Abstract
Identification of receptor activator of nuclear factor-κB (RANK) and RANK-ligand (RANKL) has provided new insights into the osteoclast differentiation pathway. Osteoclast precursor cells were isolated using monoclonal antibodies against c-Fms and RANK, and the effect of adherence on the in vitro differentiation and proliferation of these cells was examined in 2 different types of stromal-cell–free culture systems: a semisolid culture medium (a nonadherent system) and a liquid culture medium (an adherent system). Osteoclast precursor cells were not able to differentiate into mature osteoclasts efficiently in the semisolid culture system. Trimerized RANKL enhanced osteoclast differentiation in semisolid cultures, but not to the extent seen when cells were allowed to adhere to plastic. Initial precursor cells were capable of differentiating into macrophages or osteoclasts. Once these cells were transferred to adherent conditions, striking differentiation was induced. Multinuclear cells were observed even after they had displayed phagocytic activity, which suggests that cell adhesion plays an important role in the differentiation of osteoclast precursor cells. Integrins, especially the arginine-glycine-aspartic acid (RGD)–recognizing integrins αv and β3, were needed for osteoclast-committed precursor cells to proliferate in order to form multinuclear osteoclasts, and the increase in cell density affected the formation of multinuclear cells. A model of osteoclast differentiation with 2 stages of precursor development is proposed: (1) a first stage, in which precursor cells are bipotential and capable of anchorage-independent growth, and (2) a second stage, in which the further proliferation and differentiation of osteoclast-committed precursor cells is anchorage-dependent.
Introduction
Osteoclasts are bone-resorbing multinuclear cells derived from hematopoietic stem cells.1-3 Recent studies have shown that receptor activator of nuclear factor κB ligand (RANKL), which is also called ODF/OPGL/TRANCE,4-7is essential for osteoclast differentiation,8,9 and interaction between RANK and RANKL is indispensable for osteoclast differentiation and activation.10-13 Macrophages and osteoclasts develop from the same monocyte-lineage progenitor cells,14,15 and RANKL is considered a key molecule for the lineage switch. The distribution of osteoclasts is mostly limited to bone surfaces, but this cannot be attributed to restricted expression of RANKL because this molecule is expressed in many other cells and tissues as well. In contrast, macrophages do not show preferential tissue distribution. It is quite difficult to explain the restricted existence of osteoclasts compared to macrophages. Another key molecule other than RANKL is macrophage colony-stimulating factor (M-CSF). M-CSF is critical for osteoclastogenesis, as shown from analyses of osteopetrotic (op/op) mice that lack functional M-CSF.16-18 We reported that M-CSF is involved in induction of RANK on progenitors in addition to its known function in supporting cell proliferation and osteoclast differentiation.19 However, because M-CSF is a ubiquitous molecule, it is assumed that osteoclasts are not differentiated even in the presence of both molecules. In fact, no ectopic osteoclasts are observed in spleen where osteoclast precursor cells exist together with both M-CSF and RANKL. It is well known that the microenvironment affects osteoclast differentiation, and osteoclastogenesis is positively stimulated by M-CSF or RANKL and negatively regulated by OPG/OCIF, which is a decoy receptor of RANKL.20-22
An adherent environment is essential for osteoclast development, and several studies have identified an important role of integrins in the bone resorption function of osteoclasts.23,24 Most integrins bind ligands that contain the arginine-glycine-aspartic acid (RGD) tripeptide, and this 3 amino acid motif is found in many extracellular matrix proteins including fibronectin, vitronectin, fibrinogen, osteopontin, and thrombospondin. Most cells express several integrins, and individual types of integrins can often bind more than one ligand.25 The αvβ3antigen, which is a vitronectin receptor,26 is expressed in bone-resorbing osteoclasts.27-29 Because the αvβ3 antigen binds ligands via the RGD sequence,30,31 osteoclastic bone resorption in vitro can be inhibited by addition of the RGD peptide,32,33RGD-containing proteins,34-36 or antibodies against αvβ3.27 37 Although integrin involvement in bone resorption by mature osteoclasts has been well investigated, the function of integrins in differentiation or proliferation of osteoclast precursor cells prior to the multinuclear stage is poorly understood.
In this study, we have examined the effect of adherence on osteoclast precursor cell differentiation and proliferation using 2 culture methods: cultivation in semisolid medium (a condition where the cells are kept nonadherent) and in liquid medium (where the cells can become adherent).
Materials and methods
Fluorescence-activated cell sorter analysis and cell sorting
Two-month-old C57/BL6 (female) mice were purchased from SCL (Shizuoka, Japan). Mouse bone marrow cells were harvested from femurs and layered onto a lympholyte-M gradient (Cedarlane, Hornby, Ontario, Canada). After centrifugation, mononuclear cells were collected from the upper layer, and cells were incubated with CD16/CD32 antibody (Fc block) (2.4 G2; PharMingen, San Diego, CA) at a concentration of 1:200 for 15 minutes on ice. Subsequently, cells were stained with biotinylated anti-RANK monoclonal antibody (muRANK-M395; gift from Immunex, Seattle, WA) for 30 minutes on ice. After 2 washes, cells were incubated with fluorescein isothiocyanate (FITC)–conjugated c-Fms (AFS98; gift from Toray Industries, Kamakura, Japan) and allophycocyanin (APC)-conjugated streptavidin (Caltag Laboratories, San Francisco, CA). After a final wash, cells were suspended with 5% fetal bovine serum (FBS) (JRH Bioscience, Lenexa, KS) and phosphate-buffered saline (PBS), and cell sorting was performed using a fluorescence-activated cell sorter (FACS) (FACS Vantage or FACS Calibur; Becton Dickinson Immunocytometry Systems, San Jose, CA). Data were analyzed using LYSYS II software on a CONSORT 32 system or Cell Quest software (all from Becton Dickinson Immunocytometry Systems). Sorted cells were aliquoted and centrifuged onto microscope slides using a cytospin centrifuge (Shandon Southern Products, Astmoor, England). Then specimens were stained with May-Grünwald-Giemsa solution (Merck KgaA, Darmstadt, Germany) or tartrate-resistant acid phosphatase (TRAP) solution (No.387; Sigma Chemical Co., St Louis, MO). For TRAP staining, cells were fixed in 1% paraformaldehyde and histochemically stained for TRAP activity.
Osteoclast differentiation in vitro
We plated 1000 or 10 000 sorted cells on 96-well culture plates (Primaria, Falcon3872; Becton Dickinson Labware, Lincoln Park, NJ) or 96-well fibronectin-coated Cellware (Biocoat40409, Becton Dickinson Labware) and cultured the cells in α-minimum essential medium (α-MEM) (Gibco-BRL, Gaithersburg, MD) containing 10% FBS with 100 ng/mL recombinant mouse M-CSF (R&D Systems, Minneapolis, MN) alone or together with 25 ng/mL sRANKL for 6 days. sRANKL was prepared as previously described.19 In some experiments various concentrations of leucine zipper RANKL (LZ-RANKL) (gift from Immunex)38 were added to the cultures instead of sRANKL (soluble form of RANKL). Cultured cells were subjected to TRAP staining or TRAP-solution assay, and cells containing more than 5 nuclei were scored as multinuclear under a microscope. For TRAP-solution assay, cells were lysed in lysis buffer (0.2% NP-40 in distilled water) and stained with TRAP solution. The TRAP activity was measured at 405 nm of absorbance using a microplate reader (Bio-Rad Laboratories, Hercules, CA).
