Abstract
Several different preparations of cross-linked hemoglobin (CLHb) are being evaluated for their efficacy and safety as red cell substitutes in a variety of preclinical and clinical settings. Because CLHb is known to sequester nitric oxide (NO) and inhibit NO-mediated processes, we hypothesized that CLHb would have a hemostatic effect by enhancing platelet reactivity, inducing vasoconstriction, or both. Infusion of o-raffinose CLHb shortened the prolonged microvascular bleeding time and decreased blood loss from ear incisions in rabbits rendered anemic and thrombocytopenic. Moreover, this hemostatic effect persisted for at least 24 hours after infusion. Phenylephrine induced a degree of vasoconstriction similar to that induced by CLHb but did not shorten the bleeding time or decrease blood loss, suggesting that vasoconstriction alone cannot account for the hemostatic effect of CLHb. There was no evidence of CLHb-induced activation of coagulation in vivo, since infusion of CLHb did not increase circulating levels of thrombin-antithrombin complex. In vitro, CLHb abolished the inhibitory effect of the NO donor 3-morpholinosydnonimine on platelet aggregation and enhanced the aggregation of stimulated but not resting platelets. This potentiating effect was not attenuated by the addition of superoxide dismutase or catalase. To evaluate the potential arterial thrombogenicity of CLHb, a model of carotid artery thrombosis was developed in rabbits without thrombocytopenia or anemia. Compared with albumin infusion, CLHb infusion shortened the time to complete carotid occlusion. These data suggest that CLHb may shift the thromboregulatory balance toward clot formation, resulting in decreased bleeding in anemic and thrombocytopenic rabbits and possibly increasing arterial thrombogenicity in normal rabbits.
Introduction
Hemoglobin-based oxygen-carrying solutions have been under development as putative red cell substitutes for decades, but they remain investigational, partly because of the complex biologic properties of these agents. The recognition that unmodified extracellular hemoglobin readily dissociates into dimers that are rapidly cleared by the kidneys has led to the development of various methods to chemically cross-link, modify by recombinant methods, and encapsulate hemoglobin to prevent rapid clearance.1
Several types of cross-linked hemoglobin (CLHb) are currently being evaluated in clinical trials to determine their efficacy and safety in a variety of medical and surgical settings. The various types of CLHb differ from each other in the chemical or molecular strategies that are used to cross-link adjacent subunits of the hemoglobin tetramer and in their species of origin.1 Cross-linking not only improves intravascular retention, it also optimizes oxygen affinity and may attenuate the vasoconstrictive side effects of cell-free hemoglobin.2,3 Although considerable improvements over earlier generations of hemoglobin-based products have been made, contemporary preparations of CLHb still produce unintentional biologic effects in vitro and in vivo. Some of these effects, such as vasoconstriction, are partly related to the ability of heme iron to sequester nitric oxide (NO), thereby interfering with NO-mediated processes.4
NO, synthesized by vascular endothelium, acts constitutively to relax vascular smooth muscle and inhibit platelet reactivity.5Although free hemoglobin has long been recognized as a potent inhibitor of NO-mediated processes in vitro, such observations became clinically relevant only with the introduction of cell-free solutions of CLHb as investigational red cell substitutes.
We hypothesized that CLHb would augment primary hemostasis and ameliorate the hemostatic defect associated with thrombocytopenia and anemia by enhancing platelet reactivity, inducing vasoconstriction, or both. Congruent with this hypothesis are previous observations that bleeding time is prolonged by NO administration6,7 and shortened by pharmacologic inhibition of NO synthase.8Such a hemostatic property, if present, may be beneficial in surgical settings in which blood loss is usually high or hemostasis is impaired. In this study, the hemostatic efficacy of o-raffinose CLHb was evaluated and characterized in thrombocytopenic and anemic rabbits. Additional studies, done in a model of carotid artery thrombosis using rabbits without thrombocytopenia or anemia, evaluated potential thrombogenicity associated with increased platelet reactivity caused by CLHb.
Materials and methods
Laboratory animals
Male New Zealand White rabbits weighing 2.5 to 3 kg and Sprague-Dawley rats weighing 250 to 300 g (Charles River, St Constant, Quebec, Canada) were housed and treated in accordance with established guidelines.9 Animal procedures were approved by the institutional animal care committees at Queen's University and McMaster University.
Materials
A stroma-free, 10% solution of o-raffinose CLHb formulated in Ringer's lactate (Hemolink) was a gift from Hemosol Inc (Etobicoke, Ontario, Canada). Single-use aliquots were stored at −70°C until use to avoid multiple cycles of thawing and refreezing. Hemolink is produced from outdated human red cell samples as described previously.10 The o-raffinose cross-linking covalently joins αβ-hemoglobin dimers, forming intramolecular linkages within tetramers and intermolecular linkages between tetramers, thereby producing hemoglobin oligomers. The characteristics and composition of the o-raffinose CLHb, as supplied by the manufacturer, are shown in Table 1.
A 5% solution of human serum albumin (HSA; Immuno AG, Vienna, Austria) was used as an oncotically matched control solution for the in vivo experiments.11 Collagen was obtained from Chronolog (Havertown, PA) and 3-morpholinosydnonimine (SIN-1) from Calbiochem (San Diego, CA). All other reagents were from Sigma (St Louis, MO).
