Abstract
The roles of the protein tyrosine kinases Pyk2 (also called RAFTK or CAK β) and Syk in the process of functional activation of human myeloid cells were examined. During granulocytic differentiation of HL-60 cells with dimethyl sulfoxide (DMSO), the amounts of Pyk2 and β2 integrin increased, whereas the amount of Syk was abundant before differentiation and did not change during differentiation. When the granulocytic cells were stimulated withN-formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP), tyrosine phosphorylation of Pyk2 occurred promptly and subsequent association of Pyk2 with β2 integrin was detected. In contrast, Syk was not tyrosine phosphorylated by fMLP stimulation but constitutively associated with β2 integrin. Stimulation with fMLP also caused the alteration of β2 integrin to an activated form, a finding that was confirmed by the observation of fMLP-induced cell attachment on fibrinogen-coated dishes and inhibition of this attachment by pretreatment with anti-β2 integrin antibody. Cell attachment to fibrinogen caused the enhanced tyrosine phosphorylation of Pyk2 and the initial tyrosine phosphorylation of Syk, which was also inhibited by pretreatment with anti-β2 integrin antibody. In vitro kinase assays revealed that Pyk2 and Syk represented kinase activities to induce tyrosine phosphorylation of several molecules in the anti-β2 integrin immunoprecipitates of the attached cells. These results showed that Pyk2 is involved in the functional activation of granulocytic cells in 2 signaling pathways: an fMLP receptor–mediated “inside-out” signaling pathway that might cause β2 integrin activation and a subsequent β2 integrin–mediated “outside-in” signaling pathway. Syk was activated in relation to cell attachment to fibrinogen as a result of “outside-in” signaling, although it was already associated with β2 integrin before fMLP stimulation.
Introduction
Several signaling molecules play important roles in the differentiation and execution of unique functions of hematopoietic cells. As a useful model of myeloid differentiation, the HL-60 cell line has been used extensively to study these roles. HL-60 cells can be differentiated with various inducing agents, and HL-60 cells treated this way have the properties of mature myeloid cells.1 TheN-formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP) receptor belongs to the family of G protein–coupled, heptahelical receptors and is one of the first markers to appear during differentiation of myeloid cells. The ligand, fMLP, mediates effects by means of its receptor, which leads to intracellular signaling followed by cell adhesion and migration.
The well-characterized cell-surface receptors for extracellular matrix proteins on neutrophils belong to the β2 integrin family. Like other integrin families, the β2 integrin family exists as an αβ heterodimer in which a unique α subunit is associated with a common β subunit. Mac-1, LFA-1, and p150/95, which have αM, αL, and αX subunits, respectively, comprise the β2 integrin family.2 They have unique ligands, such as intracellular adhesion molecule-1 and fibrinogen (Fg), and in general, integrin-mediated cell adhesion to its ligands initiates a signaling pathway that mediates protein tyrosine phosphorylation and reorganization of the cytoskeleton.3
Various protein tyrosine kinases (PTKs) have been reported to be involved in the differentiation and functions of myeloid cells. The roles of Src family PTKs have been well investigated,4-8 but only a few studies of Syk9,10 and Pyk2 have been done.11Pyk2,12 which is also called RAFTK13 or CAK β,14 was isolated as the second member of the FAK family of PTKs. Pyk2 is highly expressed in cells of the central nervous system12-14 and hematopoietic lineages15 and is involved in various signaling pathways, including integrins,15-20 G protein–coupled receptors,12,21-26 various cytokines,27,28stress signaling,29 Jak-mediated signaling,30Fcε receptor I,31 and T-cell receptor.32Syk, a cytoplasmic PTK, is expressed in almost all hematopoietic cells.33,34 Studies have indicated that Syk plays important roles in neutrophils,9,10 monocytic cells,35 platelets,36 mast cells,37 and B cells38 and may be involved in β1,35 β2,9,10 and β336,39 40 integrin-mediated signaling.
We treated HL-60 cells with dimethyl sulfoxide (DMSO) to induce differentiation toward granulocytes. The granulocytic HL-60 cells were then stimulated with fMLP and attached to Fg, one of the ligands for β2 integrin. During this process, we examined expression, tyrosine phosphorylation, and several actions of Pyk2 and Syk.
Materials and methods
Reagents and antibodies
Fg was obtained from Yoshitomi Pharmaceutical Co (Osaka, Japan). Fetal-calf serum (FCS) was purchased from Gibco Laboratories (Grand Island, NY). DMSO and fMLP were from Sigma (St Louis, MO). Pertussis toxin (PT) was from Seikagaku Co (Tokyo, Japan), and bovine serum albumin (BSA) was from Intergen Co (Purchase, NY). Polyvinylidene difluoride (PVDF) was from Millipore (Bedford, MA), and protein A–Sepharose beads were from Amersham Pharmacia Biotech Inc (Uppsala, Sweden). Rabbit anti-fMLP receptor polyclonal antibody was previously generated.41 Antiphosphotyrosine monoclonal antibody 4G10 was obtained from Upstate Biotechnology Inc (Lake Placid, NY). Anti-β2 integrin monoclonal antibody IB4 for immunoprecipitation and blocking experiments was from Ancell Co (Bayport, MN). The following antibodies were products of Santa Cruz Biotechnology (Santa Cruz, CA): goat anti-Pyk2 polyclonal antibody for both immunoblotting and immunoprecipitation (SC-1515), rabbit anti-Syk polyclonal antibody for immunoblotting (SC-1077) and immunoprecipitation (SC-929 or SC-573), goat anti-β2 integrin polyclonal antibody for immunoblotting (SC-6623), normal mouse IgG for immunoprecipitation and blocking experiments (SC-2025), and normal goat IgG for immunoprecipitation (SC-2028).