For integrin-blocking assay, we added the following to culture on day 0 or day 1: 0.5 mg/mL glycine-arginine-glycine-aspartic acid-serine (GRGDS) peptide or glycine-arginine-glycine-glutamic acid-serine (GRGES) peptide (American Peptide, Sunnyvale, CA) or blocking monoclonal antibodies against control-purified immunoglobulin G (IgG) (rat, R35-95; hamster, G235-2356; PharMingen, San Diego, CA) or against integrins αv and β3 (RMV-7 and HM-β3-1, respectively; gifts from Dr H. Yagita, Juntendo University, Tokyo, Japan)39,40; β1 (KMI6; gift from Dr K. Miyake, Saga University, Saga, Japan)41 42; and α2 and α5 (HM-α2 and HM-α5-1, respectively; PharMingen). All antibodies were freed of their sodium azide content by dialysis in cassettes (Slide-A-Lyzer; Pierce, Rockford, IL). To examine the dose-dependency or combination effect of these blocking antibodies or peptides, fibronectin-coated 96-well plates were preincubated with 2% bovine serum albumin (BSA)/PBS for 30 minutes at 37°C. Subsequently, cells were cultured with various concentrations of antibodies or peptides, and TRAP staining or TRAP activity was analyzed.
Colony assay
Colony assay was performed as described elswhere.19After 6 days of cultivation, the number of colonies present was scored under a microscope, and single colonies were placed on microscope slides one by one. The cells on the slides were then stained with TRAP solution, and the frequency of TRAP+ cells was calculated. Single colonies derived from c-Fms+RANK− cells cultured with M-CSF alone or both M-CSF and sRANKL in methylcellulose medium were removed and aliquoted for subsequent analyses. First, cells were stained with TRAP solution, and the frequency of TRAP+cells was calculated. Second, cells were replated onto fibronectin-coated 96-well plates or 35-mm culture dishes (Falcon1008) and further cultured with M-CSF alone or M-CSF and sRANKL in liquid or methylcellulose medium for 6 days. Cultured cells were stained with TRAP solution every day. Subsequently, colonies from 6-day methylcellulose cultures were collected again, and cells derived from single colonies were subcultured further in methylcellulose or liquid medium. TRAP staining was performed daily as described above.
To investigate the differentiation of cells in methylcellulose medium, single colonies were collected at first and secondary transfer and incubated with 0.027% fluorescence-labeled latex beads (diameter, 1 μm) (Polyscience, Washington, PA) for 4 hours at 37°C. Free beads were removed by washing twice with cold PBS, and then cells were cultured with M-CSF and sRANKL for 6 days. Cells were observed under a fluorescence microscope (Olympus, Tokyo, Japan) and also stained with TRAP solution.
Analysis of expression of integrins on c-Fms+RANK− cells
Primary mouse mononuclear cells or colonies derived from c-Fms+RANK− cells cultured with M-CSF and sRANKL for 6 days in methylcellulose medium were incubated with Fc block (concentration, 1:200) on ice for 15 minutes. Cells were stained with biotinylated anti-RANK antibody followed by APC-conjugated streptavidin (Caltag). To detect expression of integrins, cells were stained with hamster antimouse β3 (2C9.G2) α2 (HM-α2), and β1 (HM-β1-1) integrin antibodies (brand names in parentheses, all from PharMingen) and detected by phycoerythrin (PE)-conjugated antihamster cocktail IgG antibody (G70-204, G94-90.5, PharMingen). To detect the expression of αv integrin, cells were stained with PE-conjugated anti-αv antibody (H9.2B8, PharMingen). For isotype-matched controls, biotinylated rat IgG2a (R35-95, PharMingen) or hamster IgG (G235-2356, PharMingen) was used. The expression of integrins or RANK was analyzed on a FACS Calibur.
Reverse transcriptase–derived polymerase chain reaction analysis
Total RNA was isolated using RNeasy mini kit (Qiagen GmbH, Hilden, Germany) from freshly isolated c-Fms+RANK− cells or cells cultured with M-CSF and sRANKL in liquid or methylcellulose medium for 6 days. Single-strand complementary DNAs (cDNAs) were synthesized with a reverse transcriptase–polymerase chain reaction (RT-PCR) kit (Clontech Laboratories, Palo Alto, CA). The cDNAs were amplified using the SYBR Green PCR System (Perkin-Elmer, Norwalk, CT) in a GeneAmp PCR system (Model 9700, Perkin-Elmer) for 40 cycles. Each cycle consisted of 30 seconds denaturation at 94°C and 4 minutes annealing and/or extension at 70°C. G3PDH was used as an internal control. Primers used for RT-PCR were as follows: αv-5′; 5′-GCCTGGGATTGTAGAAGGA GGGCAAGTT-3′, αv-3′; 5′-GCTTGTGCAGTCCGTGTTGCTAATTGGT-3′, β3-5′; 5′-TGAAGAAACAGAGCGTGTCCCGTAATCG-3′, β3-3′; 5′-AGGTGGCATTGAA- GGA CAGTGACAGCTC-3′, α2-5′; 5′-GCTTTGGGGCAAGTGATTCAACT- CTGGT-3′, α2-3′; 5′-AGCTGTTGGACATCCAGGATCAGGTCAG-3′, β1-5′; 5′-GTGCACAGGAGTGCT C CC ACTTCAATCT-3′, β1-3′; 5′-GGCCACAGAGCCCCAAAACTACCCTACT-3′, α5-5′; 5′-AGAAACGAGAGGCTCCAGGGAGGAGTTC-3′, α5-3′; 5′-CATGAGTCTGA GATCAGGAGGGCTCAGG-3′, FAK (focal adhesion kinase)-5′; 5′-CCACCAGCAGCGAGGAAG- TAACCTTAGC-3′, FAK-3′; 5′-GCAACCCAATGCTTCAGTATCCACAGGA-3′, Pyk2 (proline-rich tyrosine kinase 2)-5′; 5′-GGAGCTCAGGCGGTTCTTCAAGGATATG-3′, Pyk2-3′; 5′-ATGAGGTCAGCCATGTTCTCAGCCTCTG-3′, CTR (calcitonin receptor)-5′; 5′-TGGTTGAGGTTGTGCCC- AATGGAGA-3′, CTR-3′; 5′-CTCGTGGGTTTGCCTCATCTTGGTC-3′, CD11b-5′; 5′-TCCTCTAGAGTCTTCCTGGACCACAGCA-3′, CD11b-3′; 5′-GATCTCTTTGTTGGGGACGTCACTGGTA-3′, c-Src-5′; 5′-TG- ATGTTATGAAGAACTGCTCGCACCTG-3′, c-Src-3′; 5′-TGCCCATTTGCTGGGTACTTTCTTTCTC-3′, G3PDH-5′; 5′-TGAAGGTCGGTGTGAACGGATTTGGC-3′, G3PDH-3′; and 5 ′-CATGTAGGCCATGAGGTCCACCAC-3′.
Proliferation analysis
We define the proliferative subpopulation of cultured c-Fms+RANK− cells by FACS. Freshly sorted c-Fms+RANK− cells (1 × 105) were plated on a fibronectin-coated 35-mm dish (Iwaki 4000-030; Iwaki, Chiba, Japan) and cultured in α-MEM containing 10% FBS with 100 ng/mL M-CSF alone or together with 25 ng/mL sRANKL for 6 days. Fibronectin-coated dishes were preincubated with 2% BSA/PBS for 30 minutes at 37°C. After 48 hours of incubation, cells were harvested and washed twice by cold PBS and suspended in lysing buffer comprising 0.5% TritonX-100 (Sigma), 1% BSA, and 0.2 μg/mL ethylenediamine tetraacetic acid (EDTA)/PBS. Cells were kept on ice for 15 minutes and fixed overnight in 85% methanol. Cells were collected and washed twice by 0.1% TritonX-100/PBS and stained with biotinylated anti–proliferating-cell nuclear antigen (anti-PCNA) monoclonal antibody (32552A, PharMingen) for 30 minutes on ice. After 2 washes, cells were incubated with APC-conjugated streptavidin (Caltag). After a final wash, cells were suspended with PBS, and ribonuclease (RNase) A type I-AS (Sigma) was added. After incubation for a few minutes, propidium iodine (PI) (Sigma) was added, and then FACS analysis was performed using a FACS Calibur.
