Abstract
Polymorphonuclear leukocyte (PMN) dysfunction has been reported in human immunodeficiency virus (HIV)-infected patients. Interleukin (IL)-15 is a recently discovered cytokine that potentiates antimicrobial functions of normal PMNs. We evaluated the in vitro effect of IL-15 on chemotaxis and fungicidal activity of PMNs from 9 patients with untreated advanced HIV infection, 8 patients with viral suppression after 52 to 130 weeks of highly active antiretroviral therapy (HAART), and 12 patients with treatment failure. We also studied oxidative burst and apoptosis of PMNs in 5 patients with untreated advanced HIV infection. Twelve healthy donors were included as controls. Chemotaxis and fungicidal activity of unprimed PMNs was significantly lower in patients with untreated HIV infection compared with controls. After incubation with IL-15, a significant increase in PMN chemotaxis and fungicidal activity was found; moreover, IL-15 induced a significant reduction in the number of apoptotic HIV+ PMNs. IL-15 did not modulate oxidative burst of HIV+ PMNs as measured by chemiluminescence production. The in vitro priming of PMNs with IL-15 determined a complete reversal of defective chemotaxis and killing in all HAART-treated patients with long-term HIV suppression. IL-15 significantly enhanced chemotaxis and fungicidal activity also in patients with HAART failure. In conclusion, IL-15 is an important cytokine in the activation of the functional properties of HIV+ PMNs, by delaying apoptosis and enhancing chemotaxis and fungicidal activity. The potent stimulant effect of IL-15 on PMN function was observed in antiretroviral naive patients as well as in individuals who were receiving HAART, including those with treatment failure.
Introduction
Polymorphonuclear leukocytes (PMNs) are the first line of defense in the innate immune response to invading pathogens. During the course of human immunodeficiency virus (HIV) infection, PMNs exhibit a variety of immunologic and functional defects that may contribute to increased susceptibility to bacterial and fungal infections.1 In particular, decreased chemotaxis and killing activity, as well as altered respiratory burst response and accelerated apoptosis,2-6 have been reported.
Interleukin (IL)-15 is a recently discovered cytokine that belongs to the 4 α-helix bundle cytokine family and plays an important role in the early steps of the innate immune response to infections.7,8 IL-15 shares many biologic properties with IL-2, but with no significant amino acid sequence homology. It mediates its function through the β- and γ-chains of the IL-2–receptor (IL-2R) and its own unique α-chain (IL-15Rα).9,10IL-15 has been shown to stimulate the growth of natural killer (NK) cells, activated peripheral blood T lymphocytes, tumor-infiltrating lymphocytes (TILs), and B cells.8,11,12 In addition, IL-15 has been reported to be a chemoattractant for T lymphocytes and NK cells and to induce lymphokine-activated killer (LAK) activity in NK cells, as well as be able to promote the generation of cytolytic effector cells.13-15 Recent investigations have shown that IL-15 potentiates several antimicrobial functions of normal PMNs involved in the innate immune response against invading pathogens.16 To date, there are no data on the effects of IL-15 on the function of defective PMNs, such those of patients with HIV infection.
In this study, we therefore evaluated the in vitro effect of IL-15 on functional activity of PMNs from healthy donors and HIV-infected patients by assessing chemotaxis, fungicidal activity, oxidative burst, and apoptosis. It was the purpose of the study to determine whether addition of IL-15 to HIV+ PMNs could induce a normalization of these functional parameters. We observed that the in vitro treatment with IL-15 has a profound effect on the enhancement of chemotaxis and killing activity of PMNs from both antiretroviral naive patients and individuals who were receiving highly active antiretroviral therapy (HAART), including those with virologic and immunologic treatment failure.
Patients, materials, and methods
Patients and controls
Study participants included a total of 34 patients who were infected with HIV from the Department of Infectious and Tropical Diseases of La Sapienza University of Rome. HIV seropositivity was determinated by enzyme-linked immunosorbent assay (ELISA) and confirmed by Western blot analysis (Chiron Corporation Emeryville, CA). Patients enrolled in this study were subdivided in the following 3 groups: (A) 14 patients (11 men, 3 women; age range, 30-52 years) with untreated advanced HIV infection. The median CD4+ count was 53 cells/μL (range, 7-160) and the median plasma HIV-RNA levels was 5.85 log10 copies/mL (range, 5.5-6.3); (B) 8 patients (5 men, 3 women; age range, 27-62 years) with continuous viral suppression (less than 50 copies/mL) after 52 to 130 weeks of HAART. The median pre-HAART CD4+ count and viral load was 149 cells/μL and 5.1 log10 copies/mL, respectively. The median increase in CD4+ count after HAART was 262 cells/μL (range, 40-715). The HAART regimen consisted of 2 reverse-transcriptase inhibitors and 1 protease inhibitor (indinavir in 6 patients and ritonavir in 2 patients); and (C) 12 patients (9 men, 3 women; age range, 39-69 years) who showed evidence of virologic and immunologic antiretroviral treatment failure. The median CD4+ count was 60 cells/μL (range, 14-176) and the median plasma HIV-RNA levels was 5.4 log10 copies/mL (range, 4.4-6.2). All these patients had received protease inhibitors in combination with nucleoside or non-nucleoside analogues for 48 to 96 weeks.
