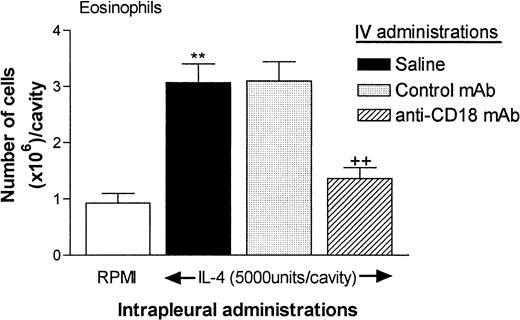
The rats used for quantification of eosinophil accumulation in skin were also injected with IL-4 by the intrapleural route for quantification of eosinophil accumulation in the pleural cavity. As was found in the skin model, a control mAb had no effect, but the anti-α4-integrins mAb TA-2 almost totally inhibited the eosinophil accumulation in the pleural cavity elicited by IL-4. Surprisingly, however, in contrast to our findings in skin, the anti–VCAM-1 mAb 5F10 had no significant effect on IL-4–induced eosinophil accumulation in the pleural cavity. Although the results shown in Figure 5 are from animals treated with both intradermally and intrapleurally administered IL-4, similar results were obtained when the animals were treated only with IL-4 given intrapleurally (data not shown). In such experiments, higher doses of 5F10 (5 mg/kg or 10 mg/kg) also had no effect on IL-4–induced eosinophil accumulation in the pleural cavity (eg, in experiments testing the effect of 10 mg/kg 5F10, the results were as follows: RPMI, 1.11 ± 0.13 × 106cells/cavity, IL-4 plus saline, 3.318 ± 0.31 × 106cells/cavity; IL-4 plus control mAb, 2.81 ± 0.47 × 106 cells/cavity; and IL-4 plus 5F10, 2.64 ± 0.25 × 106 cells/cavity [6-7 rats in each group]). Additional experiments showed that treatment of rats with an anti-β2-integrins (CD18) mAb (TA-4; 3.5 mg/kg) also resulted in almost total suppression of eosinophil accumulation in pleural cavities of rats treated with IL-4 (Figure6).
Effect of an anti-β2-integrins (CD18) mAb (TA-4) on eosinophil recruitment into rat pleural cavities.
In these experiments, 3 groups of animals were treated intravenously with saline, control mouse IgG (P1.17; 3.5 mg/kg) or anti-CD18 (TA-4; 3.5 mg/kg). After 15 minutes, the animals were injected intrapleurally with RPMI (100 μL/cavity) or IL-4 (5000 units/100 μL per cavity). Eosinophil accumulation was determined 24 hours later. The results are expressed as the mean ± SEM value for 4 to 7 animals per group. A significant increase (P < .001) over levels in RPMI-treated rats is indicated by 2 asterisks. A significant difference (P < .001) between responses in animals treated with the control antibody and those treated with anti-CD18 mAb is indicated by 2 + symbols.
Effect of an anti-β2-integrins (CD18) mAb (TA-4) on eosinophil recruitment into rat pleural cavities.
In these experiments, 3 groups of animals were treated intravenously with saline, control mouse IgG (P1.17; 3.5 mg/kg) or anti-CD18 (TA-4; 3.5 mg/kg). After 15 minutes, the animals were injected intrapleurally with RPMI (100 μL/cavity) or IL-4 (5000 units/100 μL per cavity). Eosinophil accumulation was determined 24 hours later. The results are expressed as the mean ± SEM value for 4 to 7 animals per group. A significant increase (P < .001) over levels in RPMI-treated rats is indicated by 2 asterisks. A significant difference (P < .001) between responses in animals treated with the control antibody and those treated with anti-CD18 mAb is indicated by 2 + symbols.