Results
Isolation and characterization of osteoclast precursor cells
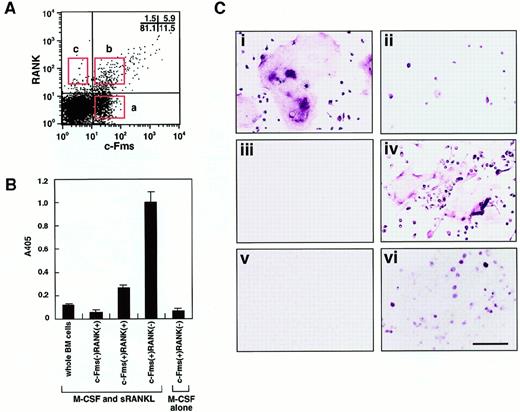
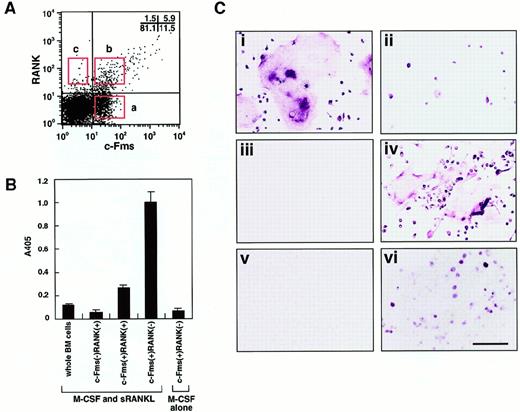
To isolate osteoclast precursor cells from murine bone marrow, cells were stained with monoclonal antibodies against a receptor of M-CSF (c-Fms) and a receptor of RANKL (RANK), and 3 populations were then fractionated by FACS on the basis of the expression of c-Fms and RANK. The frequency of each fraction was 11.5%±2.2% (c-Fms+RANK−), 5.9%±1.5% (c-Fms+RANK+), and 1.5%±0.6% (c-Fms−RANK+) in mononuclear murine bone marrow cells (Figure 1A).
Characterization of osteoclast precursor cells.
(A) Bone marrow mononuclear cells were subdivided into 3 fractions: c-Fms+RANK−, c-Fms+RANK+, and c-Fms−RANK+ cells. Isolated osteoclast precursor cells were cultured with M-CSF alone or M-CSF and sRANKL for 6 days and subjected to TRAP-solution assay (B) or TRAP staining (C). Note that the TRAP activity was greatest in c-Fms+RANK− cells cultured with M-CSF and sRANKL (panel B). Large numbers of multinuclear TRAP+ cells were observed among c-Fms+RANK− cells (i), but only mononuclear TRAP+ cells developed from c-Fms+RANK+ cells (ii), and c-Fms−RANK+ cells did not proliferate in this culture (iii). (iv) Many multinuclear TRAP+ cells were also observed when 10 times more c-Fms+RANK+ cells were cultured. (v) c-Fms+RANK− cells cultured with M-CSF alone were TRAP−. (vi) A significant reduction in the frequency of TRAP+ cells and multinuclear cell formation was observed in methylcellulose medium compared to liquid culture in the presence of M-CSF and sRANKL. The bar indicates 100 μm.
Characterization of osteoclast precursor cells.
(A) Bone marrow mononuclear cells were subdivided into 3 fractions: c-Fms+RANK−, c-Fms+RANK+, and c-Fms−RANK+ cells. Isolated osteoclast precursor cells were cultured with M-CSF alone or M-CSF and sRANKL for 6 days and subjected to TRAP-solution assay (B) or TRAP staining (C). Note that the TRAP activity was greatest in c-Fms+RANK− cells cultured with M-CSF and sRANKL (panel B). Large numbers of multinuclear TRAP+ cells were observed among c-Fms+RANK− cells (i), but only mononuclear TRAP+ cells developed from c-Fms+RANK+ cells (ii), and c-Fms−RANK+ cells did not proliferate in this culture (iii). (iv) Many multinuclear TRAP+ cells were also observed when 10 times more c-Fms+RANK+ cells were cultured. (v) c-Fms+RANK− cells cultured with M-CSF alone were TRAP−. (vi) A significant reduction in the frequency of TRAP+ cells and multinuclear cell formation was observed in methylcellulose medium compared to liquid culture in the presence of M-CSF and sRANKL. The bar indicates 100 μm.
To elucidate which cell fraction has the highest capacity to differentiate into osteoclasts, sorted cells were cultured in the presence of M-CSF and sRANKL, and TRAP activity was examined (Figure1B). The c-Fms+RANK− cells showed the highest TRAP activity among the 3 fractions and had 5-fold higher TRAP activity than c-Fms+RANK+ cells. However, when c-Fms+RANK− cells were cultured with M-CSF alone, little TRAP activity or no TRAP+ cells were detected (Figure 1C). These TRAP− cells were judged to be macrophages because they had a large amount of cytoplasm, strong phagocytic activity, and non-specific esterase activity. This result suggests that c-Fms+RANK− cells have the high capacity to differentiate into osteoclasts or macrophages via a stage when they first become c-Fms+RANK+. RANK was expressed on c-Fms+ RANK− cells during their cultivation in the presence of M-CSF alone or M-CSF and sRANKL within 24 hours.19
Multinucleation is a marker for osteoclast maturation along with positivity for TRAP staining. Multinuclear osteoclasts differentiated from c-Fms+RANK− cells, but only mononuclear TRAP+ cells differentiated from c-Fms+RANK+ cells (Figure 1Ci-ii). Neither TRAP+ nor multinucleated cells developed from c-Fms−RANK+ cells (Figure 1Ciii). Interestingly, c-Fms+RANK+ cells could differentiate into multinuclear TRAP+ cells, when 10-fold higher numbers of cells were cultured (Figure 1Civ). These results suggest that both c-Fms+RANK− cells and c-Fms+RANK+ cells have the potential to differentiate into mature multinuclear osteoclasts. However, as shown in Figure 1Ci-ii, the cell density was quite different between these 2 fractions on day 6 in the presence of M-CSF and sRANKL. c-Fms+RANK+ cells may have less ability to proliferate than c-Fms+RANK− cells, and cell density might be too low to undergo multinucleation.
To investigate the proliferative activity in each fraction, methylcellulose colony assays were performed (Table1). The c-Fms+RANK− cells had the greatest colony-forming ability in the presence of M-CSF with or without sRANKL, and only a few colonies developed from c-Fms+RANK+ or c-Fms−RANK+ cells. This result is consistent with the cell densities observed in liquid cultures containing M-CSF and sRANKL (Figure 1C). The c-Fms+RANK− cells were used for further analyses as osteoclast precursor cells because of their high capacity for differentiating into multinuclear osteoclasts.
Analysis of differentiation of osteoclast precursor cells cultured in methylcellulose or liquid medium
To examine how adherence affects osteoclast development, isolated osteoclast precursor cells (c-Fms+RANK− cells) were assayed in methylcellulose and liquid cultures. Interestingly, cell fusion was not observed in the methylcellulose cultures containing M-CSF and sRANKL even though the cells were already present in aggregates within each colony, whereas these same cytokines were able to stimulate multinuclear osteoclast formation in liquid culture. Approximately 50 colonies derived from c-Fms+RANK− cells cultured in methylcellulose medium in the presence of M-CSF and sRANKL were removed individually and stained with TRAP on day 6 (Figure 1Cvi). A large number of TRAP− cells were observed in all colonies, and 2.7% to 87.5% (mean ± SD: 23.4%±17.8%) of cells in each colony were TRAP+. These results suggest that cell adhesion together with M-CSF and sRANKL play an important role in osteoclast development. RT-PCR analysis revealed that messenger RNA (mRNA) for the calcitonin receptor (CTR), TRAP, and c-Src could be detected in pooled colony cells as well as in cells produced in liquid culture (data not shown). These results indicate that the cells can commit to the osteoclast lineage in methylcellulose cultures.