Cell culture and differentiation-induction studies
HL-60 cells were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 2 mmol/L l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin in 5% carbon dioxide (CO2) humidified air at 37°C. The cells were induced to undergo differentiation to granulocytes by seeding them on dishes at a concentration of 5 × 105 cells/mL in the presence of 1.25% DMSO. Cell differentiation was confirmed morphologically by evaluating cytospin preparations stained with May-Grünwald-Giemsa solution.
Cell stimulation and adhesion assays
Various concentrations of fMLP were added to HL-60 cell suspensions. In some experiments, HL-60 cells were pretreated with either 20 μg/mL mouse anti-β2 integrin monoclonal antibody IB4 for 45 minutes on ice, 20 μg/mL normal mouse IgG for 45 minutes on ice, or 50 ng/mL PT for 4 hours at 37°C in polypropylene tubes.
For adhesion assays, 100-mm culture dishes (3020-100; Iwaki Glass Co, Chiba, Japan) were precoated with Fg (100 μg/mL; 10 mL/dish) overnight at 4°C. Nonspecific binding was blocked with 5% BSA for 1 hour at 37°C. After the BSA was removed, the dishes were washed once gently with PBS and 5 × 106 cells were seeded on the dishes in a 10-mL volume of culture medium. After incubation with 1 μmol/L fMLP for 30 minutes at 37°C, the cells were harvested for cell lysate preparation and cell counting.
Immunoprecipitation procedures
Cells in suspension in polypropylene tubes (5 × 106 cells) were stimulated with fMLP for various times and spun down by centrifugation (10 000g in a flash at 4°C). The incubation medium was removed by aspiration. The pelleted cells were then lysed in 1 mL nonionic lysis buffer (1% Triton X-100, 50 mmol/L Tris–hydrochloric acid [HCl] at pH 7.4, 150 mmol/L sodium chloride (NaCl), 5 mmol/L EDTA, 1 mmol/L sodium vanadate, and 1 mmol/L phenylmethyl sulfonyl fluoride) and kept on ice for 15 minutes. Cells that detached spontaneously or failed to attach after the stimulation were collected and resuspended in polypropylene tubes; these cells were handled in the same manner as cells in suspension. Cells that attached to Fg-coated dishes (5 × 106 cells) on stimulation with fMLP were lysed directly in 1 mL lysis buffer and disrupted by scraping. The lysates were transferred to polypropylene tubes, kept on ice for 15 minutes, and then centrifuged at 15 000g for 10 minutes at 4°C. The supernatants were incubated with 2.5 μg of an antibody for 1 hour at 4°C, and 20 μL protein A–Sepharose diluted in PBS was added. After another hour at 4°C, the immunoprecipitates were washed 3 times with 1 mL lysis buffer. For immunoblotting, the immunoprecipitates were boiled with sodium dodecyl sulfate (SDS) sample buffer (2% SDS, 10% glycerol, 5% 2-mercaptoethanol, 0.001% bromophenol blue, and 62.5 mmol/L Tris-HCl [pH 6.8]) for 3 minutes.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting analyses
For day-course studies to detect expression of proteins, whole cell lysates were prepared by boiling with SDS sample buffer for 3 minutes. Whole cell lysates or immunoprecipitates were separated by SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with 5% skim milk in low-salt Tween 20-Tris-buffered saline (T-TBS) (10 mmol/L Tris-HCl [pH 7.4] and 100 mmol/L NaCl containing 0.1% Tween 20) for 30 minutes at 37°C. This was followed by incubation with primary antibodies at either 0.1 μg/mL (for anti-Pyk2, anti-Syk, and anti-β2 integrin antibodies) or 1 μg/mL (for antiphosphotyrosine monoclonal antibody 4G10) in low-salt T-TBS for 1 hour at room temperature. The membranes were then washed and incubated with horseradish peroxidase–conjugated secondary antibodies at 0.5 μg/mL in low-salt T-TBS for 30 minutes at room temperature. After washing with low-salt T-TBS and subsequently with low-salt TBS (10 mmol/L Tris-HCl [pH 7.4] and 100 mmol/L NaCl), enhanced chemiluminescence (ECL) assays were performed to visualize positive bands on x-ray films.