Induction of thrombocytopenia and anemia in rabbits
Severe thrombocytopenia was produced by using γ irradiation and heterologous platelet antiserum as previously described.12 Rabbits were exposed to 930 cGy from a cesium 137 source for 30 minutes, with their ears shielded with lead. Eight days after irradiation, heterologous antiplatelet serum from sheep was administered intravenously. Anemia was produced in a separate group of rabbits without thrombocytopenia by serial phlebotomies that obtained 30 mL of blood 2 to 3 times per week. With each phlebotomy, the citrated whole blood was centrifuged and autologous platelet-rich plasma was reinfused into the rabbits.
Infusion of test substances
For all in vivo experiments in rabbits, o-raffinose CLHb (0.8 g/kg; 8 mL/kg) or an equivalent volume of 5% HSA was infused through a marginal ear vein at a rate of 1 mL/min. To determine the isolated effects of vasoconstriction on bleeding time and blood loss, phenylephrine was administered in an intravenous bolus dose (1.5 μg/kg) followed by a continuous intravenous infusion (1.5 μg/kg per minute).
Determination of microvascular bleeding time
Assessment of microvascular bleeding time was performed as previously described.12 Rabbits were anesthetized with sodium pentobarbital (30 mg/kg given intravenously) and one of their ears was prewarmed by immersion in a 37°C saline bath stirred with a magnetic stirrer. A full-thickness incision through the ear was made with a scalpel blade (#6) at a site devoid of macroscopically visible vessels. The ear was then immediately reimmersed in the stirred saline bath, and the time until visible bleeding stopped was recorded. Bleeding times exceeding 60 minutes were recorded as more than 60 minutes.
Determination of blood loss
Blood loss from the ear incisions made to assess bleeding time was measured by removing aliquots from the saline bath after the incised ear had been immersed for 60 minutes. After centrifugation at 800g for 10 minutes, Drabkin solution was added to the red cell pellet and incubation allowed to continue at room temperature for 30 minutes. The optical density at 540 nm was subsequently measured. In control experiments, known volumes of rabbit blood of known hematocrit were added to Drabkin solution to construct standard curves from which the volume of blood lost was calculated.
Measurement of microvascular perfusion
To determine the degree of vasoconstriction in the rabbits' ears, microvascular perfusion was measured noninvasively by using a laser Doppler flowmeter with a right-angle probe (Transonic Systems, Ithaca, NY). Each ear was shaved and the probe fastened to it at a site that was devoid of macroscopically visible vessels. The probe surface was in direct contact with the ear. Microvascular perfusion was measured during and after infusion of CLHb, HSA, and phenylephrine, under the identical conditions of infusion, anesthesia, and ear immersion used for the bleeding-time and blood-loss determinations.
Measurement of arterial thrombogenicity
Normal rabbits were anesthetized with fentanyl and fluanisone (Hypnorm [0.3 mL/kg given intravenously]; Janssen, Beerse, Belgium) and diazepam (2 mg/kg given intravenously) followed by a continuous intravenous infusion of fentanyl and fluanisone (0.4 mL/hour) through a marginal ear vein. Rabbits were kept supine on a heating pad to maintain normothermia. A 2-cm segment of the right carotid artery was exposed, and carotid blood flow was measured by using a transit-time ultrasound flowmeter and 2-mm probe (model T206 with 2RB probe; Transonic Systems). Ultrasound gel was applied to the vessel as needed to maintain a satisfactory probe signal. Rabbits were given an infusion of CLHb (0.8 g/kg) or an equivalent volume of 5% HSA 40 minutes before the induction of thrombosis.
Thrombosis was induced by first cross-clamping the carotid artery with a hemostat for 5 seconds to induce endothelial injury. After release, an anodal 500-μA direct current (DC) from a 9-V source was applied across the injured vessel segment until complete occlusion occurred (zero flow) and was sustained for 10 minutes. The electrical circuit was constructed by using a digital multimeter and 50-Ω potentiometer joined in series to a transformer converting alternating current to 9-V DC. Thirty-gauge electrodes made of stainless steel wire were hooked around the carotid artery, separated by 1 cm, and kept in place by using a thin plastic strip as scaffolding and insulation from surrounding tissue. Care was taken not to cause a kink in the vessel. The electrodes were attached upstream from the flow probe. After the cessation of carotid flow, a 1-cm carotid segment containing the injured region was immediately excised, fixed in formalin, and processed in paraffin. Cross-sections were stained with hematoxylin, phloxin, and eosin and examined with use of light microscopy.
