Abstract
Growth factor–dependent hematopoietic cell lines expressing the BCR/ABL oncoprotein of the Ph chromosome show growth factor–independent proliferation and resistance to apoptosis. Apoptosis resistance of BCR/ABL-expressing cells may depend on enhanced expression of anti-apoptotic proteins as well as reduced expression and/or inactivation of pro-apoptotic proteins. Compared to myeloid precursor 32Dcl3 cells expressing wild type BCR/ABL, cells expressing a BCR/ABL mutant lacking amino acids 176-426 in the BCR domain (p185ΔBCR) are susceptible to apoptosis induced by interleukin-3 (IL-3) deprivation. These cells exhibited the hypophosphorylated apoptotic BAD and markedly reduced levels of Bcl-2. Upon ectopic expression of Bcl-2, these cells showed no changes in BAD phosphorylation, but they became apoptosis-resistant and proliferated in the absence of IL-3, albeit more slowly than cells expressing wild type BCR/ABL. Moreover, the p185ΔBCR/Bcl-2 double transfectants were leukemogenic when injected into immunodeficient mice, but Bcl-2 expression did not restore the leukemia-inducing effects of p185ΔBCR to the levels of wild type BCR/ABL. Leukemic cells recovered from the spleen of mice injected with p185ΔBCR/Bcl-2 cells did not show rearrangements in the Bcl-2 genomic locus, but they exhibited enhanced proliferation in culture and induced a rapidly fatal disease process when inoculated in secondary recipient mice. Together, these data support the importance of anti-apoptotic pathways for BCR/ABL-dependent leukemogenesis and suggest that Bcl-2 expression promotes secondary changes leading to a more aggressive tumor phenotype.
Introduction
The BCR/ABL oncoproteins (p185, p210, and p230) are encoded by fusion genes arising from the t(9;22) chromosomal translocation in which different segments of the bcrgene juxtapose with sequences upstream of the second exon of c-abl. p185 is found in nearly 20% of acute lymphocytic leukemia (ALL),1 p210 is typically found in chronic myeloid leukemia (CML),2 and the recently identified p230 is associated with chronic neutrophilic leukemia and CML.3 4
Ectopic expression of BCR/ABL proteins in growth factor–dependent hematopoietic cell lines causes growth factor–independent proliferation5,6 and reduced susceptibility to apoptosis.7-10 Moreover, BCR/ABL-expressing cells are leukemogenic when injected in immunodeficient mice.11-13The transforming potential of the BCR/ABL oncoproteins depends on their tyrosine kinase activity,14 which allows the recruitment and activation of intracellular signal transducers independent of extracellular cytokine regulation.9
BCR/ABL-expressing cells show constitutive activation of Ras,15,16 Jun,17 the phosphoinositide (PI)-3K/Akt pathway,18-20 and STAT521-25(signal transducer and activator of transcription). Disruption of these pathways by a variety of strategies, including expression of dominant-negative molecules or antisense transcripts, or by chemical inhibitors markedly suppresses transformation of hematopoietic cells.15,16,20,25 Some of these pathways are also required for the anti-apoptotic activity of BCR/ABL,20,25,26 which suggests that the reduced susceptibility to apoptosis of BCR/ABL-expressing cells might contribute significantly to the transforming potential of the BCR/ABL oncoproteins. Conversely, hematopoietic cells expressing BCR/ABL mutants defective in transformation show changes in the expression pattern of anti- and pro-apoptotic proteins,10 which is consistent with the notion that leukemogenic potential and activation of anti-apoptotic pathways are closely linked.
Hematopoietic precursor 32Dcl3 cells expressing p185ΔBCR, a mutant deleted in amino acids 176-426 of the BCR segment, are growth factor–dependent for proliferation and survival and have a low leukemogenic potential when injected in immunodeficient mice.9,10 These cells express Bcl-2 at low levels and exhibit the non-phosphorylated, pro-apoptotic form of BAD.10 However, upon expression of a constitutively active, mitochondria-targeted Raf-1, these cells show expression of the phosphorylated non-apoptotic form of BAD, survive when cultured in the absence of interleukin-3 (IL-3), and are able to induce leukemia in mice.10 These studies further point to the role of anti-apoptosis mechanisms in the leukemia-inducing effects of BCR/ABL.
The anti-apoptotic effects of BCR/ABL are not limited to the inactivation of BAD, but they also involve the ability to up-regulate Bcl-2 and Bcl-XL expression,27,28 perhaps by RAS- and STAT5-dependent mechanisms, respectively.29 30 Thus, restoring Bcl-2 expression in 32Dcl3 cells transfected with p185ΔBCR is expected to render these cells resistant to growth factor-deprivation–induced apoptosis and possibly cause them to be leukemogenic. Because these double-transfected cells may remain defective in their ability to phosphorylate and inactivate pro-apoptotic BAD, they may provide a model to dissect the contribution of individual anti-apoptosis pathways to BCR/ABL-dependent leukemogenesis.