In vitro kinase assays
Immunoprecipitates were washed 3 times with 1 mL lysis buffer and incubated in a 20-μL reaction mixture (50 mmol/L HEPES–sodium hydroxide [pH 8.0], 10 μmol/L sodium vanadate, 50 mmol/L magnesium acetate, 150 mmol/L NaCl, and 10 μmol/L adenosine triphosphate [ATP]) for 30 minutes at 30°C. The reaction was terminated by adding SDS sample buffer and boiling for 3 minutes. The samples were separated by SDS-PAGE, transferred to PVDF membranes, and subjected to immunoblotting analysis with antiphosphotyrosine antibody. The increase in tyrosine phosphorylation was detected by the ECL assay.
Results
Expression of Pyk2 and Syk during granulocytic differentiation of HL-60 cells
HL-60 cells can be induced to differentiate by various agents, including DMSO, which induces these cells to differentiate toward granulocytes.1 We first examined the expression of Pyk2 and Syk during DMSO-induced differentiation of HL-60 cells by using immunoblotting analyses. The expression Pyk2 was low in undifferentiated cells, but an apparent induction occurred on day 1 and reached the peak level in differentiated cells on day 4 (Figure1A). On the other hand, Syk was already abundant in undifferentiated cells, and the expression level did not change during differentiation (Figure 1B). Morphologic evaluation with May-Grünwald-Giemsa staining indicated that about 90% of the cells differentiated toward granulocytes after exposure to DMSO for 4 days. Our immunoblotting analysis did not detect expression of FAK during granulocytic differentiation of HL-60 cells (data not shown).
Day-course study of Pyk2 and Syk induction in HL-60 cells in the presence of dimethyl sulfoxide (DMSO).
HL-60 cells were treated with 1.25% DMSO for the indicated days, and whole cell lysates were subjected to immunoblotting analysis with anti-Pyk2 (A) or anti-Syk (B) polyclonal antibody. In each lane, the equivalent amounts of protein (8.5 μg) from whole cell lysates were loaded. In each panel, the positions of molecular markers are shown on the left in kilodaltons. Arrows indicate the position of a Pyk2 (A) or Syk (B) protein band.
Day-course study of Pyk2 and Syk induction in HL-60 cells in the presence of dimethyl sulfoxide (DMSO).
HL-60 cells were treated with 1.25% DMSO for the indicated days, and whole cell lysates were subjected to immunoblotting analysis with anti-Pyk2 (A) or anti-Syk (B) polyclonal antibody. In each lane, the equivalent amounts of protein (8.5 μg) from whole cell lysates were loaded. In each panel, the positions of molecular markers are shown on the left in kilodaltons. Arrows indicate the position of a Pyk2 (A) or Syk (B) protein band.
Tyrosine phosphorylation of Pyk2 on stimulation with fMLP
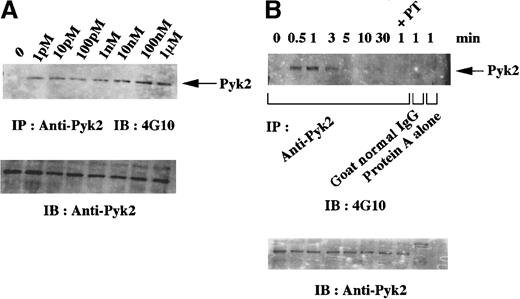
Previous studies indicated that the number of fMLP receptors and the ability to respond to fMLP increase in DMSO-differentiated granulocytic HL-60 cells.42,43 These results were confirmed in our experiments by immunoblotting and flow cytometric analyses with anti-fMLP receptor polyclonal antibody (data not shown). We then assessed whether Pyk2 is involved in signaling by means of the fMLP receptor in granulocytic cells. Figure2 shows results of dose-dependence and time-course studies of tyrosine phosphorylation of Pyk2 on stimulation with fMLP. Tyrosine phosphorylation of Pyk2 was detected in the presence of as little as 1 pmol/L fMLP and depended on the amount of fMLP used, with the maximal level reached at an fMLP concentration of 100 nmol/L (Figure 2A). After stimulation with 1 μmol/L fMLP (Figure2B), an increase in tyrosine phosphorylation of Pyk2 was detected at 30 seconds, reached the maximal level at 1 minute, and then gradually returned to the baseline level. Tyrosine phosphorylation was not detected in the immunoprecipitates with normal goat IgG or those with protein A alone (Figure 2B). To confirm that Pyk2 is involved in fMLP receptor–mediated signaling, an effect of PT, which inhibits the signaling of the Gi-protein α subunit by ADP ribosylation in HL-60 cells,44 was examined. Pretreatment with PT for 4 hours, which was sufficient to inhibit the signal mediated by the fMLP receptor,45 inhibited tyrosine phosphorylation of Pyk2 (Figure 2B). These data indicate that Pyk2 is involved in fMLP receptor–mediated signaling.