Analysis of the differentiation ability of cells cultured in methylcellulose medium
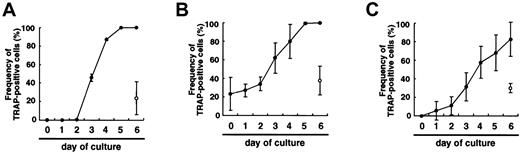
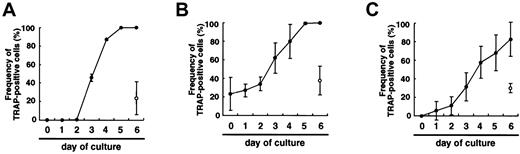
If the differentiation was just delayed in methylcellulose medium compared to liquid culture, prolonged culture might induce differentiation of osteoclasts, as observed in the liquid medium. To test this possibility, single colonies derived from osteoclast precursor cells cultured in methylcellulose medium were transferred to secondary liquid or methylcellulose medium in the presence of M-CSF and sRANKL, and the frequency of TRAP+ cells was then determined at daily intervals. This showed that the frequency of TRAP+ cells increased in liquid culture from day 2 and reached 100% by day 5 (Figure 2A). In contrast, only 24%±18% of cells subcultured in the presence of M-CSF and sRANKL in methylcellulose were TRAP+ on day 6. Next, 50 colonies were removed from each dish, and the frequency of TRAP+ cells was determined. When the cells cultured in methylcellulose were transferred into liquid medium, the frequency of TRAP+ cells increased and reached a plateau on day 5 (Figure 2B), and multinuclear osteoclasts were observed in the presence of M-CSF and sRANKL from day 2. In contrast, when the cells cultured in methylcellulose were transferred into methylcellulose medium again, the frequency of TRAP+ cells did not change, and multinuclear cells were not observed in methylcellulose with M-CSF and sRANKL (Figure 2B). To confirm these results, the same experiments were performed using secondary colonies. As expected, the results were the same as the first experiments (data not shown). Moreover, 12- to 18-day culture in methylcellulose culture with M-CSF and sRANKL did not increase the frequency of TRAP+ cells. These results demonstrate that the differentiation of osteoclasts is not delayed in a nonadherent environment, but it is arrested. However, the cells in methylcellulose still have the ability to differentiate into osteoclasts.
Comparison of osteoclast development in liquid culture and in methylcellulose medium.
(A) Time course of the change in frequency of TRAP+ cells when c-Fms+RANK− cells were cultured with M-CSF and sRANKL in liquid (●) or methylcellulose medium (○) for 6 days. (B) Colonies cultured with M-CSF and sRANKL in methylcellulose were replated into liquid (●) or methylcellulose (○) and cultured with M-CSF and sRANKL for 6 days. Note that the frequency of TRAP+ cells increased in liquid culture but not in methylcellulose culture. (C) Colonies cultured with M-CSF alone were replated into liquid (●) or methylcellulose (○) individually and further cultured with M-CSF and sRANKL. Note that the cells in methylcellulose medium cultured with M-CSF alone still had a potency to differentiate into TRAP+ cells, especially in liquid culture.
Comparison of osteoclast development in liquid culture and in methylcellulose medium.
(A) Time course of the change in frequency of TRAP+ cells when c-Fms+RANK− cells were cultured with M-CSF and sRANKL in liquid (●) or methylcellulose medium (○) for 6 days. (B) Colonies cultured with M-CSF and sRANKL in methylcellulose were replated into liquid (●) or methylcellulose (○) and cultured with M-CSF and sRANKL for 6 days. Note that the frequency of TRAP+ cells increased in liquid culture but not in methylcellulose culture. (C) Colonies cultured with M-CSF alone were replated into liquid (●) or methylcellulose (○) individually and further cultured with M-CSF and sRANKL. Note that the cells in methylcellulose medium cultured with M-CSF alone still had a potency to differentiate into TRAP+ cells, especially in liquid culture.
When osteoclast precursor cells were cultured in the presence of M-CSF alone, there were no TRAP+ cells observed in either liquid or methylcellulose cultures. When single colonies from methylcellulose cultures containing only M-CSF were transferred to secondary liquid cultures containing M-CSF and sRANKL, the frequency of TRAP+ cells increased, and multinuclear cells were also formed. In contrast, when the cells were transferred into secondary methylcellulose cultures, approximately 30% of cells became TRAP+ in the presence of M-CSF and sRANKL (Figure 2C). Repeated analyses using single colonies demonstrated that an adherent environment is crucial for osteoclast differentiation. Cells cultured with M-CSF alone or with M-CSF and sRANKL in methylcellulose for 12 days were still able to differentiate into multinuclear TRAP+ cells when transferred to liquid cultures containing M-CSF and sRANKL.
Comparison of osteoclast differentiation in the presence of leucine zipper sRANKL and sRANKL
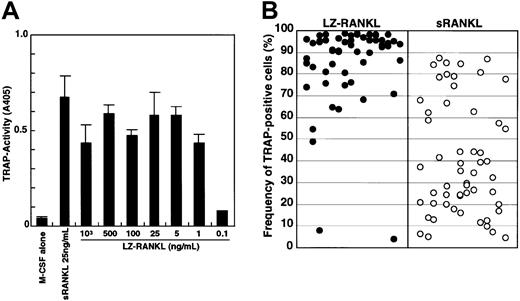
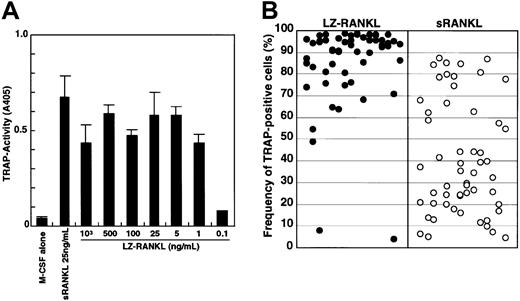
To exclude the possibility that differentiation of osteoclasts in methylcellulose culture might be suppressed because of insufficient differentiation signals, we added the leucine zipper form of sRANKL (LZ-RANKL), which is thought to facilitate and stabilize oligomerization, and determined the frequency of TRAP+cells. First, the dose-dependency of LZ-RANKL was examined in liquid culture by comparison with sRANKL (Figure3A). TRAP activity reached a plateau at a concentration of 1 ng/mL LZ-RANKL, whereas it peaked at 25 ng/mL sRANKL. Second, we examined how LZ-RANKL affects the differentiation of osteoclast precursor cells when sufficient LZ-RANKL is added to methylcellulose culture (Figure 3B). Although, there was no significant difference observed between 25 ng/mL sRANKL and LZ-RANKL in liquid culture, a difference was observed in methylcellulose culture. Although the frequency of TRAP+ cells varied in each colony, it was much higher (63% ± 27% [mean ± SD]) in the presence of 25 ng/mL LZ-RANKL compared with cultures containing 25 ng/mL sRANKL (32% ± 24%). Multinuclear TRAP+ cells (1%) were formed even in methylcellulose medium with LZ-RANKL, but no multinucleation was observed with sRANKL (data not shown).