We show here that cells coexpressing p185ΔBCR and Bcl-2 are resistant to growth factor-deprivation–induced apoptosis and are leukemogenic in mice. These effects occur independently of changes in the phosphorylation status of BAD. Indeed, Bcl-2 expression appears to be more potent than mitochondrial Raf-1–dependent BAD phosphorylation in restoring the leukemogenic potential of p185ΔBCR-expressing cells.
Materials and methods
Plasmids
The human Bcl-2 cDNA was subcloned into the blunt-endedEcoRI site of the LXSP retroviral vector. Constitutively active (M-Raf) and dominant-negative (K375W) mitochondrial Raf-110 were subcloned into the IRES-GFP bicistronic retroviral vector MIG-R131 as follows: (1) plasmids LXSP/Mas 70p/Raf and LXSP/Mas 70p/K375Wraf were digested withBamHI, blunt-end filled with Klenow, and redigested withXhoI, and (2) the releasedBamHI-blunted/XhoI fragments were subcloned in the EcoRI-blunted/XhoI-digested MIG-R1 vector.
Cell cultures and transfections
The murine IL-3–dependent cell line 32Dcl331 was maintained in Iscove modified Dulbecco medium (IMDM-CM) containing 10% heat-inactivated fetal bovine serum (FBS) (Sigma Chemical, St Louis, MO), 2 mmol/L L-glutamine (Gibco BRL Life Technologies, Grand Island, NY), 100 μg/mL each penicillin and streptomycin, and 10% WEHI-conditioned medium as a source of IL-3. Previously we established p185ΔBCR- or p185BCR/ABL-expressing 32Dc13 cells in our laboratory, and they were maintained as described.20
To obtain cell clones coexpressing p185ΔBCR and Bcl-2, a clone of p185ΔBCR-expressing cells was cocultured with packaging cells BOSC-23 previously transfected by calcium phosphate precipitation with the Bcl-2 plasmid. After 24 hours of coculture, 32Dc13 cells were harvested, plated in fresh IMDM-CM, and selected in the presence of 2.5 μg/mL puromycin (Calbiochem, Nottingham, England) and 1 mg/mL G418 (Gibco BRL).
Constitutively active (M-Raf)–expressing and dominant-negative (K375WM-Raf)–expressing p185ΔBCR/Bcl-2 32Dcl3 cells were generated as follows: high titer MIG-M-Raf and MIG-M-K375Wraf retroviral supernatants obtained by transfection of the 293-derivative Phoenix packaging cells were used to infect p185ΔBCR/Bcl-2–expressing 32Dcl3 cells. After infection, GFP+ cells were sorted and expanded in cultures supplemented with puromycin and G418. Expression of ectopic M-Raf proteins was detected by Western blot with a polyclonal anti-Raf antibody (sc-133; Santa Cruz Biotech, Santa Cruz, CA).
Cell viability and apoptosis assays
Cells were washed 4 times with phosphate-buffered saline (PBS) and plated (105 cells per mL) in IMDM in the presence or absence of IL-3, and the percentage of viable cells was evaluated by trypan blue dye exclusion. Apoptosis was evaluated by flow cytometry determination of DNA content in PI-stained nuclei.
Differentiation assay
Cells were washed 4 times with PBS and plated (5 × 104 cells per mL) in IMDM in the presence of granulocyte–colony-stimulating factor (G-CSF)–containing medium from U87 cells. U87-conditioned medium was prepared by seeding 105 cells per mL in IMDM. After 7 days, cell-free supernatant was collected and filtered through a 0.22-μm filter. Morphology of differentiating cells was evaluated by May-Grünwald-Giemsa staining of cytospin preparations.
Isolation of mitochondria
Mitochondria were isolated from p185ΔBCR-, p185ΔBCR/Bcl-2–, and p185BCR/ABL–expressing cells by sucrose density gradient centrifugation as described.32 Briefly, 4 × 107 cells were harvested with PBS; centrifuged at 1200 rpm for 10 minutes; and resuspended in isotonic mitochondrial buffer (MB) comprising 210 mmol/L D-mannitol, 70 mmol/L sucrose, 1 mmol/L ethylenediamine tetraacetic acid (EDTA), and 10 mmol/L Hepes (4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid) (pH 7.4) supplemented with protease inhibitors.
Cells were broken by repeated passage through a 25G1 0.5 × 25 needle syringe and centrifuged at 1200 rpm for 10 minutes at 4°C. Supernatants obtained after each step were collected and centrifuged at 13 000 rpm for 10 minutes at 4°C. Pellets were resuspended in 1 mL isotonic MB; loaded on 20 mL discontinuous sucrose gradient (1.2 mol/L sucrose, 10 mmol/L Hepes, 1 mmol/L EDTA, and 0.1% bovine serum albumin [BSA]) on top of 17 mL of 1.6 mol/L sucrose, 10 mmol/L Hepes, 1 mmol/L EDTA, and 0.1% BSA; and centrifuged at 27 000 rpm for 2 hours at 4°C in a Beckman SW28 rotor. Mitochondria were recovered at the 1.6 mol/L to 1.2 mol/L sucrose interface, centrifuged at 13 000 rpm for 30 minutes, washed, and resuspended in 1% Triton MB. Proteins (50 μg) were loaded on a 12.5% SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis); transferred to a PROTRAN nitrocellulose membrane (Schleicher and Schuell); and Western blotted with anti–Raf-1, anti–Bcl-2, and anti–α-HSP90 monoclonal antibodies (all from Transduction Laboratories, Lexington, KY) and anticytochrome oxidase (COX) subunit IV (Molecular Probes, Eugene, OR).