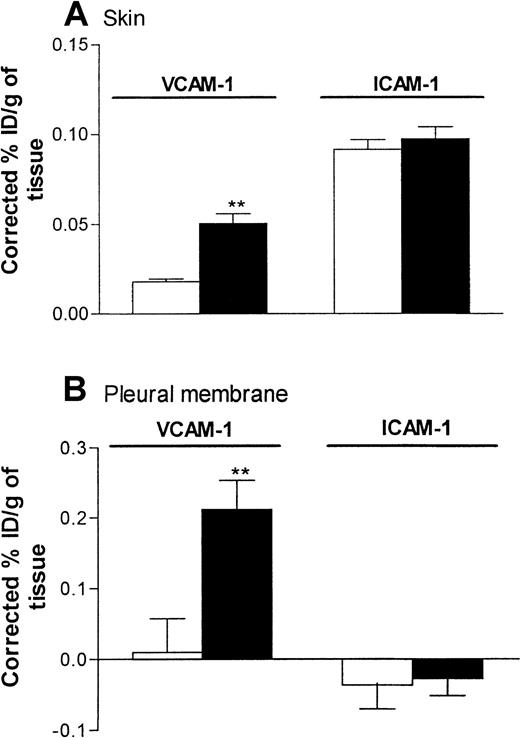
Quantification of VCAM-1 expression in rat skin and pleural membrane in response to IL-4
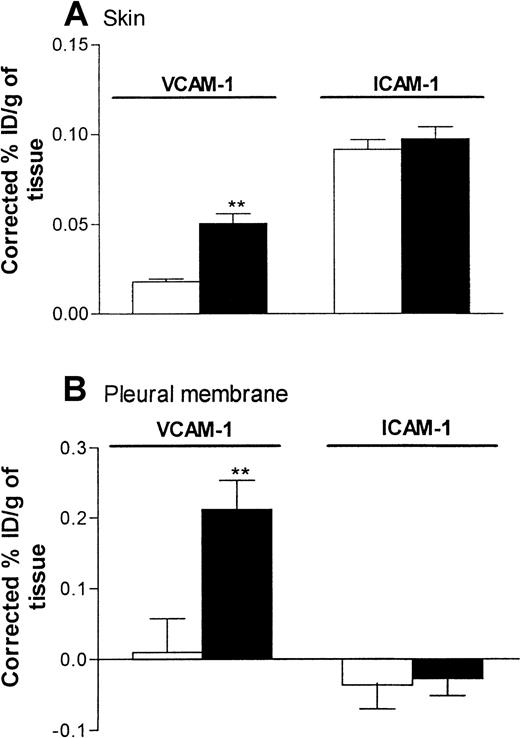
Because the anti–VCAM-1 mAb 5F10 was found to inhibit IL-4–induced eosinophil accumulation in skin but not pleural cavities, the following experiments were carried out to investigate the expression of VCAM-1 in the 2 inflammatory models. In these studies, expression of ICAM-1 was studied in parallel with that of VCAM-1. Expression of endothelial-cell–associated VCAM-1 and ICAM-1 in rat skin and pleural membrane 24 hours after administration of RPMI or IL-4 (5000 units/skin site or 5000 units/pleural cavity) was quantified by localization of intravenously administered radiolabeled anti–VCAM-1 mAb (5F10) and anti-ICAM-1 mAb (1A29).
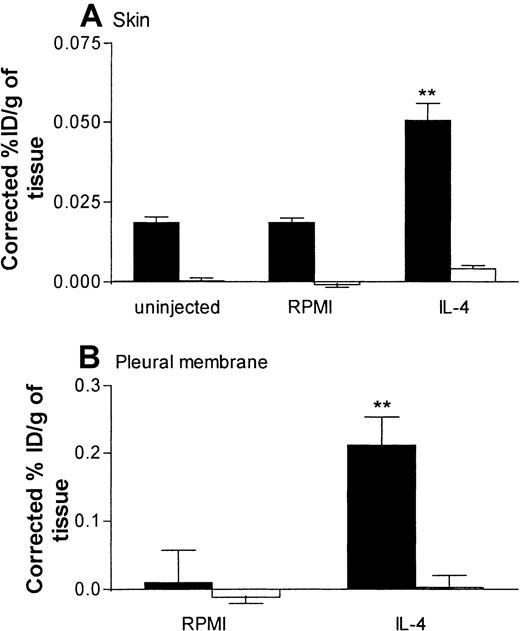
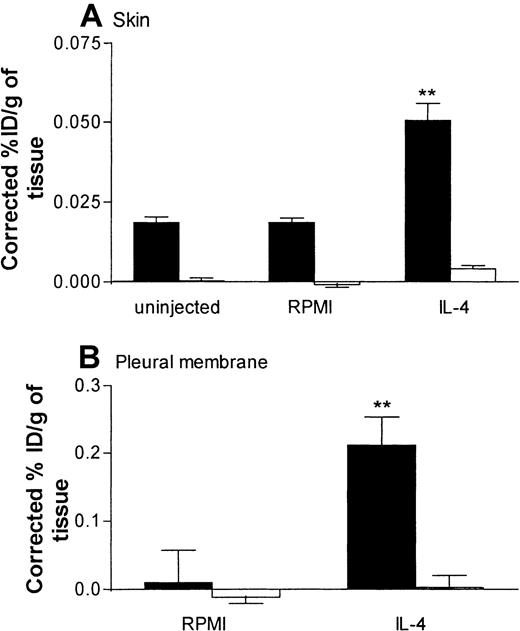
In skin, a low level of VCAM-1 expression was detected in sites injected with RPMI. Such expression was significantly enhanced after injection of IL-4 (Figure 7A). In the same rats, expression of ICAM-1 in skin sites injected with RPMI was not significantly different from that in skin sites injected with IL-4 (Figure 7A). In pleural membranes, as in skin, a significantly higher level of VCAM-1 expression, but not ICAM-1 expression, was detected in response to IL-4 than in RPMI-injected control tissues (Figure 7B). Interestingly, in contrast to the results in skin, there appeared to be no basal expression of VCAM-1 or ICAM-1 in pleural membranes. We demonstrated that the basal and elicited expressions of VCAM-1 in skin and pleural membranes were “true” expressions of VCAM-1, since binding of the labeled 5F10 was blocked completely in rats injected intravenously with excess unlabeled 5F10 (equivalent to ∼4.2 mg/kg; Figure 8). These findings also show that the maximum dose of 5F10 mAb used to block VCAM-1 in the recruitment experiments (10 mg/kg) was more than sufficient to saturate endothelial-cell VCAM-1 expression in the 2 inflammatory models.
Expression of VCAM-1 and intracellular adhesion molecule 1 (ICAM-1) in rat skin and pleural membranes as determined by the uptake of radiolabeled anti–VCAM-1 and anti-ICAM-1 mAbs, respectively.