Dose dependence and time-course studies of tyrosine phosphorylation of Pyk2 on stimulation with
N-formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP) in granulocytic cells. HL-60 cells were treated with 1.25% DMSO for 4 days, and the differentiated (granulocytic) cells were then incubated in suspension in polypropylene tubes for 1 minute with the indicated concentrations of fMLP (A) or for the indicated times with 1 μmol/L fMLP (B). Lysates from the equivalent number of cells (5 × 106 cells) were immunoprecipitated with anti-Pyk2 polyclonal antibody (A) or with anti-Pyk2 polyclonal antibody, normal goat IgG, or protein A alone (B). In the experiment shown in Figure 2B, an aliquot of cells was pretreated with pertussis toxin (PT) before stimulation with fMLP. Immunoblotting analysis was performed with antiphosphotyrosine monoclonal antibody 4G10. Each blot was stripped and reprobed with anti-Pyk2 polyclonal antibody (A-B, lower panels). Arrows indicate the position of a Pyk2 protein band (A-B, upper panels).
Dose dependence and time-course studies of tyrosine phosphorylation of Pyk2 on stimulation with
N-formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP) in granulocytic cells. HL-60 cells were treated with 1.25% DMSO for 4 days, and the differentiated (granulocytic) cells were then incubated in suspension in polypropylene tubes for 1 minute with the indicated concentrations of fMLP (A) or for the indicated times with 1 μmol/L fMLP (B). Lysates from the equivalent number of cells (5 × 106 cells) were immunoprecipitated with anti-Pyk2 polyclonal antibody (A) or with anti-Pyk2 polyclonal antibody, normal goat IgG, or protein A alone (B). In the experiment shown in Figure 2B, an aliquot of cells was pretreated with pertussis toxin (PT) before stimulation with fMLP. Immunoblotting analysis was performed with antiphosphotyrosine monoclonal antibody 4G10. Each blot was stripped and reprobed with anti-Pyk2 polyclonal antibody (A-B, lower panels). Arrows indicate the position of a Pyk2 protein band (A-B, upper panels).
fMLP-induced attachment of granulocytic cells to Fg is mediated by β2 integrin
We observed that stimulation with fMLP promoted attachment of granulocytic cells to Fg-coated dishes. Less than 3% of all cells were attached to the dishes before stimulation, but about 20% of cells became attached after 30 minutes of stimulation. Most of the attached cells had spontaneously detached after about 180 minutes of stimulation. Such attachment was not observed on dishes coated with BSA. Because Fg is one of the ligands for β2 integrin, we examined whether the attachment was mediated by β2 integrin. First, we examined the expression of β2 integrin in granulocytic cells by using immunoblotting analysis. As shown in Figure3A, β2 integrin was induced in the course of granulocytic differentiation of HL-60 cells. Next, we evaluated the effect of pretreatment with mouse anti-β2 integrin monoclonal antibody IB4 on cell attachment to Fg-coated dishes. We observed that IB4 inhibited fMLP-induced cell attachment by about 95%. This inhibition was not observed when the cells were pretreated with normal mouse IgG. These results suggest that fMLP-induced attachment of granulocytic cells to Fg is mediated by β2 integrin.
Studies in granulocytic cells attaching to fibrinogen (Fg)-coated dishes on stimulation with fMLP.
(A) Day-course study of β2 integrin induction in HL-60 cells in the presence of DMSO. HL-60 cells were treated with 1.25% DMSO for the indicated days, and whole cell lysates were subjected to immunoblotting analysis with anti-β2 integrin polyclonal antibody. In each lane, the equivalent amounts of protein (8.5 μg) from whole cell lysates were loaded. The positions of molecular markers are shown on the left in kilodaltons. Arrow indicates the position of a β2 integrin protein band. (B-C) Pyk2 and Syk are tyrosine phosphorylated in relation to attachment to Fg. Granulocytic cells were incubated for the indicated times with 1 μmol/L fMLP on Fg-coated dishes (attached/detached) or in suspension in polypropylene tubes (suspension). Lysates from the equivalent number of cells (5 × 106 cells) were immunoprecipitated with anti-Pyk2 (B) or anti-Syk (SC-929; C) polyclonal antibody. Immunoblotting analysis was performed with antiphosphotyrosine monoclonal antibody 4G10. Each blot was stripped and reprobed with the indicated antibodies (B-C, lower panels). Arrows indicate the position of a Pyk2 (B, upper panel) or Syk (C, upper panel) protein band. (D-E) Tyrosine phosphorylation of Pyk2 and Syk in fMLP-stimulated, Fg-attached cells was inhibited by pretreatment of the cells with anti-β2 integrin antibody. Granulocytic cells were incubated for 30 minutes on dishes coated with Fg or bovine serum albumin (BSA) in the presence or absence of 1 μmol/L fMLP. In blocking experiments, some aliquots of cells were pretreated with anti-β2 integrin monoclonal antibody IB4 or normal mouse IgG before stimulation with fMLP. Lysates from the equivalent number of cells (5 × 106 cells) were immunoprecipitated with anti-Pyk2 (D) or anti-Syk (SC-573; E) polyclonal antibody. Immunoblotting analysis was performed with antiphosphotyrosine monoclonal antibody 4G10. Each blot was stripped and reprobed with the indicated antibodies (D-E, lower panels). Arrows indicate the position of a Pyk2 (D, upper panel) or Syk (E, upper panel) protein band.
Studies in granulocytic cells attaching to fibrinogen (Fg)-coated dishes on stimulation with fMLP.