Analysis of the effect of LZ-RANKL or sRANKL on the differentiation of osteoclast precursor cells.
(A) The dose-dependency of LZ-RANKL was examined by measuring TRAP activity in liquid culture. (B) c-Fms+RANK−cells were cultured in methylcellulose in the presence of 1000 ng/mL LZ-RANKL or 25 ng/mL sRANKL with 100 ng/mL M-CSF for 6 days. Then single colonies were collected, and the frequency of TRAP+cells was examined.
Analysis of the effect of LZ-RANKL or sRANKL on the differentiation of osteoclast precursor cells.
(A) The dose-dependency of LZ-RANKL was examined by measuring TRAP activity in liquid culture. (B) c-Fms+RANK−cells were cultured in methylcellulose in the presence of 1000 ng/mL LZ-RANKL or 25 ng/mL sRANKL with 100 ng/mL M-CSF for 6 days. Then single colonies were collected, and the frequency of TRAP+cells was examined.
These results indicate that the RANK signal is reduced in cells maintained under nonadherent conditions compared to when they are adherent, although a signal that is strong enough can support osteoclast differentiation even under nonadherent conditions. In the presence of 1000 ng/mL LZ-RANKL, the frequency of TRAP+cells increased markedly (85% ± 19%), and the number of multinuclear cells increased proportionately. However, the number of nuclei in the multinuclear TRAP+ cells was still lower in the methylcellulose cultures (nuclei per cell, 7% ± 4%; n = 448 cells) than in the liquid cultures (nuclei per cell, 12% ± 14%; n = 466 cells), and no multinuclear cells possessing more than 25 nuclei were detected (data not shown).
Bifurcated differentiation of osteoclast precursor cells in vitro
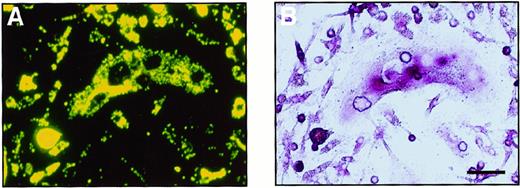
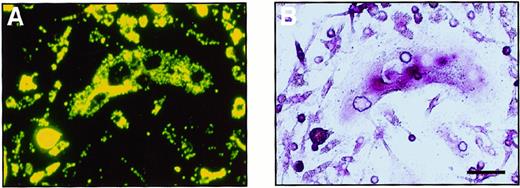
To investigate whether the cells cultured in methylcellulose are able to differentiate into monocyte-macrophages, the ability to phagocytose (which is a characteristic of macrophages) was examined. Colonies cultured with M-CSF alone or M-CSF and sRANKL were collected and cultured with fluorescence-labeled latex beads. After a 4-hour incubation with the beads, most of the cells could be seen to have phagocytosed some beads. Further cultivation was performed with M-CSF and sRANKL to examine whether cells carrying latex beads are able to differentiate into osteoclasts (Figure4). A large number of cells containing latex beads became TRAP+ after 6 days of cultivation, and some were multinuclear. On RT-PCR analysis, cells cultured in methylcellulose for 6 days expressed not only CTR and c-Src mRNA, which is characteristic of osteoclasts, but also CD11b mRNA, which is characteristic of monocyte-macrophages (data not shown). Thus, it is suggested that once osteoclast precursor cells adhere, they can differentiate into osteoclasts even after they have shown an ability to phagocytose.
Bipotential differentiation activity of osteoclast precursor cells cultured in methylcellulose.
A cultured osteoclast visualized (A) with a fluorescence microscopy and (B) by TRAP staining. After 6 days of culture with M-CSF and sRANKL, c-Fms+RANK− cells were incubated with fluorescence-labeled latex beads for 4 hours. After removal of the free latex beads, cells were cultured with M-CSF and sRANKL for 6 days. Note that precursor cells containing latex beads were able to differentiate into multinuclear osteoclasts that were positive for TRAP. The bar indicates 50 μm.
Bipotential differentiation activity of osteoclast precursor cells cultured in methylcellulose.
A cultured osteoclast visualized (A) with a fluorescence microscopy and (B) by TRAP staining. After 6 days of culture with M-CSF and sRANKL, c-Fms+RANK− cells were incubated with fluorescence-labeled latex beads for 4 hours. After removal of the free latex beads, cells were cultured with M-CSF and sRANKL for 6 days. Note that precursor cells containing latex beads were able to differentiate into multinuclear osteoclasts that were positive for TRAP. The bar indicates 50 μm.
Analysis of expression of integrins in cultured osteoclast precursor cells
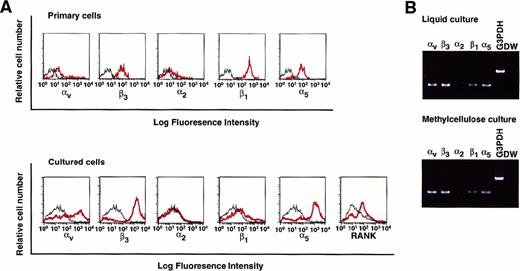
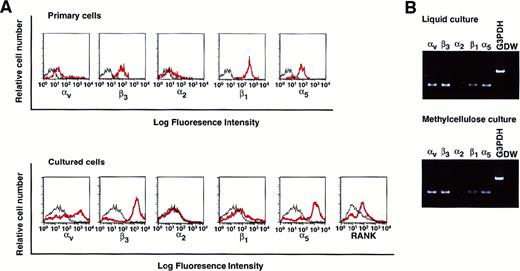
To elucidate whether isolated osteoclast precursor cells express integrins, the expression of various integrins such as αv, β3, α2, β1, and α5 on c-Fms+RANK− cells was analyzed. All tested integrins, especially those that recognize the RGD motif, were expressed on c-Fms+RANK− cells (Figure 5A, top). Colonies derived from c-Fms+RANK− cells cultured with both M-CSF and sRANKL in methylcellulose were collected, and the expression of integrins or RANK was examined (Figure 5A, bottom). Expression of αv, β3, and α5, as well as RANK, were up-regulated during cultivation even in methylcellulose medium, and the expression patterns of the integrins were similar to those for liquid culture, as revealed by RT-PCR (Figure 5B).
Analysis of the expression of various integrins on freshly isolated c-Fms+RANK− cells or cultured cells.
(A) Freshly isolated c-Fms+RANK− cells or colony cells derived from c-Fms+RANK− cells cultured with M-CSF and sRANKL for 6 days were stained with anti-αvβ3, anti-α2, anti-β1, and anti-α5 integrin antibodies or anti-RANK antibody, and expressions of integrins were analyzed by FACS. Red lines show the expression of integrins or RANK, and black lines show the isotype-matched control. (B) Expressions of αv, β3, α2, β1, and α5 integrins were examined by RT-PCR in cells cultured in liquid or methylcellulose for 6 days in the presence of M-CSF and sRANKL.
Analysis of the expression of various integrins on freshly isolated c-Fms+RANK− cells or cultured cells.
(A) Freshly isolated c-Fms+RANK− cells or colony cells derived from c-Fms+RANK− cells cultured with M-CSF and sRANKL for 6 days were stained with anti-αvβ3, anti-α2, anti-β1, and anti-α5 integrin antibodies or anti-RANK antibody, and expressions of integrins were analyzed by FACS. Red lines show the expression of integrins or RANK, and black lines show the isotype-matched control. (B) Expressions of αv, β3, α2, β1, and α5 integrins were examined by RT-PCR in cells cultured in liquid or methylcellulose for 6 days in the presence of M-CSF and sRANKL.