Opportunistic infections occurred in the patients during advanced untreated disease (Pneumocystis carinii pneumonia = 3, pulmonary tuberculosis = 2, progressive multifocal leukoencephalopathy = 2; cytomegalovirus disseminated disease = 2; cerebral toxoplasmosis = 1) and in those with treatment failure (Mycobacterium avium-complex disease = 1, pulmonary tuberculosis = 1). No infectious complications were found in the patients during continuous suppression of viral load after HAART. Nevertheless, all HIV-seropositive individuals included in the study had no evidence of active opportunistic infections at the time of blood sample handling.
Twelve healthy blood donors (9 men, 3 women; age range, 27-69 years) were included as HIV-negative controls. Samples from healthy donors were run on different days to determine day-to-day variations in assay. Control and HIV-positive PMNs were tested in parallel, using identical reagents under identical conditions. All subjects gave informed consent for the study.
Cytokines and stimuli
Recombinant human IL-15 was supplied by PeproTech (Rocky Hill, NJ). Interferon (IFN)-γ was from Sigma (St Louis, MO). Recombinant human IL-8 was from R&D Systems (Minneaopolis, MN). The following product was used as stimulus: N-formyl-L-methionyl-L-leucyl-L-phenylalanine-benzylamide (fMLPB) (Sigma). Endotoxin contamination was ruled out by measurement of endotoxin concentration by using standard Limulus amebocyte lysate assay (Sigma).
Microorganisms
Candida albicans was a clinical isolate obtained from the Microbiology Service of the Department of Infectious and Tropical Diseases of La Sapienza University of Rome. Cultures were maintained by serial passage on Sabouraud agar plates (Bio-Mérieux, Lyon, France). Yeast cells were cultured for 24 hours at 37°C on Sabouraud broth, harvested by suspending in RPMI 1640 medium (Sigma), and adjusted to the desired concentration. C albicans were opsonized by incubating yeasts with human type AB serum (Sigma) for 30 minutes at 37°C, followed by washing twice in phosphate-buffered saline (PBS).
Specimen collection and polymorphonuclear leukocyte isolation
Venous blood samples were collected into heparinized glass tubes and ethylenediamine tetracetic acid (EDTA)-containing tubes and plasma and cells were separated immediately. PMNs were prepared from heparinized blood by Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden) density gradient centrifugation, followed by dextran sedimentation. Contaminating erythrocytes were removed by a single hypotonic lysis in sterile distilled water for 30 seconds at room temperature. PMNs were suspended in Hanks balanced salt solution (HBSS) without Ca++ or Mg++ (Sigma) and adjusted to the desired concentration. The purity of PMNs isolated was always more than 98%, as determined by Giemsa staining. PMN preparations consistently contained fewer than 1% monocytes. The PMN viability was more than 98%, as estimated by trypan blue exclusion.
HIV RNA levels and CD4+ and CD8+lymphocyte counts
HIV RNA levels were measured in −70°C stored plasma prepared from blood obtained in EDTA-containing tubes. A quantitative polymerase chain reaction (Amplicor HIV Monitor; Roche Diagnostics Systems, Branchburg, NJ), was used. The limit of detection was 50 copies/mL. The enumeration of CD4+ and CD8+ lymphocyte numbers was assayed in blood collected in EDTA-containing tubes; 2-color flow cytometric analysis was performed with the Becton Dickinson flow cytometer FACScan using the whole-blood lysis procedure and SimulTEST reagents (Becton Dickinson, San Jose, CA).