(A) Day-course study of β2 integrin induction in HL-60 cells in the presence of DMSO. HL-60 cells were treated with 1.25% DMSO for the indicated days, and whole cell lysates were subjected to immunoblotting analysis with anti-β2 integrin polyclonal antibody. In each lane, the equivalent amounts of protein (8.5 μg) from whole cell lysates were loaded. The positions of molecular markers are shown on the left in kilodaltons. Arrow indicates the position of a β2 integrin protein band. (B-C) Pyk2 and Syk are tyrosine phosphorylated in relation to attachment to Fg. Granulocytic cells were incubated for the indicated times with 1 μmol/L fMLP on Fg-coated dishes (attached/detached) or in suspension in polypropylene tubes (suspension). Lysates from the equivalent number of cells (5 × 106 cells) were immunoprecipitated with anti-Pyk2 (B) or anti-Syk (SC-929; C) polyclonal antibody. Immunoblotting analysis was performed with antiphosphotyrosine monoclonal antibody 4G10. Each blot was stripped and reprobed with the indicated antibodies (B-C, lower panels). Arrows indicate the position of a Pyk2 (B, upper panel) or Syk (C, upper panel) protein band. (D-E) Tyrosine phosphorylation of Pyk2 and Syk in fMLP-stimulated, Fg-attached cells was inhibited by pretreatment of the cells with anti-β2 integrin antibody. Granulocytic cells were incubated for 30 minutes on dishes coated with Fg or bovine serum albumin (BSA) in the presence or absence of 1 μmol/L fMLP. In blocking experiments, some aliquots of cells were pretreated with anti-β2 integrin monoclonal antibody IB4 or normal mouse IgG before stimulation with fMLP. Lysates from the equivalent number of cells (5 × 106 cells) were immunoprecipitated with anti-Pyk2 (D) or anti-Syk (SC-573; E) polyclonal antibody. Immunoblotting analysis was performed with antiphosphotyrosine monoclonal antibody 4G10. Each blot was stripped and reprobed with the indicated antibodies (D-E, lower panels). Arrows indicate the position of a Pyk2 (D, upper panel) or Syk (E, upper panel) protein band.
Pyk2 and Syk are tyrosine phosphorylated in relation to cell attachment to Fg
To determine the effect of cell attachment to Fg on cell signaling, tyrosine phosphorylation of Pyk2 and Syk was examined. On stimulation with fMLP, tyrosine phosphorylation of Pyk2 was detected at 1 minute and reduced at 30 minutes in cells in suspension (Figure 2B), but it was augmented at 30 minutes in cells attached to Fg-coated dishes (Figure 3B). Tyrosine phosphorylation was not detected when the cells detached spontaneously after 180 minutes of stimulation (Figure3B). In the studies of Syk, tyrosine phosphorylation was not detected at either 1 minute or 30 minutes in cells in suspension after stimulation with fMLP, but it was detected in cells attached to Fg-coated dishes (Figure 3C).
To evaluate whether tyrosine phosphorylation of Pyk2 and Syk in the fMLP-stimulated cells attached to Fg-coated dishes was mediated by β2 integrin, we examined the effect of pretreatment with anti-β2 integrin monoclonal IB4. As shown in Figure 3D, tyrosine phosphorylation of Pyk2 after 30 minutes of stimulation with fMLP was completely inhibited with IB4 but not with normal mouse IgG in Fg-attached cells. Tyrosine phosphorylation of Pyk2 was not detected in cells on BSA-coated dishes, regardless of whether or not they had been stimulated with fMLP, nor was it detected at 30 minutes in cells on Fg-coated dishes in the absence of stimulation with fMLP (Figure 3D). Similar results were obtained in studies of Syk (Figure 3E). These results suggest that both Pyk2 and Syk participate in a β2 integrin–mediated signaling pathway in granulocytic cells.
Association of Pyk2 and Syk with β2 integrin in fMLP-stimulated granulocytic cells
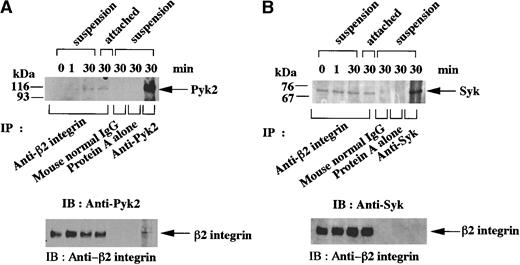
We conducted coprecipitation studies to examine whether Pyk2 and Syk are associated with β2 integrin in fMLP-stimulated granulocytic cells. Pyk2 was not detected in anti-β2 integrin immunoprecipitates of unstimulated cells or cells stimulated for 1 minute, but it became detectable both in cells kept in suspension and in cells attached to Fg-coated dishes after 30 minutes of stimulation with fMLP (Figure 4A). On the other hand, Syk was constitutively coprecipitated with anti-β2 integrin antibody, regardless of whether or not the cells were stimulated with fMLP or whether they were in suspension or attached (Figure 4B). This association of Pyk2 and Syk was not detected in immunoprecipitates with normal mouse IgG or those with protein A alone (Figure 4A-B). Conversely, β2 integrin was barely detectable in anti-Pyk2 immunoprecipitates of cells kept in suspension (Figure 4A) and cells attached to Fg-coated dishes after 30 minutes of stimulation with fMLP (Figure 5A). In anti-Syk immunoprecipitates, β2 integrin was not detected in similar time-course studies (Figure 4B and 5A).