Measurement of plasma thrombin-antithrombin (TAT) levels
Rats anesthetized by intravenous administration of fentanyl and fluanisone (Hypnorm [0.3 mL/kg]; Janssen) and diazepam (2 mg/kg) were given an infusion of CLHb (0.8 g/kg) or an equivalent volume of HSA. A continuous intravenous infusion of thromboplastin (Thromboplastin C Plus [500 μL/kg per hour]; Dade Behring, Deerfield, IL) was administered to rats in the positive control group. Plasma TAT concentrations were measured in duplicate by enzyme immunoassay (Enzygnost TAT; Dade Behring) before and 1 hour after infusion.13
Whole-blood impedance-aggregation studies
Whole blood from normal rabbits was collected into tubes containing 3.8% trisodium citrate (1:9 vol/vol) and incubated with CLHb or Ringer's lactate for 10 minutes at 37°C before testing with collagen, U46619, adenosine diphosphate (ADP), and arachidonic acid. SIN-1 (final concentration, 50 μmol/L) or phosphate-buffered saline (pH 7.4) was added 1 minute before addition of the agonist in experiments evaluating the effect of CLHb on NO-mediated platelet inhibition. Platelet aggregation in whole blood was measured with an impedance aggregometer (model 592; Chronolog). The impedance values reported are those observed 6 minutes after addition of the agonist.
Statistical methods
For comparison of continuous variables between treatment groups, the Student t test, Wilcoxon-Mann-Whitney test, one-way analysis of variance, and Kruskal-Wallis test were used as appropriate. All reported P values are 2-tailed.
Results
Effect of CLHb on microvascular bleeding time in anemic rabbits
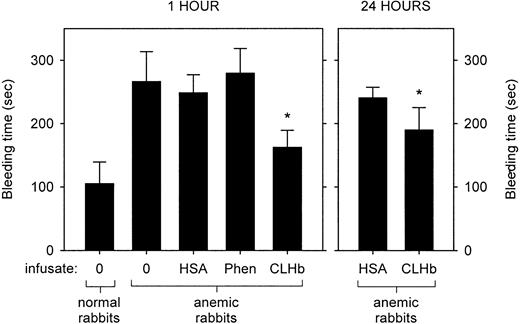
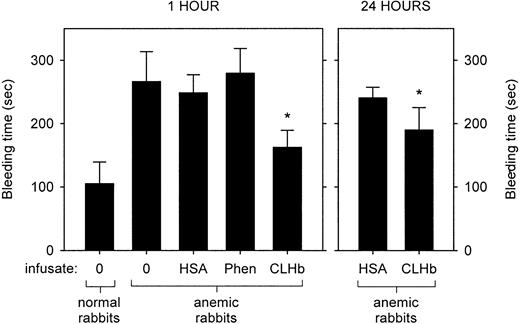
The baseline characteristics of the normal and anemic rabbits are shown in Table 2. Anemia was associated with a prolongation in bleeding time (Figure1). This hemostatic defect was ameliorated by infusion of CLHb, but not HSA or phenylephrine, measured 1 hour after infusion. This effect persisted when bleeding times were measured 24 hours after infusion. The mean ± SD plasma hemoglobin concentrations 1 and 24 hours after infusion were 13.7 ± 1.8 g/L and 5.1 ± 0.5 g/L, respectively.
Cross-linked hemoglobin (CLHb) shortened the microvascular bleeding time in anemic rabbits.
Microvascular bleeding times were measured in anemic rabbits 1 hour after infusion (left panel) of o-raffinose CLHb, 5% human serum albumin (HSA), phenylephrine (Phen), or nothing (0). For the CLHb and HSA groups, bleeding-time assessments were repeated 24 hours after infusion (right panel). The data are expressed as means; error bars denote 1 SD. *P < .001 compared with HSA, Phen, and results in untreated anemic rabbits at 1 hour; P < .001 compared with HSA at 24 hours.
Cross-linked hemoglobin (CLHb) shortened the microvascular bleeding time in anemic rabbits.
Microvascular bleeding times were measured in anemic rabbits 1 hour after infusion (left panel) of o-raffinose CLHb, 5% human serum albumin (HSA), phenylephrine (Phen), or nothing (0). For the CLHb and HSA groups, bleeding-time assessments were repeated 24 hours after infusion (right panel). The data are expressed as means; error bars denote 1 SD. *P < .001 compared with HSA, Phen, and results in untreated anemic rabbits at 1 hour; P < .001 compared with HSA at 24 hours.