Chemotaxis assay
Isolated PMNs at 2 × 106 cells/mL in DMEM (BioWhittaker, Boeringher Ingelheim, Belgium) were incubated with human recombinant IL-15 (10 to 1000 ng/mL) at 37°C under 5% CO2 atmosphere. In preliminary experiments, a time-course study was performed at 3, 6, and 18 hours to select the optimal incubation time. We found that maximal priming effect of IL-15 was seen at 6 hours (data not shown). Control cells were incubated in medium alone. After cytokine treatment, the cells were resuspended in DMEM and used at a final concentration of 1 × 106. PMN migration toward fMLPB (10−7M) or IL-8 (100 ng/mL) was carried out as previously reported.17 18 Briefly, 200 μL aliquots of chemoattractant was placed in the lower well of a blind-well chamber (Neuroprobe, Cabin John, MD), separated by polyvinylpyrrolidone-free micropore polycarbonate filter (Neuroprobe, Corp, Pleasanton, CA) from 300 μL of cell suspensions placed in the upper well. The filter pore sizes were 5 μm. After 90 minutes incubation at 37°C in 5% CO2 in a humidified atmosphere, the filters were removed. After cleaning the upper side, they were fixed and stained by adding DiffQuik (Baxter Diagnostics AG, Dudingen, Switzerland). All assays were carried out in triplicate. Migrated cells were then counted microscopically in 10 randomly selected, oil-immersion fields. Cell migration was expressed as the mean number of neutrophils that migrated per field. Spontaneous migration in the absence of chemoattractant was also calculated (8-10 cells per field) and was subtracted from migration in response to fMLPB or IL-8. In some experiments, direct PMN migration in response to IL-15 (10 ng/mL) as chemoattractant was analyzed.
Fungicidal assay
The 2 × 106 PMNs suspended in DMEM were incubated for 6 hours at 37°C under 5% CO2 atmosphere with human recombinant IL-15 (10 to 1000 ng/mL) or IFN-γ (500 U/mL) as positive control. After treatment, the fungicidal activity of PMNs was assessed by the methylene blue staining technique.19 Untreated PMNs were subjected to the same experimental conditions. Briefly, 2 × 106 PMNs were mixed with opsonized C albicans in a 1:5 ratio in MEM (BioWhittaker) containing 10% human type AB serum (Sigma) and incubated at 37°C on a shaker for 60 minutes. Deoxycholic acid (250 μL of a 2.5% solution in distilled water) was added to immediately lyse the cells. No alteration of viability of C albicans was detectable. Methylene blue (2 mL of 0.01% solution in PBS) was then added and the suspension was kept on ice for 10 minutes. After centrifugation, the pellet was resuspended in PBS and observed under light microscopy (×40) to determine the number of blue-stained (dead) versus the number of unstained (alive)C albicans yeasts (a total of 300 yeasts were counted for each slide). The killing activity against C albicans was calculated by the following formula: % killing activity = [stained yeasts/(stained + unstained yeasts)] × 100.
Measurement of oxidative burst by chemiluminescence assay
Isolated PMNs at 2.5 × 106 cells/mL in DMEM were incubated with human recombinant IL-15 (10 ng/mL) for 3 hours at 37°C under 5% CO2 atmosphere.16 Control cells were incubated in medium alone. After cytokine treatment, luminol-enhanced chemiluminescence (CL) production was recorded on a luminometer (Luminometer DCR-1; Digene Diagnostic, Inc), as previously reported.17 In each assay, the reaction mixture was made by adding 100 μL of cell suspension (2.5 × 106/mL PMN), 10 μL of 0.2 mmol/L luminol (5-amino-2,3deydro-1,4-phthalazinedione) (Sigma), an aliquot of stimulus, and DMEM to bring the final volume of 1 mL. Two different stimuli were used: fMLPB (10−9 mol/L); opsonized C albicans (40 μL of 5 × 107 yeasts/mL). CL production was measured after 30 minutes of incubation at 37°C in agitation. The results of each assay were expressed as relative light units (RLU)/103 cells. In some experiments, we studied the direct effect of IL-15 (10 ng/mL) on CL production.
Apoptosis
Isolated PMNs (1 × 106) in RPMI 1640 supplemented with 10% human type AB serum (Sigma) were incubated for 18 hours at 37°C under 5% CO2 atmosphere in 24-well tissue culture plates in the absence or presence of human recombinant IL-15 (10 ng/mL). After incubation, 200 μL of cell preparation were cytocentrifuged and fixed with 4% paraformaldehyde. Apoptosis was evaluated by in situ cell death detection kit, POD (Boerhinger Mannheim, Germany), according to the manufacturer's instructions. This assay measured DNA fragmentation by immunocytochemistry using TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling) method. Cell death was confirmed by immunofluorescence analysis of phosphatidylserine on the outer leaflet of apoptotic cell-membranes (Annexin-V-FLUOS, Boerhinger Mannheim). One hundred cells were counted per sample and results are expressed as percentage of apoptotic cells.
Statistical analysis
The difference between PMNs treated or untreated with cytokines and PMNs from HIV-infected patients or healthy donors was evaluated using analysis of variance, followed by Dunnett's test. Linear regression analysis was used to determine whether correlation existed between numbers of CD4+ and PMN chemotaxis or fungicidal activity.