BAD phosphorylation assay
p185ΔBCR-, p185ΔBCR/Bcl-2–, and p185BCR/ABL-expressing cells (107 cells each) were washed twice with Roswell Park Memorial Institute (RPMI 1640) medium without sodium phosphate (Gibco BRL). Cells were then centrifuged at 1200 rpm for 10 minutes, resuspended in 10 mL of the same medium supplemented with 0.1% BSA and 25 mmol/L Hepes (pH 7.4), and incubated at 37°C in a Hybad mini-oven. After 3 hours, the cells were centrifuged, resuspended in 2 mL of the same incubation medium containing 111 MBq/mL (0.3 mCi/mL) of phosphorous 32 (32P)-orthophosphate (New England Nuclear, Boston, MA), and incubated at 37°C in the Hybad mini-oven for 3 hours.
After washing cells with PBS, proteins were extracted in lysis buffer comprising 20 mmol/L Hepes, 150 mmol/L sodium chloride (NaCl), 1 mmol/L EDTA, and 0.5% NP-40, and 1 mg cell lysate was used for immunoprecipitation with anti-BAD polyclonal antibody (C-20; Santa Cruz Biotechnology). Immunoprecipitated proteins were loaded in a 12.5% SDS-PAGE and transferred on a PROTRAN nitrocellulose membrane. BAD phosphorylation was visualized by autoradiography.
Leukemogenesis in severe combined immune deficiency mice
Using 4- to 6-week-old severe combined immune deficiency (SCID) mice (Taconic Farms, Germantown, NY), we intravenously injected (5 × 106 cells per mouse) 10 mice with p185ΔBCR clones, 24 mice with p185ΔBCR/Bcl-2 clones, and 5 mice with both BCR/ABL and 32Dc13/Bcl-2 clones. At 4 and 6 weeks after injection, mice representative of each group were killed, and their organs were analyzed for the presence of leukemia. Tissues were fixed with 3% paraformaldehyde in PBS, and embedded in paraffin. Sections (5 μm in thickness) were stained with hematoxylin and eosin.
Isolation of cells from mouse spleen
Three mice (one of each p185ΔBCR/Bcl-2 clone) were killed when visibly ill, and cells were purified from the spleen suspension. Briefly, spleen was minced, homogenized by scraping with a pestle on a grid generating a cell suspension in PBS plus 0.1% BSA, and kept on ice.
Red blood cells were removed by lysis in hypotonic solution comprising 0.85% NH4Cl and 17 mmol/L Tris-HCl (tris[hydroxymethyl] aminomethane–hydrochloride) (pH 7.4) for 10 minutes on ice. The cells were then washed and plated in IMDM in the absence of IL-3. p185ΔBCR/Bcl-2–expressing cells were selected in the presence of 1.25 μg/mL puromycin and 500 μg/mL G418. After obtaining a uniform cell population, high molecular weight DNA was extracted for Southern blot analysis, and the cells were used for injection in secondary recipient mice.
Southern blot analysis
p185ΔBCR- and p185ΔBCR/Bcl-2–expressing 32Dc13 cells and splenocytes isolated from leukemic mice (5 × 106 cells each) were washed with PBS and resuspended in 500 μL of 100 mmol/L NaCl, 10 mmol/L Tris-HCl (pH 8.0), 25 mmol/L EDTA (pH 8.0), 0.5% SDS, and 0.1 mg/mL Proteinase K. High molecular weight DNA was obtained by phenol extraction as described.34
For Southern blot, 10 μg of each DNA sample was digested overnight with restriction enzymes HindIII, BamHI, orXbaI (all from Boehringer), separated on a 0.8% agarose gel, and transferred to a Hybond-N+ membrane (Amersham Life Sciences, Uppsala, Sweden). A full-length Bcl-2 complementary DNA (cDNA) labeled by random priming (Boehringer) was used as hybridization probe.
Results
Bcl-2 overexpression restores the anti-apoptotic potential of the p185ΔBCR mutant
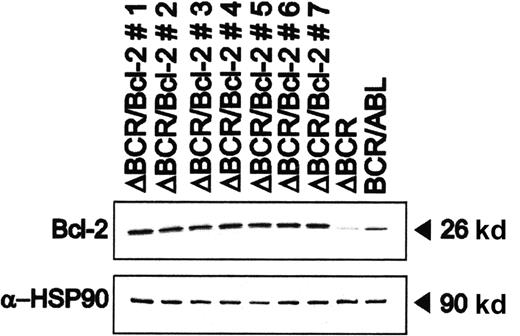
When cultured in the absence of IL-3–containing conditioned medium, 32Dcl3 cells expressing the p185ΔBCR mutant undergo apoptosis with kinetics very similar to that of parental cells.10Moreover, these cells exhibit Bcl-2 levels markedly lower than those of 32Dc13 cells expressing wild-type BCR/ABL.10 Transfection of p185ΔBCR-expressing cells with a plasmid carrying a human Bcl-2 cDNA restored Bcl-2 expression (Figure1).