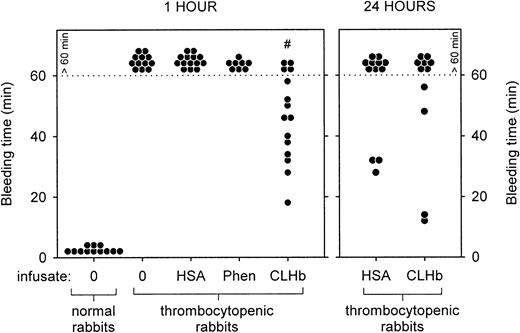
Effect of CLHb on bleeding time and blood loss in thrombocytopenic rabbits
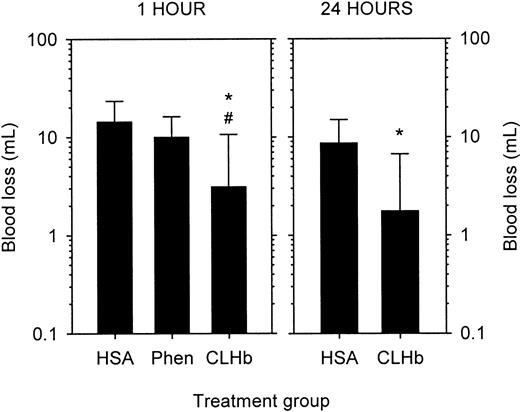
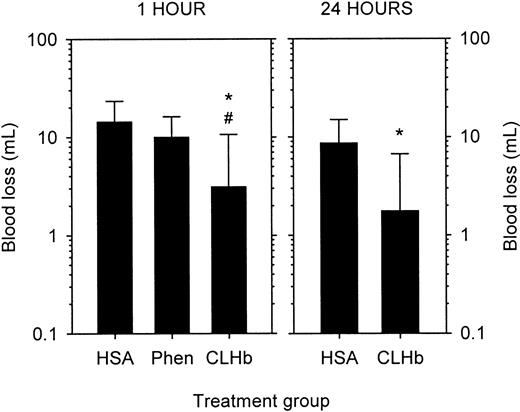
The baseline characteristics of the thrombocytopenic rabbits are shown in Table 2. Thrombocytopenic rabbits that did not receive infusions had bleeding times that exceeded 60 minutes, in contrast to a mean bleeding time of 1.75 minutes in healthy rabbits (Figure2). The prolonged bleeding times were shortened by infusion of CLHb, but not HSA or phenylephrine, measured 1 hour after infusion. By 24 hours after infusion, there was no detectable difference in bleeding times between the HSA and CLHb groups. Mean blood loss from ear incisions 1 hour after infusion was lower in CLHb-treated rabbits than in those treated with HSA or phenylephrine (Figure 3). Twenty-four hours after infusion, the difference in blood loss between the HSA and CLHb groups was still detectable.
CLHb shortened the microvascular bleeding time in thrombocytopenic rabbits.
Microvascular bleeding times were measured in thrombocytopenic rabbits 1 hour after infusion (left panel) with o-raffinose CLHb, 5% HSA, Phen, or nothing (0). For the CLHb and HSA groups, bleeding-time assessments were repeated 24 hours after infusion (right panel). Exact bleeding times are shown for data below the dotted line. Data points above the dotted line represent bleeding times that exceeded 60 minutes but were not followed to cessation of bleeding. #P < .001 compared with results of all other treatments in thrombocytopenic rabbits at 1 hour.
CLHb shortened the microvascular bleeding time in thrombocytopenic rabbits.
Microvascular bleeding times were measured in thrombocytopenic rabbits 1 hour after infusion (left panel) with o-raffinose CLHb, 5% HSA, Phen, or nothing (0). For the CLHb and HSA groups, bleeding-time assessments were repeated 24 hours after infusion (right panel). Exact bleeding times are shown for data below the dotted line. Data points above the dotted line represent bleeding times that exceeded 60 minutes but were not followed to cessation of bleeding. #P < .001 compared with results of all other treatments in thrombocytopenic rabbits at 1 hour.
CLHb decreased blood loss from thrombocytopenic rabbits.
Blood loss from standardized ear incisions in thrombocytopenic rabbits was measured 1 hour (left panel) and 24 hours (right panel) after infusion of o-raffinose CLHb, 5% HSA, or Phen. The data shown are geometric means; error bars denote 1 SD. *P < .01 compared with HSA; #P = .02 compared with Phen.
CLHb decreased blood loss from thrombocytopenic rabbits.
Blood loss from standardized ear incisions in thrombocytopenic rabbits was measured 1 hour (left panel) and 24 hours (right panel) after infusion of o-raffinose CLHb, 5% HSA, or Phen. The data shown are geometric means; error bars denote 1 SD. *P < .01 compared with HSA; #P = .02 compared with Phen.
Effect of treatment on microvascular perfusion
Infusions of CLHb, HSA, and phenylephrine were all associated with a decrease in microvascular perfusion of the ear in rabbits without anemia or thrombocytopenia (Figure 4). Although maximum vasoconstriction did not occur until after administration of the anesthetic agent, decreased microvascular perfusion was observed in all groups even before anesthesia was induced. During the 60 minutes the ear was immersed, less vasoconstriction occurred in rabbits given an infusion of HSA than in those given an infusion of CLHb (P = .003) or phenylephrine (P = .05). Phenylephrine infusion induced a degree of vasoconstriction similar to that induced by CLHb (Figure 4).
Extent of vasoconstriction induced by CLHb and Phen.
Changes in microvascular ear perfusion in rabbits given an infusion ofo-raffinose CLHb (solid circles; n = 6), 5% HSA (open squares; n = 6), or Phen (open triangles; n = 5) were measured by using a laser Doppler flowmeter. Baseline microvascular perfusion was that observed before infusion of the test substances. The solid bar indicates the period of infusion of the test substance (either CLHb or HSA), “A” denotes the time of administration of anesthesia, and the open bar denotes the period of ear immersion into the 37°C saline bath for bleeding-time testing. Phen was administered as an intravenous bolus followed by continuous infusion for the duration of the experiment. The data shown are means; error bars denote 1 SD.
Extent of vasoconstriction induced by CLHb and Phen.