Results
Effect of IL-15 on chemotaxis, fungicidal activity, oxidative burst, and apoptosis of PMNs from normal subjects and patients with untreated advanced HIV infection
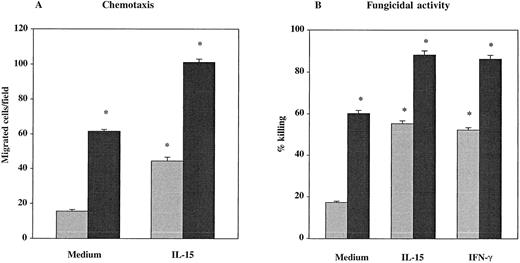
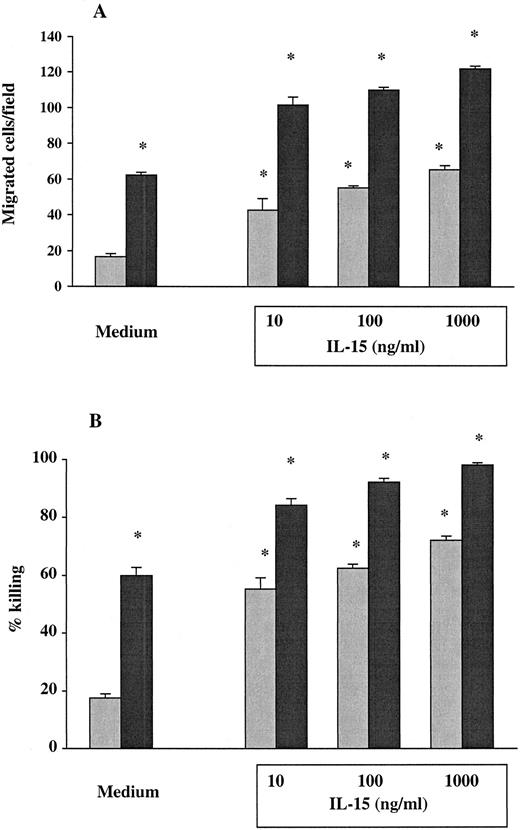
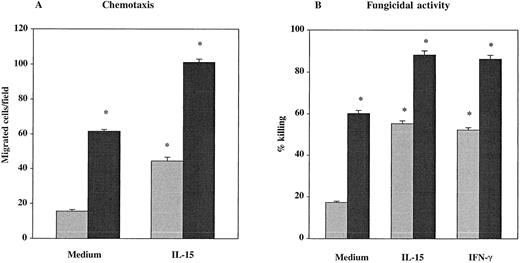
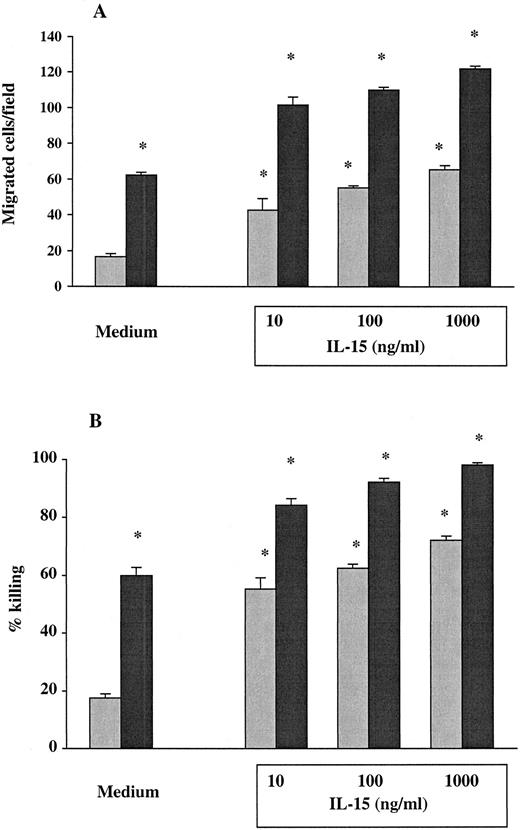
The PMNs from 7 healthy donors and from 9 untreated HIV-seropositive individuals were studied for their chemotactic responsiveness toward fMLPB as chemoattractant, as well as killing activity against C albicans. Results showed that the baseline chemotactic activity of unstimulated PMNs from HIV-infected patients was significantly decreased compared with seronegative controls (mean ± SEM, 15.44 ± 1 versus 61.43 ± 1.1) (P < .001) (Figure 1A). The percentage killing of C albicans by PMNs from HIV-infected subjects was also significantly lower compared with healthy controls (mean ± SEM, 17.22 ± 0.6 versus 60.14 ± 1.4) (P < .001) (Figure 1B). To evaluate the effect of IL-15 on the PMN chemotaxis and fungicidal activity, PMNs from the same subjects were cultured with the cytokine for 6 hours. As shown in Figure 1A, in vitro priming of PMNs with IL-15 significantly enhanced the chemotactic activity in response to fMLPB of normal PMNs (101 ± 1.92) as well as that of PMNs from HIV-infected patients with defective chemotaxis (44.4 ± 2.21) (P < .05). To investigate whether IL-15 was equally effective in enhancing chemotactic response to other chemoattractants, we evaluated PMN chemotaxis toward IL-8 in 5 untreated HIV+ patients and 5 healthy controls. The results showed that IL-15–primed PMNs from HIV+ subjects and controls exhibited a significant increase in chemotactic activity toward IL-8 compared with unprimed PMNs (mean ± SEM, 44.4 ± 0.74 and 102 ± 2.7 versus 17.6 ± 0.7 and 65.5 ± 1.65, respectively; P < .05). When chemotaxis was directly assessed in response to IL-15 as chemoattractant (10 ng/mL), we found that IL-15 did not have any direct chemoattractant effect on PMNs (data not shown). Figure 1B illustrates the enhanced fungicidal activity of PMNs preincubated with IL-15 from both healthy donors and patients with untreated advanced HIV infection. IL-15 significantly augmented PMN-mediated killing activity against C albicans to 88.14% ± 1.96% in healthy controls and to 55.22% ± 1.43% in HIV-seropositive individuals (P < .05 for both). The effect of IL-15 on the candidacidal activity of PMNs was comparable with the effect of IFNγ (500 U/mL), which was used as positive control.16In some experiments, chemotaxis and killing activity were assessed after stimulation of PMNs with increased concentrations of IL-15. As reported in Figure 2, IL-15 enhanced the chemotactic and antifungal activity of PMNs from HIV+subjects and healthy controls in a dose-dependent manner.