Bcl-2 expression in representative clones generated by transfection of p185ΔBCR/32Dc13 cells with a human Bcl-2 cDNA.
Levels of HSP90 were measured as control of equal loading.
Bcl-2 expression in representative clones generated by transfection of p185ΔBCR/32Dc13 cells with a human Bcl-2 cDNA.
Levels of HSP90 were measured as control of equal loading.
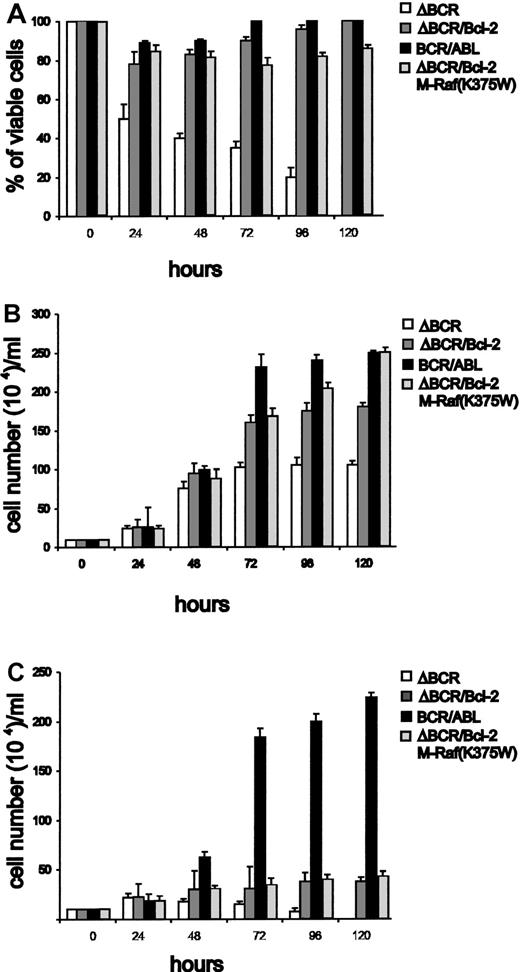
Three clones expressing comparable levels of Bcl-2 were selected and analyzed for viability and proliferation after IL-3 deprivation. Bcl-2–overexpressing cells were resistant to apoptosis (Figure2A, representative example). Similar results were obtained in representative samples when apoptosis was evaluated by DNA content analysis in PI-stained nuclei (not shown). The Bcl-2–overexpressing cells also proliferated in a growth factor–independent manner, albeit at a markedly slower rate compared to wild-type BCR/ABL-expressing cells (Figure 2C). In IL-3–containing medium, proliferation of p185ΔBCR/Bcl-2 coexpressing cells was faster than that of p185ΔBCR cells but slower than that of wild-type BCR/ABL-expressing cells (Figure 2B). As expected, p185ΔBCR-expressing cells did not survive in the absence of IL-3, and were all dead within 5 days (Figure 2A).
Viability and proliferation of 32Dc13 cells expressing wild-type BCR/ABL, p185ΔBCR, or both p185ΔBCR and Bcl-2.
(A) Numbers of viable cells (triplicate counts) were determined by trypan blue exclusion at the indicated times after removal of IL-3. Results are representative of 3 independent experiments using 3 p185ΔBCR/Bcl-2 clones. Cells (105/mL) were plated in 25-mL flasks (B) with or (C) without IL-3–containing conditioned medium and counted by trypan blue exclusion at the indicated times. Results are representative of 3 independent experiments using 3 individual clones.
Viability and proliferation of 32Dc13 cells expressing wild-type BCR/ABL, p185ΔBCR, or both p185ΔBCR and Bcl-2.
(A) Numbers of viable cells (triplicate counts) were determined by trypan blue exclusion at the indicated times after removal of IL-3. Results are representative of 3 independent experiments using 3 p185ΔBCR/Bcl-2 clones. Cells (105/mL) were plated in 25-mL flasks (B) with or (C) without IL-3–containing conditioned medium and counted by trypan blue exclusion at the indicated times. Results are representative of 3 independent experiments using 3 individual clones.
32Dc13 cells expressing wild-type, p185ΔBCR, Bcl-2, or both p185ΔBCR/Bcl-2 were also assessed for their ability to differentiate in the presence of G-CSF–containing conditioned medium (Figure3). Parental 32Dc13 cells were all terminally differentiated after 10 days of culture in G-CSF–containing medium (not shown); however, terminally differentiated granulocytes represented only a small fraction of the cells after 7 days. Terminal differentiation of 32Dc13 cells expressing Bcl-2 was accelerated, while cells expressing wild-type BCR/ABL or the ΔBCR mutant did not show morphologic features of differentiation. Interestingly, differentiating cells were more numerous in cultures of p185ΔBCR/BCL-2-coexpressing cells (Figure 3).
G-CSF–induced differentiation of parental and transfected 32Dc13 cells.
Representative microphotographs of May-Grünwald-Giemsa–stained cytospins.