Changes in microvascular ear perfusion in rabbits given an infusion ofo-raffinose CLHb (solid circles; n = 6), 5% HSA (open squares; n = 6), or Phen (open triangles; n = 5) were measured by using a laser Doppler flowmeter. Baseline microvascular perfusion was that observed before infusion of the test substances. The solid bar indicates the period of infusion of the test substance (either CLHb or HSA), “A” denotes the time of administration of anesthesia, and the open bar denotes the period of ear immersion into the 37°C saline bath for bleeding-time testing. Phen was administered as an intravenous bolus followed by continuous infusion for the duration of the experiment. The data shown are means; error bars denote 1 SD.
Arterial thrombogenicity of o-raffinose CLHb
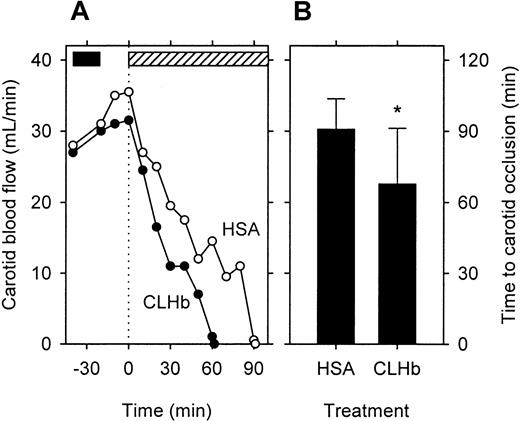
Carotid artery blood flow and the time until complete vessel occlusion in the rabbit model of carotid artery thrombosis is shown in Figure 4. Before application of the electrical current, baseline carotid blood flow in CLHb- and HSA-treated animals did not differ, although there was a trend toward higher flow in the HSA group (P = 0.39 at time 0). After the electric current was applied, carotid blood flow decreased steadily in both groups; in rabbits given an infusion of CLHb, the time until complete carotid occlusion was shorter than that in those given an HSA infusion (Figure5). Cyclic flow variations were observed in both groups before sustained complete occlusion. Histopathologic examinations of occluded carotid artery segments confirmed the presence of platelet-rich thrombus, with intimal and medial disruption.
CLHb shortened the time to complete arterial occlusion in a rabbit model of carotid thrombosis.
Carotid blood flow was measured in rabbits given an infusion ofo-raffinose CLHb (n = 10) or 5% HSA (n = 10). At time 0 (dotted line), the right carotid artery was clamped briefly and a 500-μA current was applied to the artery until complete and sustained occlusion occurred. (A) Median carotid blood flow in rabbits given an infusion of CLHb (solid circles) or HSA (open circles). The solid bar denotes the period of infusion of the test substance; the hatched bar denotes the application of current. (B) Time to complete carotid occlusion in CLHb- and HSA-treated rabbits. The data shown are means; error bars denote 1 SD. *P < .05 compared with HSA.
CLHb shortened the time to complete arterial occlusion in a rabbit model of carotid thrombosis.
Carotid blood flow was measured in rabbits given an infusion ofo-raffinose CLHb (n = 10) or 5% HSA (n = 10). At time 0 (dotted line), the right carotid artery was clamped briefly and a 500-μA current was applied to the artery until complete and sustained occlusion occurred. (A) Median carotid blood flow in rabbits given an infusion of CLHb (solid circles) or HSA (open circles). The solid bar denotes the period of infusion of the test substance; the hatched bar denotes the application of current. (B) Time to complete carotid occlusion in CLHb- and HSA-treated rabbits. The data shown are means; error bars denote 1 SD. *P < .05 compared with HSA.
Effect of CLHb infusion on thrombin generation
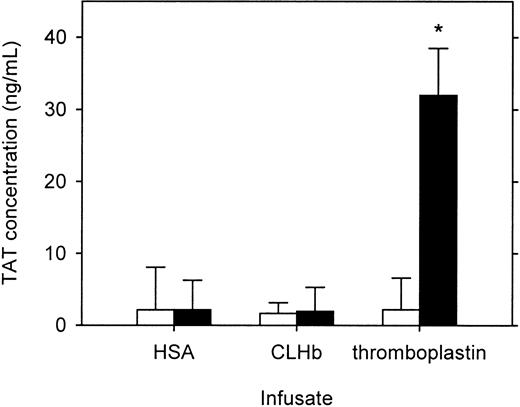
In comparison with preinfusion values, infusion of CLHb into rats was not found to increase plasma TAT concentration 1 hour after infusion (Figure 6). In contrast, thromboplastin infusion induced a significant rise in TAT concentration.
CLHb infusion did not produce increased thrombin generation in vivo.
Plasma thrombin-antithrombin concentrations were measured before (open bars) and 1 hour after (solid bars) a bolus infusion ofo-raffinose CLHb or 5% HSA or a continuous infusion of thromboplastin in rats (n = 6 per treatment arm). The data are expressed as means; error bars denote 1 SD. *P < .001 compared with preinfusion value.
CLHb infusion did not produce increased thrombin generation in vivo.
Plasma thrombin-antithrombin concentrations were measured before (open bars) and 1 hour after (solid bars) a bolus infusion ofo-raffinose CLHb or 5% HSA or a continuous infusion of thromboplastin in rats (n = 6 per treatment arm). The data are expressed as means; error bars denote 1 SD. *P < .001 compared with preinfusion value.