Because integrins were expressed on cells cultured in methylcellulose medium, a ligand of integrin, 0.5 mg/mL GRGDS peptide, was added to the methylcellulose culture. The number of colonies and frequency of TRAP+ cells were not affected by the addition of GRGDS peptide (data not shown). FAK and Pyk2 are known as signal transducers acting downstream of integrins,43,46 and Pyk2 has been reported to be expressed mostly in osteoclasts.45 It is possible that if these molecules were not expressed in cells cultured in methylcellulose, osteoclast differentiation would not be induced in methylcellulose, unlike induced differentiation in liquid culture. However, by RT-PCR analysis, the expression of both FAK and Pyk2 in methylcellulose culture seemed not to differ from that in liquid culture (data not shown). These results clearly indicate that the expression of integrins and their signal molecules are not affected by a nonadherent environment, and a ligand of integrin (GRGDS) does not affect osteoclast differentiation in spite of the expression of integrins on cells cultured in methylcellulose.
GRGDS peptide and antibody against αv or β3 integrin inhibit osteoclastogenesis in vitro
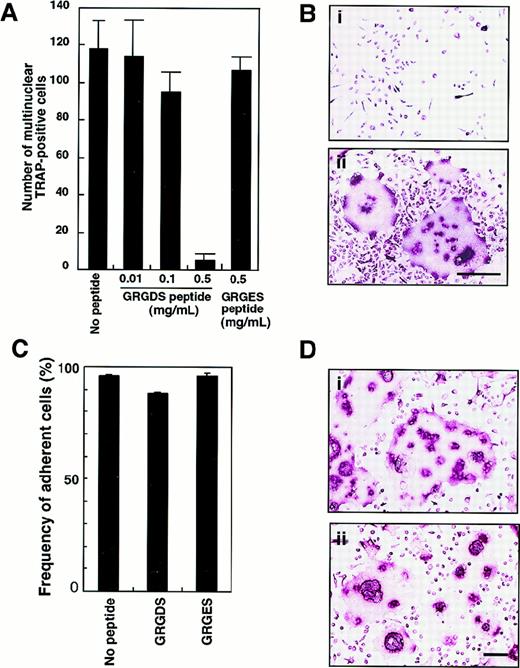
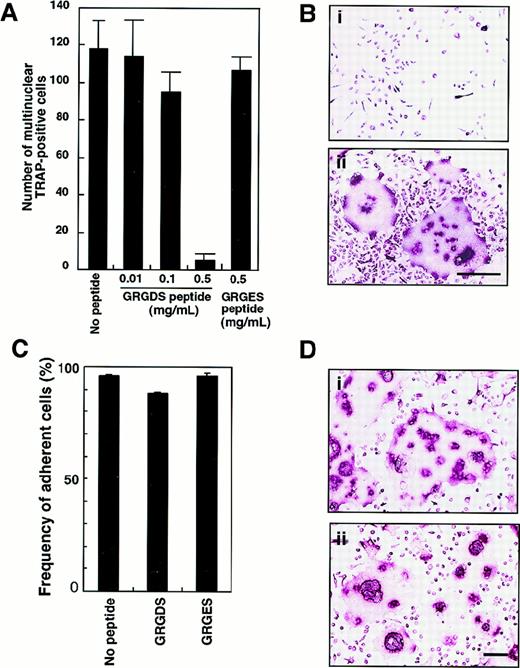
To confirm the role of adhesion molecules in osteoclast proliferation and/or differentiation in liquid culture, isolated c-Fms+RANK− cells were plated onto fibronectin-coated plates and cultured with 0.5 mg/mL GRGDS or GRGES peptide in the presence of M-CSF and sRANKL. The number of multinuclear TRAP+ cells was dose-dependently reduced after 6 days of cultivation by the addition of GRGDS peptide but not GRGES peptide (Figure 6A). Because GRGDS peptide was initially added to culture, it might affect initial cell attachment and inhibit osteoclast proliferation. Even when GRGDS peptide was added 24 hours after starting the cell culture, the number of multinuclear TRAP+ cells was reduced to 16% of the number seen in control cultures. Although the multinuclear cells were not seen, 100% of the cultured cells were TRAP+ in the presence of GRGDS peptide (Figure 6B). On the other hand, GRGES peptide did not affect multinuclear osteoclast formation at all (Figure 6A,B). To test the effect of the GRGDS peptide on cell adhesion, c-Fms+RANK− cells were cultured in the presence of M-CSF and sRANKL with GRGDS peptides for 8 hours, and then the frequency of adherent cells was scored. Compared with controls (no peptide or GRGES peptide), initial attachment was not affected by the presence of the GRGDS peptide (Figure 6C).
Inhibition of multinuclear osteoclast formation by GRGDS peptide.
(A) c-Fms+RANK− cells were cultured in the presence of M-CSF and sRANKL with various concentration of GRGDS peptide or 0.5 mg/mL GRGES peptide for 6 days and stained with TRAP. The number of multinuclear TRAP+ cells was scored. (B) Osteoclast precursor cells (× 103) were cultured in the presence of M-CSF and sRANKL with 0.5 mg/mL (a) GRGDS peptide or (b) GRGES peptide for 6 days and stained with TRAP. Note that all cells were TRAP+ in the presence of GRGDS peptide; however, a marked decrease in the cell density and multinuclear cells was observed with GRGDS. The bar indicates 50 μm. (C) c-Fms+RANK− cells were cultured in the presence of M-CSF and sRANKL with either 0.5 mg/mL GRGDS peptide, 0.5 mg/mL GRGES peptide, or without peptide for 8 hours, then the number of adherent cells was scored. Note that initial attachment was not affected by GRGDS peptide. (D) A large number of c-Fms+RANK− cells (1 × 104) were cultured in the presence of M-CSF and sRANKL with 0.5 mg/mL (a) GRGES peptide or (b) GRGDS peptide for 4 days. The cell fusion was observed even in the presence of GRGDS in this case. The bar indicates 100 μm.
Inhibition of multinuclear osteoclast formation by GRGDS peptide.
(A) c-Fms+RANK− cells were cultured in the presence of M-CSF and sRANKL with various concentration of GRGDS peptide or 0.5 mg/mL GRGES peptide for 6 days and stained with TRAP. The number of multinuclear TRAP+ cells was scored. (B) Osteoclast precursor cells (× 103) were cultured in the presence of M-CSF and sRANKL with 0.5 mg/mL (a) GRGDS peptide or (b) GRGES peptide for 6 days and stained with TRAP. Note that all cells were TRAP+ in the presence of GRGDS peptide; however, a marked decrease in the cell density and multinuclear cells was observed with GRGDS. The bar indicates 50 μm. (C) c-Fms+RANK− cells were cultured in the presence of M-CSF and sRANKL with either 0.5 mg/mL GRGDS peptide, 0.5 mg/mL GRGES peptide, or without peptide for 8 hours, then the number of adherent cells was scored. Note that initial attachment was not affected by GRGDS peptide. (D) A large number of c-Fms+RANK− cells (1 × 104) were cultured in the presence of M-CSF and sRANKL with 0.5 mg/mL (a) GRGES peptide or (b) GRGDS peptide for 4 days. The cell fusion was observed even in the presence of GRGDS in this case. The bar indicates 100 μm.