G-CSF–induced differentiation of parental and transfected 32Dc13 cells.
Representative microphotographs of May-Grünwald-Giemsa–stained cytospins.
Anti-apoptosis effects of Bcl-2 do not involve phosphorylation-dependent BAD inactivation
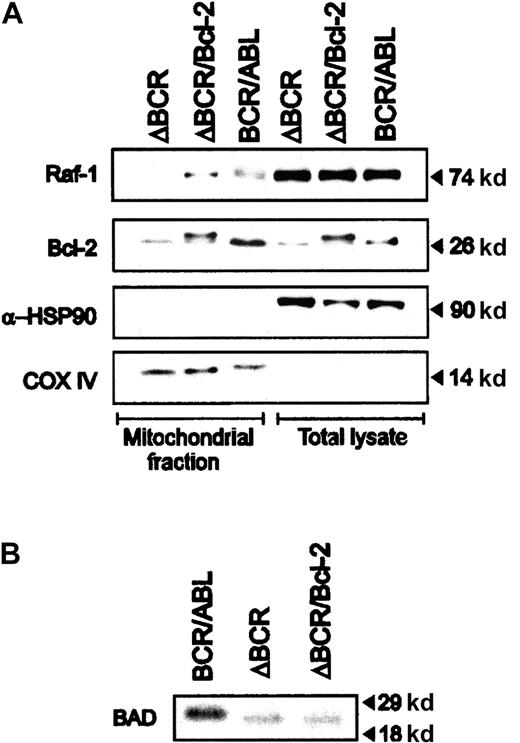
The interaction of Bcl-2 and Raf-135 and the subsequent targeting of Raf-1 to mitochondria36 have been proposed as a mechanism underlying the anti-apoptotic activity of Bcl-2. The observation that a mitochondria-targeted Raf-1 promotes the phosphorylation and inactivation of BAD10 raised the possibility that Bcl-2 overexpression may be accompanied by BAD phosphorylation via a Raf-1–dependent mechanism. Expression of mitochondrial Raf-1 was detected in 32Dcl3 cells expressing wild-type BCR/ABL and in cells coexpressing the ΔBCR mutant and Bcl-2, but not in cells expressing only p185ΔBCR (Figure4A). However, immunoprecipitation with either the antihuman Bcl-2 (hBcl-2, for hBcl-2–expressing cells) or the antimouse Bcl-2 (mBcl-2, for p185ΔBCR- and BCR/ABL-expressing cells) antibody and Western blots with the anti–Raf-1 antibody did not reveal Raf-1/Bcl-2 coimmunoprecipitates even in Bcl-2–overexpressing cells (not shown).
Expression of mitochondrial Raf-1 in BCR/ABL and p185ΔBCR/Bcl-2–coexpressing cells.
(A) Levels of HSP90 and subunit IV of COX were measured as control of loading and subcellular fractionation. (B) Phosphorylation of BAD in BCR/ABL (wild-type and mutant) and p185ΔBCR/Bcl-2–coexpressing cells. Cells were starved of both IL-3 and serum for 3 hours and then cultured in 32P-orthophosphate–containing medium for 3 hours. Cell lysates were immunoprecipitated with anti-BAD polyclonal antibody, and phosphorylation was assessed by autoradiography.
Expression of mitochondrial Raf-1 in BCR/ABL and p185ΔBCR/Bcl-2–coexpressing cells.
(A) Levels of HSP90 and subunit IV of COX were measured as control of loading and subcellular fractionation. (B) Phosphorylation of BAD in BCR/ABL (wild-type and mutant) and p185ΔBCR/Bcl-2–coexpressing cells. Cells were starved of both IL-3 and serum for 3 hours and then cultured in 32P-orthophosphate–containing medium for 3 hours. Cell lysates were immunoprecipitated with anti-BAD polyclonal antibody, and phosphorylation was assessed by autoradiography.
BAD phosphorylation was directly assessed in anti-BAD immunoprecipitates from cells metabolically labeled with32P-orthophosphate. As shown in Figure 4B, BAD phosphorylation was readily detected in cells expressing wild-type BCR/ABL, but not in p185ΔBCR-expressing cells or in cells coexpressing p185ΔBCR and Bcl-2. Thus, while Bcl-2 overexpression might promote the association of Raf-1 with the mitochondria, this does not appear to be sufficient for BAD phosphorylation. Consistent with these findings, expression of mitochondria-targeted, dominant-negative (K375W) Raf-1 in p185ΔBCR/Bcl-2 cells had no effect on their survival or proliferation (Figure 2). Interestingly, expression of Bcl-XL was essentially identical in wild-type BCR/ABL-, p185ΔBCR-, and p185ΔBCR/Bcl-2–expressing cells (not shown).