Association of Pyk2 and Syk with β2 integrin in fMLP-stimulated granulocytic cells.
Granulocytic cells were incubated for the indicated times with 1 μmol/L fMLP on Fg-coated dishes (attached) or in suspension in polypropylene tubes (suspension). Lysates from the equivalent number of cells (5 × 106 cells) were immunoprecipitated with anti-β2 integrin monoclonal antibody, normal mouse IgG, protein A alone (A-B), anti-Pyk2 polyclonal antibody (A), or anti-Syk (SC-929) polyclonal antibody (B). Immunoblotting analysis was performed with anti-Pyk2 (A) or anti-Syk (B) polyclonal antibody. Each blot was stripped and reprobed with anti-β2 integrin polyclonal antibody (A-B, lower panels). The positions of molecular markers are shown on the left in kilodaltons. Arrows indicate the position of a Pyk2 (A, upper panel), Syk (B, upper panel), or β2 integrin (A-B, lower panels) protein band.
Association of Pyk2 and Syk with β2 integrin in fMLP-stimulated granulocytic cells.
Granulocytic cells were incubated for the indicated times with 1 μmol/L fMLP on Fg-coated dishes (attached) or in suspension in polypropylene tubes (suspension). Lysates from the equivalent number of cells (5 × 106 cells) were immunoprecipitated with anti-β2 integrin monoclonal antibody, normal mouse IgG, protein A alone (A-B), anti-Pyk2 polyclonal antibody (A), or anti-Syk (SC-929) polyclonal antibody (B). Immunoblotting analysis was performed with anti-Pyk2 (A) or anti-Syk (B) polyclonal antibody. Each blot was stripped and reprobed with anti-β2 integrin polyclonal antibody (A-B, lower panels). The positions of molecular markers are shown on the left in kilodaltons. Arrows indicate the position of a Pyk2 (A, upper panel), Syk (B, upper panel), or β2 integrin (A-B, lower panels) protein band.
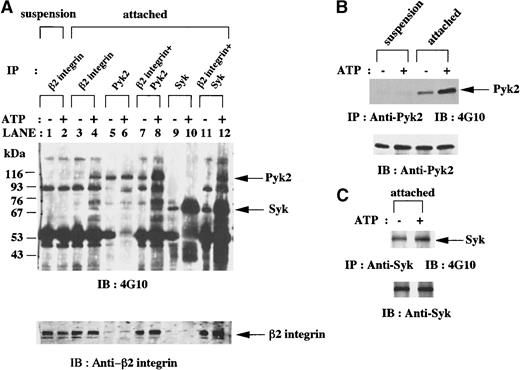
In vitro kinase assays of anti-β2 integrin, anti-Pyk2, and anti-Syk immunoprecipitates from lysates of fMLP-stimulated granulocytic cells.
(A) Granulocytic cells were incubated for 30 minutes with 1 μmol/L fMLP on Fg-coated dishes (attached) or in suspension in polypropylene tubes (suspension). Anti-β2 integrin, anti-Pyk2, and anti-Syk (SC-573) immunoprecipitates from lysates of the equivalent number of cells (5 × 106 cells) were mixed as indicated and subjected to in vitro kinase assays in the presence or absence of adenosine triphosphate (ATP) and immunoblotting with antiphosphotyrosine monoclonal antibody 4G10. Each blot was stripped and reprobed with anti-β2 integrin polyclonal antibody. The positions of molecular markers are shown on the left in kilodaltons. Arrows indicate the position of a Pyk2, Syk (upper panel), or β2 integrin (lower panel) protein band. (B-C) Granulocytic cells were incubated for 30 minutes with 1 μmol/L fMLP on Fg-coated dishes (attached) or in suspension in polypropylene tubes (suspension). Anti-Pyk2 (B) and anti-Syk (SC-929; C) immunoprecipitates from lysates of the equivalent number of cells (5 × 106 cells) were subjected to in vitro kinase assays in the presence or absence of ATP and immunoblotting with antiphosphotyrosine monoclonal antibody 4G10. Each blot was stripped and reprobed with the indicated antibodies (B-C, lower panels). Arrows indicate the position of a Pyk2 (B, upper panel) or Syk (C, upper panel) protein band.
In vitro kinase assays of anti-β2 integrin, anti-Pyk2, and anti-Syk immunoprecipitates from lysates of fMLP-stimulated granulocytic cells.