Skin sites (A) and pleural cavities (B) were injected with RPMI (100 μL; ■) or IL-4 (5000 units/100 μL per cavity or site; ▪). Twenty-four hours later, the animals were injected with a mixture (25 μg each) of iodine 125–labeled 5F10 (anti–VCAM-1), technetium 99m–labeled 1A29 (anti–ICAM-1), and indium 111–labeled P1.17 (control mAb) 5 minutes before they were killed. The circulation was then perfused with phosphate-buffered saline to minimize the contribution of blood-pool activity to tissue-specific isotope counts. The pleura and skin sites were removed, weighed, and counted. Expression of adhesion molecules is indicated as the percentage of injected dose per gram of tissue (ID/g), corrected for the nonspecific uptake of the control mAb. Results are expressed as the mean ± SEM value for 7 or 8 rats per group. Two asterisks indicate a significant difference (P < .001) from uptake of antibodies in RPMI-treated sites.
Expression of VCAM-1 and intracellular adhesion molecule 1 (ICAM-1) in rat skin and pleural membranes as determined by the uptake of radiolabeled anti–VCAM-1 and anti-ICAM-1 mAbs, respectively.
Skin sites (A) and pleural cavities (B) were injected with RPMI (100 μL; ■) or IL-4 (5000 units/100 μL per cavity or site; ▪). Twenty-four hours later, the animals were injected with a mixture (25 μg each) of iodine 125–labeled 5F10 (anti–VCAM-1), technetium 99m–labeled 1A29 (anti–ICAM-1), and indium 111–labeled P1.17 (control mAb) 5 minutes before they were killed. The circulation was then perfused with phosphate-buffered saline to minimize the contribution of blood-pool activity to tissue-specific isotope counts. The pleura and skin sites were removed, weighed, and counted. Expression of adhesion molecules is indicated as the percentage of injected dose per gram of tissue (ID/g), corrected for the nonspecific uptake of the control mAb. Results are expressed as the mean ± SEM value for 7 or 8 rats per group. Two asterisks indicate a significant difference (P < .001) from uptake of antibodies in RPMI-treated sites.
Specific binding of radiolabeled anti–VCAM-1 mAb in rat skin and pleural membrane.