Bcl-2 overexpression restores the leukemogenic potential of p185ΔBCR-expressing cells
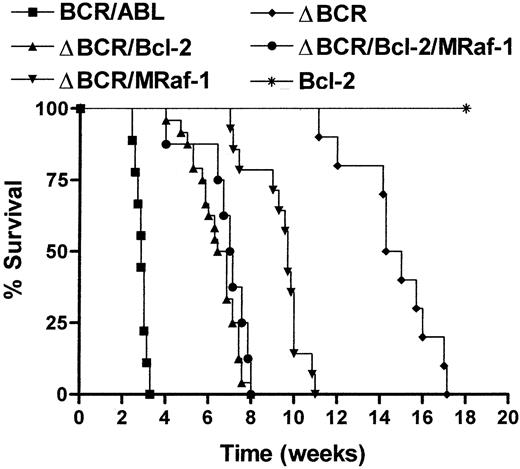
To determine whether overexpression of Bcl-2 restores a survival pathway sufficient for the leukemia-inducing effect of the BCR/ABL oncoprotein, immunocompromised SCID mice were injected with an equal number (5 × 106 cells per mouse) of 32Dcl3 cells expressing either wild-type BCR/ABL or p185ΔBCR mutant or coexpressing p185ΔBCR and Bcl-2. As additional controls, mice were also injected with 32Dc13 cells overexpressing Bcl-2 or with cells coexpressing p185ΔBCR and constitutively active M-Raf-110and with cells coexpressing p185ΔBCR, Bcl-2, and M-Raf-1. Mice inoculated with cells expressing wild-type BCR/ABL were all dead 3 weeks after injection.
At necropsy, these mice exhibited an enlarged spleen. Histopathological analysis showed multiorgan involvement by acute myelogenous leukemia (AML). The bone marrow showed effaced architecture and replacement of normal hematopoietic elements by sheets of immature myeloid cells. The spleen and liver also showed marked involvement by AML, and scattered foci of AML cells were detected in the kidneys. Four weeks after injection, 1 or 2 mice of each remaining group were killed, and hematopoietic and nonhematopoietic organs were analyzed for the presence of leukemia. None of the organs examined showed infiltration by leukemic cells (not shown). However, 6 weeks after injection, most mice injected with cells coexpressing p185ΔBCR and Bcl-2 showed a markedly enlarged spleen. Upon microscopic examination, all organs were infiltrated with leukemic cells exhibiting a somewhat higher degree of differentiation than that shown by cells expressing wild-type BCR/ABL (not shown). The more prominent differentiation was particularly evident in the spleen and liver. The spleen showed almost complete effacement of the architecture, while the liver showed a combination of sinusoidal and parenchymal involvement. p185ΔBCR/Bcl-2–coexpressing cells displayed a rather aggressive phenotype as indicated by large areas of necrosis and extensive tissue infiltration and disruption. Accordingly, the leukemia involving the bone marrow displayed infiltration of the cortical bone and adjacent muscle (not shown).
All mice in the p185ΔBCR/Bcl-2 group died of leukemia 6 to 8 weeks after cell injection (Figure 5). Likewise, all mice in the p185ΔBCR/Bcl-2/M-Raf-1 group died of leukemia by 8 weeks after cell injection (Figure 5). At 4 and 6 weeks after injection, mice in the p185ΔBCR/M-Raf-1 group were apparently normal, but subsequent analyses revealed leukemic cells in the organs examined, and the mice died of leukemia 10 to 12 weeks after injection (Figure 5).
Survival of SCID mice injected with 32Dc13 cells expressing BCR/ABL, Bcl-2, p185ΔBCR/Bcl-2, p185ΔBCR/M-Raf-1, or p185ΔBCR/Bcl-2/M-Raf-1.
Mice were injected intravenously with 5 × 106 cells from parental or transfected 32Dc13 cells. Nine mice were included in the wild-type BCR/ABL group; 5 mice, the Bcl-2 group; 10 mice, the p185ΔBCR group; 24 mice, the p185ΔBCR/Bcl-2 group (8 mice for each of 3 clones); 14 mice, the p185ΔBCR/M-Raf group (7 mice for each of 2 clones); and 8 mice, the p185ΔBCR/Bcl-2/M-Raf-1 group.
Survival of SCID mice injected with 32Dc13 cells expressing BCR/ABL, Bcl-2, p185ΔBCR/Bcl-2, p185ΔBCR/M-Raf-1, or p185ΔBCR/Bcl-2/M-Raf-1.
Mice were injected intravenously with 5 × 106 cells from parental or transfected 32Dc13 cells. Nine mice were included in the wild-type BCR/ABL group; 5 mice, the Bcl-2 group; 10 mice, the p185ΔBCR group; 24 mice, the p185ΔBCR/Bcl-2 group (8 mice for each of 3 clones); 14 mice, the p185ΔBCR/M-Raf group (7 mice for each of 2 clones); and 8 mice, the p185ΔBCR/Bcl-2/M-Raf-1 group.
The 10 mice injected with p185ΔBcl-expressing cells developed leukemia after long latency and died 16 to 17 weeks after cell inoculation (Figure 5); none of the mice in the 32Dc13/Bcl-2 group developed leukemia. 32Dcl3 cells recovered from the spleen of leukemic mice (p185ΔBCR/Bcl-2 S cells) proliferated faster than noninjected p185ΔBCR/Bcl-2 cells (Figure 6A), suggesting that Bcl-2–dependent changes potentially leading to a more aggressive phenotype were selected during in vivo growth. Indeed, upon reinoculation of 15 SCID mice with 5 × 106p185ΔBCR/Bcl-2–coexpressing cells recovered from the spleen of leukemic mice, leukemia development was accelerated because all 15 mice were dead 4 to 6 weeks after injection (Figure 6B).