(A) Granulocytic cells were incubated for 30 minutes with 1 μmol/L fMLP on Fg-coated dishes (attached) or in suspension in polypropylene tubes (suspension). Anti-β2 integrin, anti-Pyk2, and anti-Syk (SC-573) immunoprecipitates from lysates of the equivalent number of cells (5 × 106 cells) were mixed as indicated and subjected to in vitro kinase assays in the presence or absence of adenosine triphosphate (ATP) and immunoblotting with antiphosphotyrosine monoclonal antibody 4G10. Each blot was stripped and reprobed with anti-β2 integrin polyclonal antibody. The positions of molecular markers are shown on the left in kilodaltons. Arrows indicate the position of a Pyk2, Syk (upper panel), or β2 integrin (lower panel) protein band. (B-C) Granulocytic cells were incubated for 30 minutes with 1 μmol/L fMLP on Fg-coated dishes (attached) or in suspension in polypropylene tubes (suspension). Anti-Pyk2 (B) and anti-Syk (SC-929; C) immunoprecipitates from lysates of the equivalent number of cells (5 × 106 cells) were subjected to in vitro kinase assays in the presence or absence of ATP and immunoblotting with antiphosphotyrosine monoclonal antibody 4G10. Each blot was stripped and reprobed with the indicated antibodies (B-C, lower panels). Arrows indicate the position of a Pyk2 (B, upper panel) or Syk (C, upper panel) protein band.
To examine whether Pyk2 and Syk are functionally associated with β2 integrin with kinase activities, we performed in vitro kinase assays of anti-β2 integrin, anti-Pyk2, and anti-Syk immunoprecipitates. In the presence of ATP, an increase in tyrosine phosphorylation of several proteins, including 110-kd, 74-kd, 70-kd, and 60-kd protein bands, was detected in anti-β2 integrin immunoprecipitates from the lysates of attached cells, whereas little phosphorylation was observed in cells in suspension (Figure 5A, lanes 1-4). When in vitro kinase assays of anti-Pyk2 and anti-Syk immunoprecipitates were performed, an increase in tyrosine phosphorylation of Pyk2 and Syk was observed in attached cells (Figure 5B-C) but not in suspended cells (Figure 5B and data not shown). These results led us to postulate that Pyk2 and Syk in attached cells exert kinase activities on anti-β2 integrin immunoprecipitates.
Additional kinase assays were performed in the combination of anti-β2 integrin immunoprecipitates and anti-Pyk2 immunoprecipitates or the combination of anti-β2 integrin immunoprecipitates and anti-Syk immunoprecipitates in the presence and absence of ATP. In the presence of ATP, tyrosine phosphorylation of proteins with molecular weights of 110 kd, 90 kd, 74 kd, and 60 kd in the first combination of immunoprecipitates (Figure 5A, lane 8) was quantitatively greater than that in anti-β2 integrin immunoprecipitates (Figure 5A, lane 4) plus that in anti-Pyk2 immunoprecipitates (Figure 5A, lane 6). Tyrosine phosphorylation of proteins with molecular weights of 110 kd, 90 kd, and 60 kd in the second combination of immunoprecipitates (Figure 5A, lane 12) was quantitatively greater than that in anti-β2 integrin immunoprecipitates (Figure 5A, lane 4) plus that in anti-Syk immunoprecipitates (Figure 5A, lane 10). In the absence of ATP, these combinations of immunoprecipitates did not bring about enhanced tyrosine phosphorylation of any of the proteins mentioned above. Because we assume that the additional tyrosine phosphorylation of some proteins associated with β2 integrin in attached cells was mediated by Pyk2 or Syk, it appears that anti-β2 integrin immunoprecipitates of the attached cells contain the substrates of Pyk2 and Syk.
Discussion
In the current study, we examined the involvement of Pyk2 and Syk in functional activation of HL-60 cells. During granulocytic differentiation, Pyk2 was induced, but its tyrosine phosphorylation was not enhanced (Figure 1A and data not shown). Pyk2 was also induced in the course of 12-o-tetradecanoyl-phorbol-13-acetate (TPA)–induced monocytic differentiation of HL-60 cells, with apparent tyrosine phosphorylation (data not shown). Because it has been demonstrated that Pyk2 is activated when it is tyrosine phosphorylated,20,32 Pyk2 might not be activated during DMSO-induced granulocytic differentiation, but it may be activated during TPA-induced monocytic differentiation of HL-60 cells. Various cytoplasmic PTKs have been found to be related to differentiation of HL-60 cells. Src family PTKs Lyn and Fgr are expressed and tyrosine phosphorylated during retinoic acid–induced granulocytic differentiation, and inhibition of these PTKs by antisense oligodeoxynucleotide caused apoptosis.8 Induction and tyrosine phosphorylation of Fgr was also confirmed in DMSO-differentiated cells.7 Lyn, Fgr, and Fyn were all shown to be activated in the course of monocytic differentiation, and inhibition of these PTKs by PTK inhibitors modulated differentiation.5 6 We found that Syk was constitutively expressed but apparently not tyrosine phosphorylated during DMSO-induced differentiation (Figure 1B and data not shown). Therefore, PTKs involved in differentiation of HL-60 cells might vary in their differentiation inducers and differentiation lineages.