Effect of CLHb on platelet aggregation
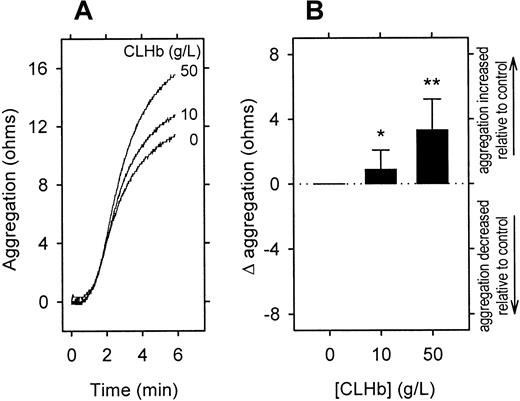
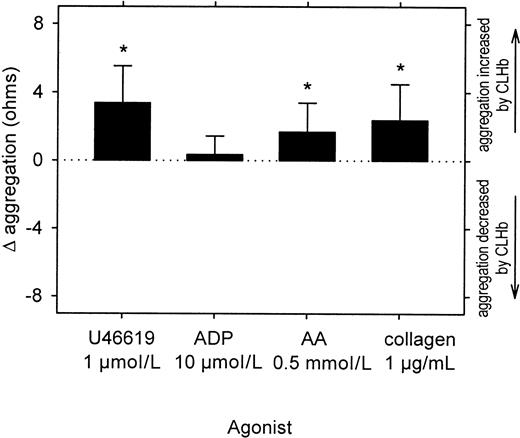
In concentrations of up to 50 g/L, CLHb did not cause resting platelets to aggregate in rabbit whole blood (data not shown). However, CLHb did enhance aggregation of platelets stimulated with collagen (1 μg/mL) in a concentration-dependent fashion (Figure7) and abolished the inhibitory effect of the NO donor SIN-1 on platelet aggregation (Figure8). This enhancement of aggregation was also observed when rabbit platelets were stimulated with U46619 or arachidonic acid but not ADP (Figure 9). Catalase (200 U/mL) and superoxide dismutase (200 U/mL) did not abrogate the effect of CLHb on platelet aggregation (Figure10). HSA had no effect on collagen-induced platelet aggregation in rabbit whole blood (data not shown).
CLHb enhanced the aggregation of rabbit platelets stimulated with collagen.
Impedance aggregometry was used to evaluate the effect ofo-raffinose CLHb on platelet aggregation in rabbit whole blood. CLHb enhanced the aggregation of platelets stimulated with collagen (1 μg/mL) in a dose-dependent fashion as shown in a representative experiment (A) and results of assessment of aggregation amplitudes at 6 minutes in 15 experiments (B). The change in platelet aggregation (Δ aggregation) was calculated as the aggregation in the presence of CLHb minus that observed in Ringer's lactate alone. The data are expressed as means; error bars denote 1 SD. *P = .01 and **P < .001 compared with results with Ringer's lactate alone.
CLHb enhanced the aggregation of rabbit platelets stimulated with collagen.
Impedance aggregometry was used to evaluate the effect ofo-raffinose CLHb on platelet aggregation in rabbit whole blood. CLHb enhanced the aggregation of platelets stimulated with collagen (1 μg/mL) in a dose-dependent fashion as shown in a representative experiment (A) and results of assessment of aggregation amplitudes at 6 minutes in 15 experiments (B). The change in platelet aggregation (Δ aggregation) was calculated as the aggregation in the presence of CLHb minus that observed in Ringer's lactate alone. The data are expressed as means; error bars denote 1 SD. *P = .01 and **P < .001 compared with results with Ringer's lactate alone.
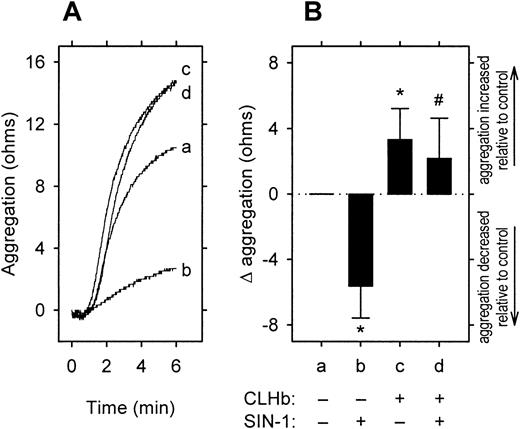
CLHb abolished the inhibitory effect of 3-morpholinosydnonimine (SIN-1) on platelet aggregation.
The effect of o-raffinose CLHb on SIN-1–mediated platelet inhibition was evaluated by using impedance aggregometry on rabbit whole blood. Collagen (1 μg/mL) was used in all experiments. (A) A representative experiment, in which aggregation curvea is that of the control; b, SIN-1 (50 μmol/L);c, CLHb (50 g/L); and d, CLHb and SIN-1. (B) The aggregation amplitudes measured at 6 minutes in 14 experiments. The change in platelet aggregation (Δ aggregation) was calculated as the aggregation in the presence of CLHb minus that observed in Ringer's lactate alone. The data are expressed as means; error bars denote 1 SD. *P = .001 and #P = .005 compared with results with Ringer's lactate alone.