Panels A and B show the localization of radiolabeled anti–VCAM-1 mAb 5F10 in uninjected skin sites and skin sites injected with RPMI (100 μL) or IL-4 (5000 units/100 μL per site) and in pleural membranes after intrapleural administration of RPMI (100 μL) or IL-4 (5000 units/100 μL per cavity), respectively. In these experiments, localization of radiolabeled 5F10 was quantified in rats injected intravenously with the radiolabeled mAbs only (▪) and in rats injected with the radiolabeled mAbs and a 50-fold (1.25 mg of protein equivalent to 4.2 mg/kg) excess of unlabeled 5F10 (■). Expression of VCAM-1 in skin (A) and pleural membranes (B) is shown as the percentage of injected dose per gram of tissue (ID/g), corrected for the nonspecific uptake of the control mAb. Results are expressed as the mean ± SEM value for 6 to 8 animals (skin) or 3 to 7 animals (pleural membrane). Two asterisks indicate a significant difference (P < .001) from the uptake in uninjected skin sites or RPMI-treated pleural membranes.
Specific binding of radiolabeled anti–VCAM-1 mAb in rat skin and pleural membrane.
Panels A and B show the localization of radiolabeled anti–VCAM-1 mAb 5F10 in uninjected skin sites and skin sites injected with RPMI (100 μL) or IL-4 (5000 units/100 μL per site) and in pleural membranes after intrapleural administration of RPMI (100 μL) or IL-4 (5000 units/100 μL per cavity), respectively. In these experiments, localization of radiolabeled 5F10 was quantified in rats injected intravenously with the radiolabeled mAbs only (▪) and in rats injected with the radiolabeled mAbs and a 50-fold (1.25 mg of protein equivalent to 4.2 mg/kg) excess of unlabeled 5F10 (■). Expression of VCAM-1 in skin (A) and pleural membranes (B) is shown as the percentage of injected dose per gram of tissue (ID/g), corrected for the nonspecific uptake of the control mAb. Results are expressed as the mean ± SEM value for 6 to 8 animals (skin) or 3 to 7 animals (pleural membrane). Two asterisks indicate a significant difference (P < .001) from the uptake in uninjected skin sites or RPMI-treated pleural membranes.
Discussion
This study investigated the involvement of α4-integrins–VCAM-1 adhesion pathways in eosinophil migration into rat pleural cavities and skin in response to IL-4. The results suggest 3 important and novel aspects of VCAM-1 expression in eosinophil accumulation in vivo: (1) the functional importance of VCAM-1 in mediating eosinophil accumulation in vivo is tissue specific; (2) under certain inflammatory conditions, endothelial-cell VCAM-1 expression can be dissociated from eosinophil accumulation; and (3) α4-integrins may interact with ligands other than VCAM-1 in mediating eosinophil accumulation in vivo. Our findings provide the basis for further investigations into tissue-specific targets for suppression of leukocyte migration in vivo.
The novel inflammatory reaction investigated in this study was IL-4–induced eosinophil accumulation in rat pleural cavities when the cytokine was administered by the intrapleural route. The rat pleurisy model has previously been used extensively for investigations into the induction and pharmacologic modulation of eosinophil migration elicited by inflammatory mediators such as platelet-activating factor and after antigen challenge in sensitized rats.35,43 Intrapleural injection of IL-4 induced an early accumulation of neutrophils (4 hours) in the pleural cavity, with neutrophil levels returning to basal values by 24 hours. This response was followed by a second phase of leukocyte migration composed of mononuclear leukocytes and eosinophils (20 hours). Whereas neutrophil-accumulation levels decreased to basal values by 24 hours after administration of IL-4, the eosinophil-accumulation response was sustained. Of relevance to this finding are studies in IL-4 transgenic mice in which expression of the cytokine was targeted to the lungs and an infiltration of leukocytes into mouse airways consisting of neutrophils, mononuclear leukocytes, and eosinophils was demonstrated.44 Because the specific aim of the current study was to investigate mechanisms of IL-4–induced eosinophil accumulation in the pleural cavity, we focused on the inflammatory reaction elicited at 24 hours. In agreement with our previous findings in rat skin injected with IL-4,16 the IL-4–stimulated eosinophil accumulation in rat pleural cavities appears to be associated with the local generation of the chemokine eotaxin.