Effect of IL-15 on chemotaxis and fungicidal activity of PMNs from healthy controls (black columns) and patients (gray columns) with untreated advanced HIV infection.
(A) PMNs were incubated with IL-15 (10 ng/mL) or medium alone for 6 hours and then tested for their ability to migrate in a blind-well chamber toward fMLPB as chemoattractant. (B) PMNs were incubated with medium alone, IL-15 (10 ng/mL) or IFN-γ (500 U/mL) for 6 hours and then assayed for killing activity against Candida by the methylene blue staining technique. Results are expressed as mean ± SEM. Asterisks indicate a significant increase in PMN chemotaxis and fungicidal activity (P < .05).
Effect of IL-15 on chemotaxis and fungicidal activity of PMNs from healthy controls (black columns) and patients (gray columns) with untreated advanced HIV infection.
(A) PMNs were incubated with IL-15 (10 ng/mL) or medium alone for 6 hours and then tested for their ability to migrate in a blind-well chamber toward fMLPB as chemoattractant. (B) PMNs were incubated with medium alone, IL-15 (10 ng/mL) or IFN-γ (500 U/mL) for 6 hours and then assayed for killing activity against Candida by the methylene blue staining technique. Results are expressed as mean ± SEM. Asterisks indicate a significant increase in PMN chemotaxis and fungicidal activity (P < .05).
IL-15 dose response.
Dose-response of IL-15 on chemotaxis (A) and fungicidal activity (B) of PMNs from untreated HIV+ patients (gray columns) and healthy controls (black columns). PMNs were incubated with medium alone or with increased concentrations of IL-15 (10 to 1000 ng/mL) for 6 hours. Results represent the mean ± SEM of values from 3 experiments. Asterisks indicate a significant increase in PMN chemotaxis and fungicidal activity (P < .05).
IL-15 dose response.
Dose-response of IL-15 on chemotaxis (A) and fungicidal activity (B) of PMNs from untreated HIV+ patients (gray columns) and healthy controls (black columns). PMNs were incubated with medium alone or with increased concentrations of IL-15 (10 to 1000 ng/mL) for 6 hours. Results represent the mean ± SEM of values from 3 experiments. Asterisks indicate a significant increase in PMN chemotaxis and fungicidal activity (P < .05).
We next studied the priming effect of IL-15 on PMN oxidative respiratory burst from 5 untreated HIV-infected patients. Results showed that there was no significant difference in CL production (mean ± SEM) after stimulation with fMLPB and opsonized C albicans when PMNs were preincubated in the presence or absence of IL-15 (1.05 ± 0.18 RLU and 1.21 ± 0.21 RLU for primed PMNs versus 1.35 ± 0.26 RLU and 1.53 ± 0.12 RLU for unprimed PMNs, respectively; P > .05). In addition, when CL production of PMNs was assessed after stimulation with IL-15, we found that this cytokine did not directly modulate oxidative burst response (data not shown).