Proliferation and leukemogenic potential of p185ΔBCR/Bcl-2–coexpressing 32Dc13 cells isolated from the spleen of leukemic mice.
(A) p185ΔBCR/Bcl-2 cells maintained in IL-3–free medium and cells recovered from the spleen of primary recipient leukemic mice (p185ΔBCR/Bcl-2 S) were plated at a density of 105 cells per mL in 25-mL flasks in the absence of IL-3–containing medium and counted by trypan blue exclusion at the indicated times. Results are representative of 3 independent experiments using 3 individual clones. (B) Survival of SCID mice injected with p185ΔBCR/Bcl-2 or p185ΔBCR/Bcl-2 S cells. Mice were injected intravenously with 5 × 106 cells. Eight mice were included in the p185ΔBCR/Bcl-2 group (1 clone), and 15 mice were included in the p185ΔBCR/Bcl-2 S group (5 mice for each of 3 clones).
Proliferation and leukemogenic potential of p185ΔBCR/Bcl-2–coexpressing 32Dc13 cells isolated from the spleen of leukemic mice.
(A) p185ΔBCR/Bcl-2 cells maintained in IL-3–free medium and cells recovered from the spleen of primary recipient leukemic mice (p185ΔBCR/Bcl-2 S) were plated at a density of 105 cells per mL in 25-mL flasks in the absence of IL-3–containing medium and counted by trypan blue exclusion at the indicated times. Results are representative of 3 independent experiments using 3 individual clones. (B) Survival of SCID mice injected with p185ΔBCR/Bcl-2 or p185ΔBCR/Bcl-2 S cells. Mice were injected intravenously with 5 × 106 cells. Eight mice were included in the p185ΔBCR/Bcl-2 group (1 clone), and 15 mice were included in the p185ΔBCR/Bcl-2 S group (5 mice for each of 3 clones).
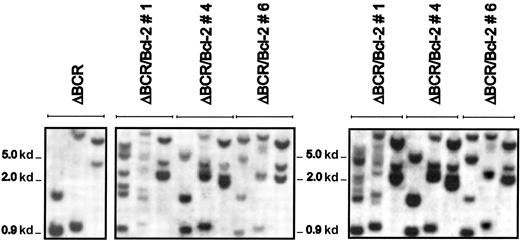
To exclude the possibility that the more aggressive leukemia in secondary recipients was accompanied by changes in the Bcl-2locus, the pattern of genomic Bcl-2 integration was compared in cells coexpressing p185ΔBCR and Bcl-2 before injection in SCID mice and after culturing spleen cells from leukemic mice in the presence of puromycin and in the absence of IL-3–containing conditioned medium to eliminate contaminating normal splenocytes. Southern blotting of genomic DNA digested with 3 different restriction enzymes revealed the same pattern of Bcl-2 integration in the cells injected in SCID mice and in those recovered from the leukemic spleen (Figure 7). Moreover, Bcl-2 levels were indistinguishable in the p185ΔBCR/Bcl-2 cells injected in SCID mice and in those recovered from the spleen of leukemic mice (not shown).
Southern blot analysis of the Bcl-2 locus in p185ΔBCR/Bcl-2 clones before injection in SCID mice and in leukemic cells recovered from the spleen.
Southern blot of genomic DNA from p185ΔBCR 32Dc13 clones (left panel), p185ΔBCR/Bcl-2 clones (middle panel), and p185ΔBCR/Bcl-2 clones recovered from the spleen of leukemic mice (right panel). Genomic DNA was digested with HindIII, BamHI, orXbaI (from left to right), electrophoresed on a 0.8% agarose gel, transferred to nylon membranes, and hybridized to a32P-labeled Bcl-2 fragment.
Southern blot analysis of the Bcl-2 locus in p185ΔBCR/Bcl-2 clones before injection in SCID mice and in leukemic cells recovered from the spleen.
Southern blot of genomic DNA from p185ΔBCR 32Dc13 clones (left panel), p185ΔBCR/Bcl-2 clones (middle panel), and p185ΔBCR/Bcl-2 clones recovered from the spleen of leukemic mice (right panel). Genomic DNA was digested with HindIII, BamHI, orXbaI (from left to right), electrophoresed on a 0.8% agarose gel, transferred to nylon membranes, and hybridized to a32P-labeled Bcl-2 fragment.