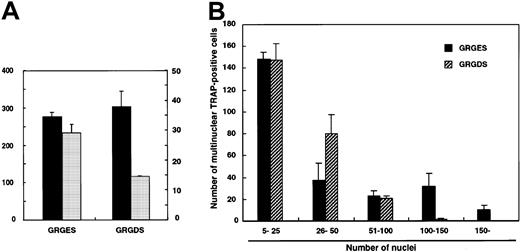
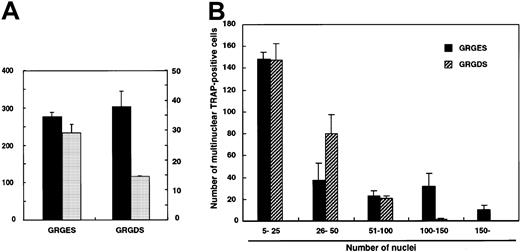
To investigate whether the GRGDS peptide affects cell fusion itself, larger numbers (104 cells per well) of c-Fms+RANK− cells were cultured with M-CSF and sRANKL. Multinuclear TRAP+ cells were observed even in the presence of GRGDS peptide (Figure 6D), and the number of multinuclear TRAP+ cells did not decrease (Figure7A). However, the size of the multinuclear TRAP+ cells cultured with GRGDS peptide was relatively small, and the average number of nuclei was reduced compared to that of the control cultures (Figure 7A). Similarly, the number of multinuclear giant cells containing more than 100 nuclei was significantly reduced in the presence of GRGDS peptide (Figure 7B). These results indicate that GRGDS peptide does not inhibit osteoclast formation by impairing initial cell attachment and differentiation into mononuclear or multinuclear TRAP+ cells and cell fusion, but rather the peptide inhibits osteoclast formation by decreasing the cell density.
Effect of GRGDS peptide or blocking antibody against αv integrin on cell-to-cell fusion.
Large numbers of c-Fms+RANK− cells (104 cells per well) were cultured in the presence of M-CSF and sRANKL with 0.5 mg/mL GRGDS or GRGES peptide, and TRAP staining was performed on day 4. (A) The numbers of multinuclear TRAP+cells and nuclei in multinuclear cells were determined. ▪, multinuclear TRAP-positive cells; ░, average number of nuclei. (B) Distribution of the number of nuclei in multinuclear TRAP+cells. Note that the number of giant cells containing more than 100 nuclei was significantly reduced in the presence of the GRGDS peptide compared with the control.
Effect of GRGDS peptide or blocking antibody against αv integrin on cell-to-cell fusion.
Large numbers of c-Fms+RANK− cells (104 cells per well) were cultured in the presence of M-CSF and sRANKL with 0.5 mg/mL GRGDS or GRGES peptide, and TRAP staining was performed on day 4. (A) The numbers of multinuclear TRAP+cells and nuclei in multinuclear cells were determined. ▪, multinuclear TRAP-positive cells; ░, average number of nuclei. (B) Distribution of the number of nuclei in multinuclear TRAP+cells. Note that the number of giant cells containing more than 100 nuclei was significantly reduced in the presence of the GRGDS peptide compared with the control.
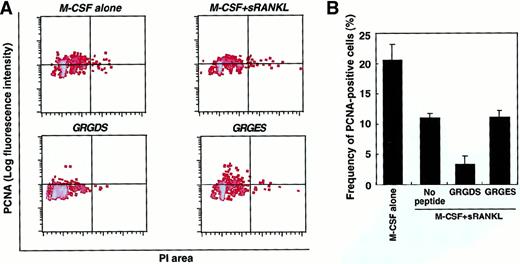
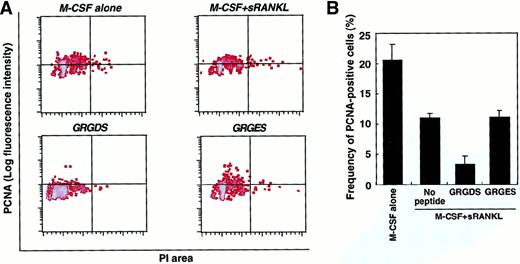
To analyze more directly whether the GRGDS peptide affects cell proliferation, cultured cells were harvested after a 48-hour exposure and stained with PI and PCNA (Figure 8). The proliferative activity of the osteoclast precursor cells was reduced to 50% by the addition of sRANKL compared to the culture with M-CSF alone. PCNA expression was markedly reduced by GRGDS peptide treatment (30% compared with GRGES treatment) in the presence of M-CSF and sRANKL. These results suggest that integrins that recognize RGD motif play an important role in regulating the proliferation of TRAP+ cells.
Proliferative activity of osteoclast progenitor cells.
(A) c-Fms+RANK− cells were cultured in the presence of M-CSF alone or M-CSF and sRANKL together with 0.5 mg/mL GRGDS or GRGES peptide for 48 hours. Then the DNA content (PI, x axis) and the expression of PCNA (y axis) were analyzed. (B) The frequency of PCNA+ cells was quantified by comparison with an isotype-matched control. Aggregated cells were excluded by PI gate. The percentage of PCNA+ cells was reduced in the presence of sRANKL and by addition of the GRGDS peptide.
Proliferative activity of osteoclast progenitor cells.
(A) c-Fms+RANK− cells were cultured in the presence of M-CSF alone or M-CSF and sRANKL together with 0.5 mg/mL GRGDS or GRGES peptide for 48 hours. Then the DNA content (PI, x axis) and the expression of PCNA (y axis) were analyzed. (B) The frequency of PCNA+ cells was quantified by comparison with an isotype-matched control. Aggregated cells were excluded by PI gate. The percentage of PCNA+ cells was reduced in the presence of sRANKL and by addition of the GRGDS peptide.
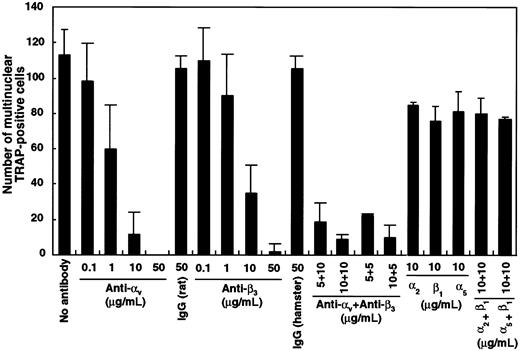
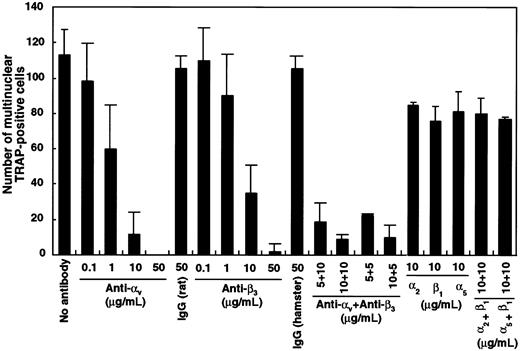
To investigate which integrin was critical, blocking antibodies against αv, β3, α2, β1, or α5 integrin were added singly or in combination to liquid cultures containing M-CSF and sRANKL. The number of multinuclear TRAP+ cells was dose-dependently reduced in the presence of antibodies against αv and β3 both alone and in combination (Figure 9). Interestingly, all cells cultured with blocking antibodies against integrins were TRAP+, the same as in cultures with GRGDS peptide (Figure6). Neither TRAP− cells nor floating cells were observed in this assay. Multinuclear formation was also inhibited by the addition of antibodies against αv or β3integrins when these were added after an initial period in culture of 24 hours. Formation of highly multinuclear osteoclasts was also affected by the addition of αv-blocking antibody.
Inhibition of multinuclear osteoclast formation by blocking antibody against αv and β3integrin.
c-Fms+RANK− cells (1 × 103) were cultured with M-CSF and sRANKL with blocking antibodies against various integrins. After 6 days of culture, the number of multinuclear TRAP+ cells was scored. Note that the number of multinuclear cells was significantly reduced in the presence of anti–αv- and/or anti–β3-blocking antibodies compared to isotype-matched control.
Inhibition of multinuclear osteoclast formation by blocking antibody against αv and β3integrin.
c-Fms+RANK− cells (1 × 103) were cultured with M-CSF and sRANKL with blocking antibodies against various integrins. After 6 days of culture, the number of multinuclear TRAP+ cells was scored. Note that the number of multinuclear cells was significantly reduced in the presence of anti–αv- and/or anti–β3-blocking antibodies compared to isotype-matched control.