Because abnormalities in PMN function during HIV infection were associated with accelerated apoptosis of these cells, we also analyzed the effect of IL-15 on PMN apoptosis of 5 patients with untreated advanced HIV infection. IL-15 in vitro treatment significantly decreased the percentage of apoptotic cells (27.6% ± 1.4%) when compared with that of unprimed PMN (59.6% ± 8.0%) (P < .05).
Chemotaxis and fungicidal activity of PMN from patients with continuous HIV suppression after HAART and effect of IL-15
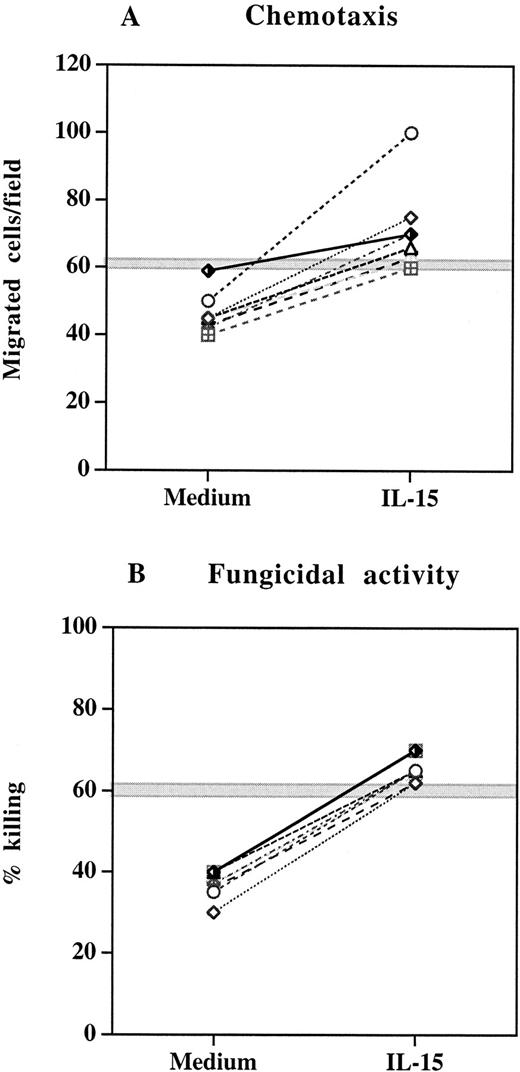
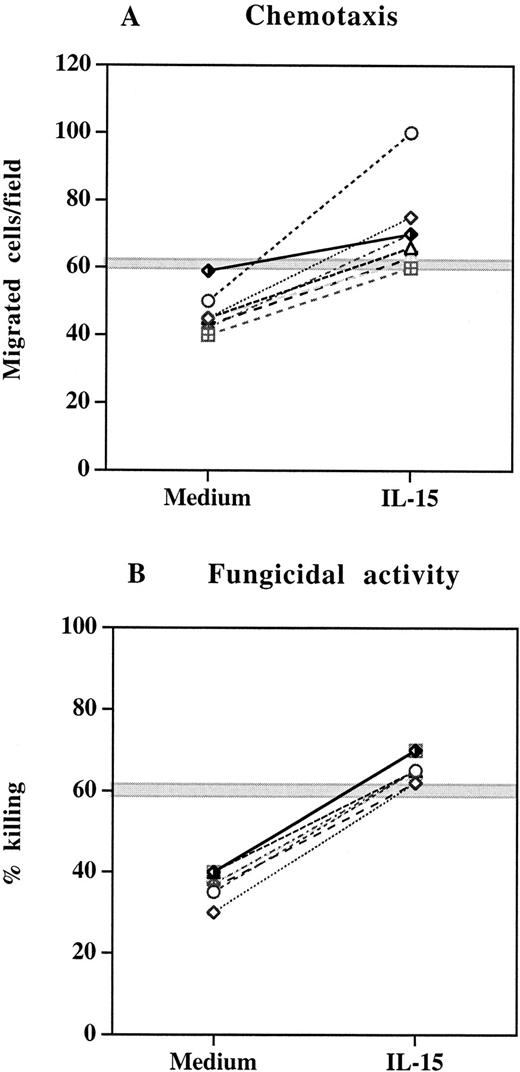
We next studied a cohort of 8 HIV-infected patients who received HAART for 52 to 130 weeks and showed continuous viral suppression and sustained increase in CD4+ T-cell count over the treatment period. PMNs were isolated from blood samples collected before and after HAART and assessed for chemotaxis and fungicidal activity. As reported in Table 1, HAART regimen was associated with a significant increase in both chemotactic response and anticandidal activity of PMNs (P < .05), which positively correlated with elevation in CD4+ T-cell counts (r = 0.70 and r = 0.71, respectively). Despite the improvement in the chemotactic and antifungal function after HAART, the values of these parameters remained below the range found for healthy controls. To investigate whether IL-15 could induce a complete restoration of the PMN chemotactic and fungicidal activity of these patients in vitro, we incubated PMNs from HAART-treated subjects with IL-15 (10 ng/mL) for 6 hours before functional studies. In all experiments, IL-15 significantly enhanced the chemotactic responsiveness and candidal activity of PMNs (Figure3). The defect that was present before the in vitro IL-15 treatment was completely reversed and the chemotaxis and killing rate reached the ranges of normal PMNs.