Discussion
The leukemogenic potential of the BCR/ABL oncoproteins appears to require the activation of several signal transduction pathways that control proliferation, apoptosis, and motility. Some of the BCR/ABL-regulated effectors (eg, RAS) have been implicated in the regulation of both proliferation and survival,9,26 while others (eg, Rac) may only regulate the motility of BCR/ABL-expressing cells without a measurable effect on survival and proliferation.37
Evidence now indicates that BCR/ABL negatively regulates the activity of proapoptotic BAD primarily via Akt-dependent phosphorylation37,38 and that it enhances the expression of Bcl-2, in part via a RAS-dependent mechanism.29Hematopoietic progenitor 32Dcl3 cells expressing the p185ΔBCR mutant are susceptible to apoptosis induced by growth factor deprivation9,10 and exhibit the hypophosphorylated proapoptotic form of BAD and low levels of Bcl-2.10Moreover, the in vivo leukemogenic potential of these cells is markedly impaired in the immunodeficient mice model.9,10 Resistance to apoptosis and leukemogenesis were, in part, restored by coexpression of a mitochondria-targeted Raf-1 that was able to induce BAD phosphorylation and inactivation but not influence the levels of Bcl-2.10
In the present study we observed restoration of apoptosis resistance and leukemogenesis of p185ΔBCR-expressing cells by ectopic expression of Bcl-2. Interestingly, the enhanced expression of Bcl-2 was not accompanied by detectable changes in the phosphorylation of BAD, even though levels of mitochondrial Raf-1 were increased in Bcl-2–expressing cells. Perhaps the level of Raf-1 activation by p185ΔBCR is not sufficient for the induction of BAD phosphorylation once Raf-1 localizes to the mitochondria via the Bcl-2–dependent mechanism.36 Consistent with the observation that Bc1-2 overexpression does not lead to M-Raf-1–dependent BAD phosphorylation, expression of dominant-negative M-Raf-1 had no effects on the ability of ectopically expressed Bcl-2 to protect p185ΔBCR/Bcl-2 cells from IL-3 deprivation-induced apoptosis.
Restoration of Bcl-2 expression in p185ΔBCR cells was followed by resistance to apoptosis and growth factor–independent proliferation. Most of the p185ΔBCR or p185ΔBCR/Bcl-2 coexpressing cells remained undifferentiated when cultured in the presence of G-CSF, but features of myeloid maturation were more prominent in the p185ΔBCR/Bcl-2 cultures, which is consistent with the accelerated differentiation observed in cultures of Bcl-2–expressing cells. The partial differentiation of in vitro–cultured p185ΔBCR/Bcl-2–coexpressing cells was mimicked by the morphological heterogeneity exhibited by the transformed cells infiltrating the hematopoietic organs of immunodeficient mice. Features of myeloid maturation were also observed when infiltrating cells were recovered from the spleen and cultured in the absence of IL-3. The partial differentiation of p185ΔBCR/Bcl-2–coexpressing cells had no obvious consequences on their malignant behavior, as indicated by the high degree of invasiveness noted in sections of different tissues and the poor survival of injected mice.
Mice injected with p185ΔBCR/Bcl-2–coexpressing cells had a longer survival than those injected with wild-type BCR/ABL-expressing cells. This suggests that restoring the Bcl-2–dependent antiapoptotic pathway does not, per se, reconstitute the full leukemogenic potential of wild-type BCR/ABL. These findings also imply that other signal transduction pathways dependent on an intact BCR domain are important for leukemogenesis. In this regard, the GRB-2–binding site located at amino acid 177 is absent in p185ΔBCR/ABL and may contribute to the reduced ability of this mutant to activate RAS10; p185ΔBCR/ABL also shows an impairment in the interaction with the p85 regulatory subunit of PI-3K and is defective in AKT activation. Moreover, the ΔBCR BCR/ABL mutant lacks the BCR sequences necessary for formation of an intramolecular complex with the SH2 domain, an association that reportedly enhances BCR/ABL tyrosine kinase activity.40 The leukemogenic potential of p185ΔBCR/Bcl-2 cells was not enhanced by M-Raf-1 expression. This observation suggests that sufficient levels of Bcl-2 (or a Bcl-2 equivalent) can provide the critical anti-apoptotic signal necessary for the leukemia-inducing effect of BCR/ABL. The finding that p185ΔBCR/M-Raf-1 cells are less potent than p185ΔBCR/Bcl-2 cells in inducing leukemia in mice also suggests that there is a gradient of antiapoptotic signals which can contribute differently to the leukemogenic potential of BCR/ABL. Moreover, even if M-Raf-1 and Bcl-2 provide nonoverlapping anti-apoptotic functions, the inability of M-Raf-1 to potentiate the effect of Bcl-2 is consistent with the existence of “plateau” levels of survival signals beyond which there is no further enhancement of the transformation potency of BCR/ABL and perhaps of other oncoproteins.
p185ΔBCR/ABL/Bcl-2–coexpressing cells recovered from the leukemic spleen of primary recipient mice induced a rapidly fatal disease process upon inoculation in secondary recipients. This suggests that Bcl-2 expression, directly or indirectly, promotes the occurrence of secondary changes responsible for the increasingly aggressive behavior of p185ΔBCR/Bcl-2 cells. Because overexpression of Bcl-2 is a common feature of advanced stage neoplasms including leukemia,41-45 the identification of such secondary changes will help in elucidating the molecular mechanisms associated with progression to an increasingly malignant phenotype.
Supported in part by a grant (PO1CA78890) from the National Institutes of Health, Bethesda, MD. M.C. was a postgraduate fellow from the University of Catania Medical School, Catania, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bruno Calabretta, the Department of Microbiology and Immunology, Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA; e-mail: b_calabretta@lac.jci.tju.edu.