Discussion
In this study we have shown that adhesion is required for osteoclast development, especially during the differentiation of osteoclast precursor cells into TRAP+ multinucleated cells. Osteoclast precursor cells prevented from adhering are unable to differentiate into multinuclear osteoclasts even in the presence of M-CSF and sRANKL, and their terminal differentiation can be arrested at a late stage when the cells may have characteristic features of both osteoclasts and macrophages. However, when transferred to conditions where they become adherent, further differentiation is induced and multinuclear osteoclasts develop. We have also shown that integrins which recognize the RGD motif are involved in the proliferation and multinuclear osteoclast formation.
There could be 2 explanations as to how osteoclast precursor cells would be maintained at an undifferentiated stage in methylcellulose medium: (1) Osteoclast precursor cells are not fully stimulated by a differentiation factor or RANKL, or (2) osteoclast precursor cells are delayed in their terminal differentiation in methylcellulose medium. To test the first possibility, trimerized RANKL (designated as LZ-RANKL) was added to methylcellulose medium instead of sRANKL. Ligands of the TNF receptor superfamily are known to form trimers, and trimerized ligands transduce signals more efficiently to the target cells by clustering of receptors.46 Stimulation of LZ-RANKL is more intense than that of the monomer form of sRANKL (D.M.A., unpublished data, 2000). Together with the shorter spacer region of LZ-RANKL, which begins at amino acid 138, compared to sRANKL, which begins at amino acid 76, LZ-RANKL induced the differentiation of TRAP+ cells more strongly than sRANKL in methylcellulose culture because the soluble form of RANKL containing a shorter length of the “spacer” region was more active than that of the longer one.5 Although LZ-RANKL induced the differentiation of TRAP+ cells more strongly than sRANKL in methylcellulose cultures, no pure osteoclast colonies were obtained, and the frequency of TRAP+ cells varied among the colonies. Even in methylcellulose medium with LZ-RANKL, no giant cells containing more than 25 nuclei were detected, in contrast to what was seen in liquid culture with sRANKL. This suggests that in a nonadherent environment, osteoclasts do not differentiate well, even if the osteoclast precursor cells are stimulated by LZ-RANKL. Trimerized RANKL may partially supplement the function of cell adhesion for efficient cell signaling.
To examine the second possibility, the effect of prolonged culture under nonadherent conditions on osteoclast differentiation was analyzed using twice serially passaged single colonies. Once replated into conditions that allowed the cells to become adherent, osteoclast precursor cells started to differentiate into TRAP+ cells in the presence of M-CSF and RANKL, and the frequency of TRAP+ cells eventually reached 100%. Although osteoclast precursor cells cultured with M-CSF plus RANKL and with M-CSF alone both generated TRAP+ cells after transfer into liquid cultures, those cells cultured with M-CSF alone took slightly longer to achieve complete differentiation (100% TRAP+ cells). Osteoclast precursor cells were thus maintained in M-CSF and were also able to differentiate into TRAP+ osteoclasts, but only if they were allowed to become adherent.
Because osteoclast precursor cells were thought to have the same ancestor as monocyte-macrophages, phagocytotic ability was examined in both primary cells and cells cultured in methylcellulose with M-CSF and sRANKL. Not only primary isolated osteoclast precursor cells but also cells cultured with M-CSF and sRANKL for 6 days took up fluorescence-labeled beads and then differentiated into TRAP+ multinuclear osteoclasts. This suggests that osteoclast precursor cells maintain the potential to differentiate into macrophages or osteoclasts in methylcellulose medium. Although freshly isolated c-Fms+RANK− cells expressed CD11b, a marker specific for monocyte-macrophage–lineage cells, they did not express mature osteoclast markers such as CTR or c-Src. However, initially, c-Fms+RANK− cells did begin to express these markers after 2 days of cultivation in methylcellulose with M-CSF and RANKL even though they were still CD11b+(data not shown). Thus, osteoclast precursor cells cultured in methylcellulose have the potential to differentiate into macrophages or osteoclasts, and terminal osteoclast differentiation may be anchorage-dependent. Consistent with this hypothesis, when osteoclast precursor cells were cultured on petri (nonculture) dishes, little development was observed (data not shown), suggesting that extracellular matrices are a key regulator for osteoclastogenesis.
Because integrins, especially the RGD-recognizing integrins αv, β3, α5, and β1, are expressed by osteoclast precursor cells, it was of interest to determine whether the GRGDS peptide, which is the active portion of osteopontin, fibronectin, or vitoronectin, would affect osteoclast differentiation in culture. When the GRGDS peptide was added to liquid cultures, osteoclast multinucleation was significantly inhibited, but no effect was observed when the same cells were cultured in methylcellulose. This suggests that the GRGDS peptide can affect anchorage-dependent osteoclast precursor proliferation. It is still not clear whether the GRGDS peptide or blocking αv or β3 antibodies directly affect the formation of multinuclear cells. We speculate that the GRGDS peptide can suppress cell proliferation rather than cell adhesion and differentiation for the following reasons. Approximately 90% of precursor cells were able to adhere to fibronectin-coated plates even in the presence of GRGDS peptide (Figure 6). Moreover, this inhibitory effect on multinuclear formation was observed when addition of the GRGDS peptide was delayed for 24 hours after the cells had been allowed to adhere to the dishes, which suggests that integrins are not involved in the initial attachment of the precursor cells. Ten times more plated osteoclast precursor cells could induce multinuclear cell formation even in the presence of GRGDS peptide, indicating that GRGDS peptide is not involved in cell fusion. However, the population of PCNA+proliferating cells was reduced by the addition of GRGDS peptide. Thus, suppression of multinuclear cell formation by GRGDS peptide or blocking antibodies is caused by inhibition of osteoclast precursor proliferation resulting in an insufficient density of TRAP+osteoclasts in the well. The αv and β3blocking antibodies significantly reduced the number of multinuclear cells compared to other integrins, even though α5 and β1 were highly expressed in osteoclast precursor cells. We conclude that αv and β3 are the most important integrins for enhancing osteoclast precursor proliferation and bone resorption.
It has been reported that adhesion or ligation of integrins is involved in activation of the MAP kinase pathway via activation of growth factor receptors, such as endothelial cell, platelet-derived, or fibroblast growth factor (ECGF, PDGF, or FGF, respectively) receptors, and this activation is involved in cell survival and proliferation.47-49 This may be relevant to the finding here that osteoclast precursor proliferation was reduced in the presence of GRGDS peptide, whereas some other mechanism might be involved in suppression of multinucleation. RGD peptides or peptides containing the RGD motif were also reported to induce the detachment35 or apoptosis50 of cultured cells. Echistatin, which contains the RGD motif, inhibits osteoclast multinucleation by impairing the cell migration but not the fusion process itself.36
We propose a model in which osteoclast differentiation proceeds through 2 stages. The first is represented by bipotential precursor cells that can proliferate in an adherence-independent manner. The second is represented by osteoclast-committed precursor cells whose subsequent differentiation is adherence-dependent. The differentiation of osteoclast precursor cells is arrested in a nonadherent environment, and when the cells are transferred into an adherent environment, differentiation proceeds in order to allow the appearance of mature osteoclasts. Integrins, especially the RGD-recognizing integrins αv and β3, are important for the proliferation of osteoclast precursor cells in an adherent environment to form multinuclear cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Toshio Suda, Department of Cell Differentiation, Institute of Molecular Embryology and Genetics (IMEG), Kumamoto University School of Medicine, 2-2-1 Honjo, Kumamoto 860-0811, Japan; e-mail: sudato@gpo.kumamoto-u.ac.jp.