CLHb abolished the inhibitory effect of 3-morpholinosydnonimine (SIN-1) on platelet aggregation.
The effect of o-raffinose CLHb on SIN-1–mediated platelet inhibition was evaluated by using impedance aggregometry on rabbit whole blood. Collagen (1 μg/mL) was used in all experiments. (A) A representative experiment, in which aggregation curvea is that of the control; b, SIN-1 (50 μmol/L);c, CLHb (50 g/L); and d, CLHb and SIN-1. (B) The aggregation amplitudes measured at 6 minutes in 14 experiments. The change in platelet aggregation (Δ aggregation) was calculated as the aggregation in the presence of CLHb minus that observed in Ringer's lactate alone. The data are expressed as means; error bars denote 1 SD. *P = .001 and #P = .005 compared with results with Ringer's lactate alone.
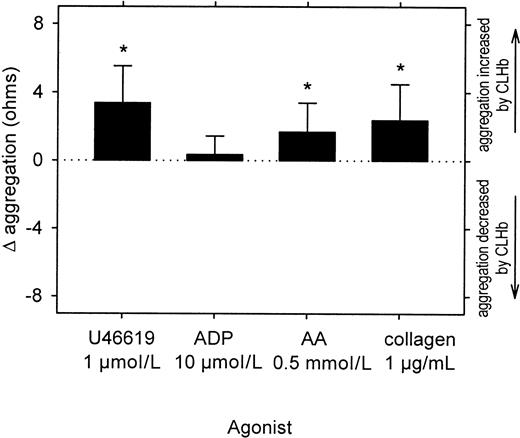
CLHb enhanced the aggregation of rabbit platelets stimulated with U46619 and arachidonic acid (AA) but not adenosine diphosphate (ADP)
. Impedance aggregometry was performed on rabbit whole blood stimulated with U46619, ADP, AA, or collagen in the presence or absence of CLHb (50 g/L). Δ aggregation was calculated as the aggregation amplitude in the presence of o-raffinose CLHb minus that observed in Ringer's lactate alone, measured 6 minutes after the addition of the agonist (n = 24 data pairs per treatment arm). The data shown are means; error bars represent 1 SD. Mean absolute impedance values forU46619, ADP, AA, and collagen in the presence of Ringer's lactate alone were 1.34, 1.87, 5.69, and 9.11 Ω, respectively. *P < .001 compared with results with Ringer's lactate alone.
CLHb enhanced the aggregation of rabbit platelets stimulated with U46619 and arachidonic acid (AA) but not adenosine diphosphate (ADP)
. Impedance aggregometry was performed on rabbit whole blood stimulated with U46619, ADP, AA, or collagen in the presence or absence of CLHb (50 g/L). Δ aggregation was calculated as the aggregation amplitude in the presence of o-raffinose CLHb minus that observed in Ringer's lactate alone, measured 6 minutes after the addition of the agonist (n = 24 data pairs per treatment arm). The data shown are means; error bars represent 1 SD. Mean absolute impedance values forU46619, ADP, AA, and collagen in the presence of Ringer's lactate alone were 1.34, 1.87, 5.69, and 9.11 Ω, respectively. *P < .001 compared with results with Ringer's lactate alone.
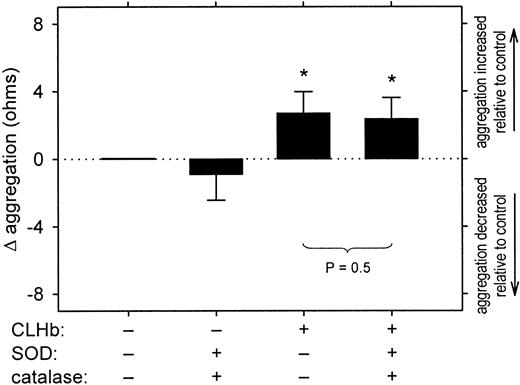
The proaggregatory effect of CLHb was not attenuated by superoxide dismutase (SOD) and catalase.
Impedance aggregometry was performed on rabbit whole blood stimulated with collagen (1 μg/mL) in the presence or absence of superoxide dismutase (200 U/mL), catalase (200 U/mL), and CLHb (50 g/L). The Δ aggregation is the aggregation amplitude in the presence of the test substances minus that observed in Ringer's lactate alone, measured 6 minutes after the addition of the agonist (n = 10 data pairs per treatment arm). The data shown are means; error bars represent 1 SD. *P < .001 compared with results with Ringer's lactate alone.
The proaggregatory effect of CLHb was not attenuated by superoxide dismutase (SOD) and catalase.
Impedance aggregometry was performed on rabbit whole blood stimulated with collagen (1 μg/mL) in the presence or absence of superoxide dismutase (200 U/mL), catalase (200 U/mL), and CLHb (50 g/L). The Δ aggregation is the aggregation amplitude in the presence of the test substances minus that observed in Ringer's lactate alone, measured 6 minutes after the addition of the agonist (n = 10 data pairs per treatment arm). The data shown are means; error bars represent 1 SD. *P < .001 compared with results with Ringer's lactate alone.