In vitro, IL-4–elicited pleural eosinophils had a greater level of responsiveness with respect to degranulation and adhesion to plates coated with fibronectin or VCAM-1, with the latter finding indicating an enhanced state of activation of eosinophil α4-integrins. We did not investigate the mechanisms responsible for this enhanced responsiveness in this study, but several can be proposed. The simplest explanation is that intrapleural administration of IL-4, along with recruiting eosinophils, also directly activates or primes the migrated eosinophils in the cavity. Of relevance to this idea is that eosinophils were shown to express IL-4 receptors45 and to respond to IL-4 in several functional assays.45-49 It is also possible that the enhanced responsiveness of IL-4–elicited eosinophils is triggered after the interaction of migrating eosinophils with soluble or endothelial-cell–associated stimulating factors generated in response to IL-4, eg, eotaxin, VCAM-1, or both.50 One implication of these findings is that in the airways of patients with asthma, in which IL-4 message and protein have been detected,18-20IL-4 may contribute not only to eosinophil recruitment but also to enhanced eosinophil activation and hence eosinophil-mediated tissue damage.
The functional importance of α4-integrins and VCAM-1 in IL-4–induced eosinophil accumulation in rat pleural cavities compared with rat skin was investigated by using the neutralizing mAbs TA-2 and 5F10, respectively. In agreement with our previous findings,17 eosinophil accumulation in rat skin induced by IL-4 was inhibited by both these antibodies. Surprisingly, however, whereas IL-4–induced eosinophil accumulation in pleural cavities was blocked by the anti-α4-integrins mAb, the anti–VCAM-1 mAb had no effect, even though it was clearly bioactive, as demonstrated by its ability to block IL-4–induced recruitment of eosinophils into the skin of the same animals.51 52 To provide a comparison with these results, the effect of an antirat β2-integrin mAb (TA-4) was also investigated and found to significantly block IL-4–induced eosinophil accumulation in rat pleural cavities.
The simplest explanation for the lack of effect of the anti–VCAM-1 mAb in the pleural model would be a lack of expression of endothelial-cell VCAM-1 in IL-4–activated pleural cavities. A previous study provided a clear demonstration of different profiles of endothelial-cell VCAM-1 expression in different tissues.40 Hence, we investigated the endothelial-cell expression of VCAM-1 in IL-4–stimulated rat pleural membranes and skin sites, as measured by the localization of intravenously administered radiolabeled 5F10. In the studies in pleural membranes, VCAM-1 expression was not detected in control rats but a significantly increased VCAM-1 level was observed in rats injected with IL-4 by the intrapleural route. In the studies in skin, both a constitutive and an IL-4–induced level of VCAM-1 expression were detected. We also showed that the localization of radiolabeled anti–VCAM-1 mAb was blocked in the presence of excess cold antibody, thereby demonstrating that the quantified expressions of VCAM-1 resulted from specific binding of the anti–VCAM-1 mAb. Furthermore, these experiments showed that the dose of mAb 5F10 (10 mg/kg) tested on IL-4–induced eosinophil accumulation in rat pleural cavities was sufficient to block endothelial-cell VCAM-1 expression and that the lack of functional effect of 5F10 in the pleural model was not due to an insufficient amount of antibody.