Effect of IL-15 on chemotaxis and fungicidal activity of PMNs from 8 patients with continuous viral suppression after 52 to 130 weeks of HAART.
(A) PMNs were incubated with IL-15 (10 ng/mL) or medium alone for 6 hours and then tested for their ability to migrate in a blind-well chamber toward fMLPB as chemoattractant. (B) PMNs were incubated with medium alone or IL-15 (10 ng/mL) for 6 hours and then assayed for killing activity against Candida by the methylene blue staining technique. Each pair of symbols represents different patients. The gray horizontal zone represents the mean ± SEM of healthy controls.
Effect of IL-15 on chemotaxis and fungicidal activity of PMNs from 8 patients with continuous viral suppression after 52 to 130 weeks of HAART.
(A) PMNs were incubated with IL-15 (10 ng/mL) or medium alone for 6 hours and then tested for their ability to migrate in a blind-well chamber toward fMLPB as chemoattractant. (B) PMNs were incubated with medium alone or IL-15 (10 ng/mL) for 6 hours and then assayed for killing activity against Candida by the methylene blue staining technique. Each pair of symbols represents different patients. The gray horizontal zone represents the mean ± SEM of healthy controls.
Enhancement of chemotactic and fungicidal activity of PMNs by IL-15 in patients with HAART-treatment failure
In another set of experiments, we also studied a selected group of 12 HIV-infected individuals with virologic and immunologic treatment failure after 48 to 96 weeks of HAART regimen. In these subjects, baseline chemotactic activity and percentage killing of C albicans by unprimed PMNs were significantly reduced when compared with those of HIV-positive subjects with long-term viral suppression after HAART (P < .001). When PMNs from patients with HAART-treatment failure were incubated with IL-15, a significant increase in both chemotactic response to bacterial peptide fMLPB and killing activity against C albicans were found (P < .05) (Table 2). Interestingly, in these subjects the enhancement of PMN chemotaxis and antifungal function by IL-15 reached values that were significantly higher than those of unprimed PMNs from HAART-responder individuals.
Discussion
Patients with HIV infection display a variety of immunologic defects that almost invariably lead to the development of a diverse array of opportunistic infections.20 In particular, the emergence of bacterial and fungal infections could in part be ascribed to functional defects of neutrophils that represent a crucial component of the innate antimicrobial immunity. Several studies have shown dysfunctional activity of PMNs from AIDS patients in their chemotactic, phagocytic, bactericidal, and fungicidal activity and oxidative metabolism processes.1,21 It has been suggested that the dysregulated production of cytokines modulating neutrophil function may be the underlying cause of decreased PMN function during HIV infection. In this respect, cytokines, such as granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF), have been reported to up-regulate functional properties of HIV+ PMNs, whereas IL-4 and IL-10 have been shown to induce a down-regulation of functional status of PMNs from HIV-infected patients.4,22 23
In this study, we evaluated the biologic role on HIV-associated neutrophil dysfunctions of IL-15, a recently discovered cytokine that is an important regulator of the innate immune response to invading microrganisms. IL-15 is produced by activated monocytes early in the course of the innate immune response and may contribute to host defense against intracellular pathogens. In addition to its biologic effects on the development, survival, and activation of NK cells, IL-15 plays an important role in the activation of immune response of phagocytic cells. Previous studies have shown that IL-15 augmented superoxide production and candidal activity of human monocytes and induced the production of IL-8 and monocyte chemotactic protein-1 (MCP-1) in the same cells.24,25 Also, PMN has been shown to be a cellular target for IL-15, which was able to induce morphologic changes and delay apoptosis in these cells.26 Recently, Musso et al16 demonstrated that IL-15 was a potent stimulant of proinflammatory and antimicrobial functions of normal PMNs by activating several functional properties of PMNs involved in the innate immune response to C albicans. In agreement with these findings, we found that IL-15 enhanced in vitro the functional activity of PMNs from healthy blood donors, as demonstrated by increased chemotactic responsiveness as well as killing activity against C albicans. PMN activation by IL-15 was dose-dependent and, with regard to the antifungal activity, was comparable with the priming effect of IFN-γ. Moreover, when functional parameters of PMNs obtained from patients with untreated advanced HIV infection were studied, we demonstrated that IL-15 was able to up-regulate the defective PMN chemotaxis and killing activity observed in such individuals. As reported by others in normal PMNs,26 we found no effect of IL-15 on the oxidative burst response of HIV+ PMN.