Discussion
In this study, o-raffinose CLHb was found to ameliorate the hemostatic defect associated with anemia and thrombocytopenia in rabbits, an effect that persisted for at least 24 hours after a single bolus infusion. CLHb infusion was also associated with vasoconstriction in vivo, an effect that is well documented,2,11,14,15although the relative role of NO sequestration in mediating this effect is controversial.16 Phenylephrine induced a degree of vasoconstriction similar to that induced by CLHb but had no effect on bleeding time or blood loss, suggesting that the hemostatic effect of CLHb cannot be attributable to vasoconstriction alone.
We found that o-raffinose CLHb enhanced the aggregation of stimulated but not resting rabbit platelets in a dose-dependent fashion. This effect was observed when collagen, U46619, or arachidonic acid, but not ADP, were used as the agonist. A similar pattern of response to these agonists has been reported for diaspirin CLHb.17 As expected, CLHb also abolished the inhibitory effect of the NO donor SIN-1 on platelet aggregation. The interaction of CLHb with NO is also known to interfere with redox processes, with generation of free-radical species18 that may modulate platelet reactivity.19 However, superoxide dismutase and catalase did not attenuate the potentiation of aggregation by CLHb, suggesting that superoxide anion and hydrogen peroxide do not mediate this effect.
These data therefore suggest that enhancement of platelet reactivity by CLHb, possibly by means of NO sequestration, is a plausible mechanism for the hemostatic effect of CLHb. Similar proaggregatory effects of hemoglobin-based oxygen carriers were observed previously by some investigators3,17 but not others.20,21 More studies are required to clarify the pathways for this effect, since the modulation of platelet reactivity by CLHb may involve mechanisms other than NO sequestration.17 22
The association between prolongation of bleeding time and anemia and the fact that this condition can be corrected by blood transfusion have long been recognized,23,24 but the mechanisms remain unknown.25 The current study suggests that this hemostatic defect is also ameliorated by CLHb infusion, an unintended but potentially beneficial property of CLHb in the setting of defective hemostasis and bleeding. However, it remains to be determined whether the mechanisms of bleeding-time correction by red cell transfusion and by CLHb transfusion are similar.
Our observation that o-raffinose CLHb can correct a prolonged bleeding time is consistent with reports that bleeding time was shortened by inhibition of NO production8 and prolonged by pharmacologic administration of NO.6,7However, diaspirin CLHb was not found to significantly shorten the normal tail-bleeding time in rats.26 Whether these differences in study findings are the result of differences in the CLHb preparation or in the animal models used is unknown.
Infusion of older preparations of cell-free hemoglobin into animals resulted in activation of the complement and coagulation pathways, causing disseminated intravascular coagulation with thrombosis.27 Such toxic effects are widely believed to be the result of contaminating phospholipids and endotoxins, since hemoglobin solutions devoid of these impurities were not found to be associated with similar in vivo toxicity.28 In the current study, we found no evidence of increased thrombin generation in vivo after o-raffinose CLHb infusion, as determined by assessment of plasma TAT levels. Other studies have also failed to demonstrate activation of coagulation or evidence of consumptive coagulopathy in animals and humans given infusions of o-raffinose CLHb.10 29
Because of the hemostatic and potentiating effects ofo-raffinose CLHb on platelet aggregation, we also investigated whether infusion of o-raffinose CLHb would lead to a decrease in thromboresistance in the arterial circulation, where thrombosis is particularly dependent on platelets.30-32Our observation that o-raffinose CLHb accelerated carotid artery thrombosis in rabbits without thrombocytopenia or anemia suggests that arterial thromboregulation is disturbed by infusion of CLHb, and this finding is consistent with a previous report describing increased platelet deposition at sites of arterial injury in rats given infusions of diaspirin CLHb.26
Our results require cautious interpretation. It remains an unproved assumption that assessment of bleeding time or blood loss from a standardized incision is a measure of in vivo hemostasis. In patients, bleeding time is a poor predictor of bleeding.33 However, in the laboratory setting, in which laboratory animals are less heterogeneous than patient populations, the microvascular ear-bleeding time in thrombocytopenic and anemic rabbits has proved to be a useful tool for evaluating novel platelet products and the physiologic aspects of primary hemostasis.12,34 35
Our results suggest that infusion of o-raffinose CLHb may shift the thromboregulatory balance toward clot formation in rabbits, although the mechanism of action on platelets requires further clarification. When primary hemostasis was compromised by thrombocytopenia or anemia, o-raffinose CLHb decreased bleeding; however, resistance to the development of arterial thrombi may be lowered in animals without thrombocytopenia or anemia. Whether these effects are generalizable to human recipients ofo-raffinose CLHb is unknown. Whereas enhancing hemostasis to decrease bleeding could be clinically useful, pathologic thrombosis is not. Ongoing surveillance for unintentional effects of CLHb preparations appears justified.
Acknowledgments
We thank Dr Gloriann Ropchan for expert surgical assistance and Hemosol Inc for providing the o-raffinose CLHb.
Supported by grants from the Heart and Stroke Foundation of Ontario (NA 3651) and the J. P. Bickell Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David H. Lee, Division of Hematology, Etherington Hall, Room 2013, Queen's University, Kingston, Ontario, Canada K7L 3N6; e-mail: leedh@meds.queensu.ca.