In contrast to the findings regarding the expression of VCAM-1, we detected a basal level of ICAM-1 expression (quantified by the localization of radiolabeled 1A29) in rat skin, but not in pleural membranes, and observed no increase in ICAM-1 expression in tissues stimulated with IL-4. These results are consistent with those of in vitro studies showing that cultured endothelial cells stimulated with IL-4 express VCAM-1 but not ICAM-112-14,21 and with in vivo studies showing a correlation between IL-4–induced leukocyte infiltration and expression of VCAM-1 but not ICAM-1.30
Migration of eosinophils into the pleural cavity involves several distinct stages. Initially, eosinophils must interact with endothelial cells that line the vessel walls in the pleural membrane. Penetration of the vessel wall is followed by eosinophil migration through the interstitial tissue and the mesothelial layer, resulting in accumulation in the pleural cavity. Hence, theoretically, the inhibition of eosinophil accumulation detected in rats treated with anti-α4- and anti-β2-integrin mAbs may have resulted from blockade of eosinophil migration at the level of the endothelial or mesothelial barrier. However, our results suggest that pleural-membrane endothelial-cell VCAM-1 does not play a functional role in the initial adhesion and transendothelial-cell migration of eosinophils in response to IL-4, thus raising the important question of the nature of the endothelial-cell adhesion molecules involved in this process. Because we showed an association between eotaxin generation and eosinophil migration in this β2-integrin–dependent model, it is possible that IL-4–induced eotaxin generation (a response that may also depend on α4- or β2-integrins) triggers a β2-integrin–mediated adhesion or transendothelial-cell migration of eosinophils. Clearly, since no basal or induced endothelial-cell ICAM-1 expression was detected in pleural membranes stimulated with IL-4, our results suggest the involvement of a non-ICAM-1 endothelial-cell ligand or ligands for β2-integrins. One possible candidate is ICAM-2, since studies in mice deficient in ICAM-2 indicated a role for this molecule in eosinophil migration in vivo.53 However, a functional role for other uncharacterized endothelial-cell β2-integrin ligands cannot be ruled out. It is relevant that, in vitro, eosinophil adhesion to and transmigration through IL-4–activated endothelial cells, although independent of ICAM-1, can be partly inhibited by anti-β2-integrin mAbs.14 54
An alternative mechanism by which eosinophils may penetrate the vascular wall in the pleural membrane is through interaction of eosinophil α4-integrins with non-VCAM-1 endothelial-cell ligands. The lack of effect of the anti–VCAM-1 mAb on α4-integrins–mediated eosinophil accumulation in rat pleural cavities suggests the existence of functionally important alternative α4 ligands in mediating eosinophil accumulation in vivo. In studies using the same mAbs as those in the current investigation, divergent results were obtained with anti-α4–integrins mAbs and anti–VCAM-1 mAbs in other rat models of inflammation.55,56 Because the anti-α4–integrins mAb used in our study recognizes both α4β1 and α4β7, it is possible that the eosinophil accumulation detected in pleural cavities was mediated by α4β7 by means of an interaction with its principal ligand mucosal addressin cell adhesion molecule 1 (MadCAM-1), a molecule that has been shown to support eosinophil rolling and firm adhesion under conditions of flow.57 Because of a lack of specific reagents, the expression of MadCAM-1 in rat pleural membranes could not be investigated. Although VCAM-1 is generally considered to be the principal endothelial-cell ligand for α4β1(very late antigen 4 [VLA-4]), several alternative ligands have been identified. The best characterized is connecting segment 1, which is present in alternatively spliced forms of fibronectin.58,59 Furthermore, in vitro studies have provided evidence for the existence of an additional α4-integrin ligand on endothelial cells activated by tumor necrosis factor α60; and other VLA-4 ligands,61 such as thrombospondin62 and osteopontin,63 have been reported. The relevance of these molecules to eosinophil migration in vivo remains to be determined.
A final important question raised by our findings is that since VCAM-1 blockade did not influence IL-4–induced eosinophil migration into the pleural cavity, what is the functional role of IL-4–induced endothelial-cell VCAM-1 expression in the pleural membrane? It is possible that pleural VCAM-1 has a role in mediating the activation of eosinophils and other leukocyte subtypes. Indeed, much in vitro evidence shows that eosinophil adhesion to VCAM-1 stimulates leukocytes for enhanced responsiveness,50 61 and we demonstrated that IL-4–elicited pleural eosinophils are more responsive in vitro. However, our attempts to determine whether this is due to VCAM-1 expression have yielded equivocal results and this possibility remains a subject for future studies.
In conclusion, we demonstrated differential effects of antibodies to α4-integrins and VCAM-1 in IL-4–induced eosinophil accumulation in rat skin and pleural cavities, suggesting the existence of tissue-specific mechanisms in the regulation of eosinophil infiltration in vivo. The results raise several important questions regarding the functional role of VCAM-1 in eosinophil migration and provide the basis for further investigations into tissue-specific targets for suppression of leukocyte-mediated tissue damage in the treatment of organ-specific inflammatory diseases.
Supported by the Wellcome Trust, United Kingdom; Biogen Inc, Cambridge, MA; and the British Heart Foundation, United Kingdom.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sussan Nourshargh, BHF Cardiovascular Medicine Unit, Imperial College School of Medicine at the National Heart and Lung Institute, Hammersmith Hospital, Du Cane Road, London W12 0NN, UK; e-mail: s.nourshargh@ic.ac.uk