Previous studies have suggested a immunoregulatory role of IL-15 during HIV infection.27-33 To our knowledge, this study represents the first data to report that the depressed chemotactic and fungicidal activity observed in PMNs from patients with advanced HIV infection could be improved by in vitro treatment with IL-15 and was associated with decreased apoptosis. IL-15 has been shown to be a chemoattractant for T-lymphocytes and NK cells, promoting transendothelial migration of T cells into perivascular tissues.34-37 In this study, we found that IL-15 exhibited no direct chemoattractant effect on PMNs by itself; on the other hand, it acts as priming agent in inducing PMN migration in response to the chemotactic bacterial peptide fMPLB and IL-8. It is known that IL-15 has the ability to stimulate neutrophils to produce IL-8, a chemokine that induces both recruitment and activation of PMNs.38Therefore, it is conceivable that IL-15 could indirectly exert its biologic effect on chemotaxis by modulating the PMN expression of chemokines or their receptors that are essential for migration of additional neutrophils to sites of inflammation. On the other hand, IL-15 has the capacity to activate PMNs, by delaying apoptosis and enhancing their fungicidal and phagocytic activity. The ability of IL-15 to elicit functional responses of PMNs correlates with the finding that these cells express on their surface all 3 components of high-affinity IL-15 receptor, such as IL-15Rα and IL-2/15Rβ and γ subunits.38
In another set of experiments, we evaluated PMN functional activity of HIV-infected patients undergoing potent antiretroviral treatments, including 2 reverse-transcriptase inhibitors and one protease inhibitor. We found that there was a significant enhancement in PMN chemotaxis and fungicidal activity in patients who had continuous viral suppression, sustained increase in CD4+ T-cell count, and lack of infectious complications during HAART treatment. These findings are in agreement with our recent investigation assessing the phagocyte function in patients with moderate-advanced HIV-infection.17 In such patients, the administration of HAART led to a considerable improvement in the neutrophil and monocyte function that had not been observed in earlier studies among patients receiving only reverse transcriptase inhibitors. Such functional improvement was associated with suppression of HIV replication, but an immunomodulating effect of HIV reverse transcriptase or protease inhibitors on PMN function has been suggested.39 40Although PMNs exhibited increasing chemotactic and killing activity after HAART, a complete recovery of these functional parameters was never achieved. In this study, we showed that the in vitro addition of IL-15 to PMNs fully corrected and normalized the chemotactic responsiveness and candidal activity of these cells. The positive effect of IL-15 on HIV+ PMN function was also documented in HIV-infected patients who developed virologic and immunologic treatment failure during HAART. In these patients, the persistence of high levels of plasma HIV-RNA and the inadequate CD4+ T-cell response was associated with the impairment in PMN functional activity. The in vitro priming of PMNs with IL-15 induced in patients with HAART-treatment failure considerable improvements in PMN chemotaxis and antifungal function that was comparable to those obtained in patients who successfully responded to HAART.
Taken together, our results show that IL-15 is an important cytokine in activating the functional properties of PMNs from both HIV-negative and HIV-positive individuals. The potent stimulant effect of IL-15 on PMN function was observed in antiretroviral naive patients as well as in individuals who were receiving HAART, including those with treatment failure. Additional studies are needed to investigate the usefulness of IL-15 alone or in combination with antiretroviral drugs for enhancing the innate immune response during HIV infection.
Supported by a grant from the Ministero della Sanità-Istituto Superiore della Sanità-Progetto AIDS 1997 (30A.0.74) and 1998 (30B.88) (Rome, Italy).
Parts of this paper were presented at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, September 26-29, 1999, San Francisco, CA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Claudio M. Mastroianni, Department of Infectious and Tropical Diseases, La Sapienza University, Policlinico Umberto I, Viale Regina Elena 331, 00161 Rome, Italy; e-mail:cm.mastroianni@tiscalinet.it.