Treatment of HL-60 cells with DMSO resulted in expression of fMLP receptor during granulocytic differentiation (data not shown). To determine whether functional activation of granulocytic cells correlates with activation of Pyk2, we treated the cells with fMLP and detected tyrosine phosphorylation of Pyk2 (Figure 2A-B). This phosphorylation was blocked by PT (Figure 2B), which indicated that Pyk2 is involved in G protein–coupled, receptor-mediated signaling as described in several studies.12 21-26
G protein–coupled, receptor-mediated signaling leads to activation of some integrins. It was demonstrated that stimulation of neutrophils with fMLP caused alteration of αM β2 integrin from a resting form to an activated form.46 Integrin-ligand binding does not occur in a resting form even if sufficient amounts of integrins are expressed on the surface of the cells. In our study, only a few granulocytic cells attached to Fg-coated dishes before stimulation with fMLP, but cell attachment was obviously promoted after stimulation. Granulocytic cells expressed β2 integrin (Figure 3A), and the cell attachment to Fg was almost completely inhibited by pretreatment with anti-β2 integrin antibody. These findings suggest that β2 integrin is activated as a result of “inside-out” signaling by means of an fMLP receptor–mediated pathway.
Stimulation of granulocytic cells with fMLP caused not only alteration of β2 integrin to an activated form but also tyrosine phosphorylation of Pyk2 (Figure 3B). In addition, association of Pyk2 with β2 integrin was detected after stimulation with fMLP (Figure 4A and 5A). These findings prompted us to postulate that Pyk2 might be involved in the alteration of β2 integrin to an activated form through an “inside-out” signaling process by means of an fMLP receptor–mediated, G protein–coupled pathway. However, there seems to be some dissociation in the time between the initial tyrosine phosphorylation of Pyk2 at 1 minute (Figure 3B) and the association of Pyk2 with β2 integrin at 30 minutes (Figure 4A) after stimulation with fMLP. Previous investigations indicated that the distribution of CAK β is diffuse in cytoplasm and not localized at focal adhesions in a rat fibroblast cell line.47 We speculate on this dissociation as follows. First, Pyk2 close to fMLP receptors becomes tyrosine phosphorylated immediately after stimulation and the following signaling streams run down toward β2 integrin (“inside-out” signaling). Second, Pyk2, not associated with β2 integrin but near it, is affected by this signaling stream and becomes associated with β2 integrin. However, the exact mechanisms of activation of β2 integrin and the roles and interrelations among Pyk2, Syk, and β2 integrin remain to be elucidated.
Cell attachment to ligand-coated dishes often gives rise to “outside-in” signaling through integrins in an activated form. Because we wondered whether Pyk2 is activated through the ligation of β2 integrin, we pretreated granulocytic cells with fMLP and placed the cells on Fg-coated dishes. The secondary signal was generated by cell attachment and enhanced tyrosine phosphorylation of Pyk2 was observed (Figure 3B,D), which was blocked by pretreatment with anti-β2 integrin antibody (Figure 3D). These results indicate that Pyk2 is activated by “outside-in” signaling through β2 integrin. Src family PTKs and Syk are involved in integrin-mediated signaling in neutrophils.9,10,48 We found here that Pyk2 is another PTK involved in β2 integrin–mediated signaling. While our study was in progress, Yan and Novak11 reported that Pyk2 becomes tyrosine phosphorylated in neutrophils. In their system, Pyk2 tyrosine phosphorylation was detected in cells attached to Fg-coated plates after stimulation with fMLP or tumor necrosis factor α and seemed to result from “outside-in” signaling through β2 integrin.
We also showed that Pyk2 and Syk are associated with β2 integrin in a different manner. Pyk2 became associated with β2 integrin after stimulation with fMLP, whereas Syk was associated constitutively with β2 integrin, regardless of stimulation or whether the cells were in suspension or attached (Figure 4A and 4B).
In summary, we speculate that the following molecular events occur. During granulocytic differentiation, fMLP receptor and β2 integrin are induced, and the amount of Pyk2 is increased but neither activated nor associated with β2 integrin. In contrast, Syk is already prepared and associated with β2 integrin but not yet activated. Stimulation with fMLP causes the initial tyrosine phosphorylation of Pyk2 and the subsequent association of Pyk2 with β2 integrin. Alteration of β2 integrin to an activated form also occurs and granulocytic cells can then receive a new stimulation from outside by means of β2 integrin. This stimulation leads to activation of Syk and additionally enhanced activation of Pyk2. Both the Pyk2 and the Syk of Fg-attached cells represented kinase activities (Figure 5B-C) and induced tyrosine phosphorylation of several proteins in anti-β2 integrin immunoprecipitates in vitro (Figure 5A). Taken together, these results indicate that Pyk2 and Syk are both functionally associated with β2 integrin in relation to cell attachment to Fg after stimulation with fMLP but in a different manner. Thus, various signals that are receptor-mediated or integrin-mediated and coupled by tyrosine kinases may be involved in the functional activation of granulocytic cells.
Acknowledgment
We thank Dr Momoyo Asahi (Fukui Prefectural University, Fukui, Japan) for discussions about fMLP signaling.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kaoru Tohyama, Department of Laboratory Medicine and Clinical Sciences, Graduate School of Medicine, Kyoto University, Sakyo-ku, Kyoto 606-8507, Japan: e-mail: ktohyama@kuhp.kyoto-u.ac.jp.