Abstract
The bone marrow is supplied with both sensory and autonomic neurons, but their roles in regulating hematopoietic and immunocompetent cells are unknown. Leukocyte growth and activity in patients with stable and complete spinal cord injuries were studied. The innervation of the bone marrow below the injury level lacked normal supraspinal activity, that is, a decentralized bone marrow. Lymphocyte functions were markedly decreased in injured patients. Long-term colony formation of all hematopoietic cell lineages, including dendritic cells, by decentralized bone marrow cells was substantially reduced. It was concluded that nonspecific and adaptive lymphocyte-mediated immunity and growth of early hematopoietic progenitor cells are impaired in patients with spinal cord injuries. Possibly, this reflects cellular defects caused by the malfunctioning neuronal regulation of immune and bone marrow function.
Introduction
Cytokines regulate hematopoiesis and immunity.1,2 In addition, sensory and autonomic neurons have been identified within the bone marrow, suggesting a neuronal regulatory function.3-6 We could not detect any functional role of these nerves,6,7 but this has been challenged.8-10 The discrepancies may result from different experimental approaches, different animal species, or both.6 Therefore, it was important to ascertain whether our findings would also apply to humans.
Complete injury to the spinal cord leads to functional loss of neuronal activity below the site of injury, termed a decentralized autonomous system.11 12 A comparison of hematopoiesis in bone marrow located above and below the injury level offers a unique opportunity to study regulatory functions of bone marrow innervation. Examination of immunocompetent cells from patients with large parts of their bodies denervated years ago might also provide clues to roles played by the nervous system in immune regulation and adaption. We, therefore, examined hematopoietic and immunocompetent cells from patients with a stable paraplegia or tetraplegia.
Patients, materials, and methods
Male study subjects
Control subjects (age range, 29-37 years) and patients with either paraplegia (age range, 35-54 years; injury level, Th5-Th10) or tetraplegia (age range, 36-49 years; injury level, C5-C7) who had been injured 7 to 40 years ago were studied. The level and completeness of their injuries met the criteria of the American Spinal Injury Association.13 They were otherwise healthy and received no regular medication, and their body temperatures, reactive protein C levels, and erythrocyte sedimentation rates were normal, as were their urine test results and their morning levels of cortisol and testosterone. We collected venous blood and bone marrow aspirates from the sternum and the iliac crest.
Lymphocyte assays
Natural killer (NK) and T cells were isolated from the blood sample by density centrifugation and staining with monoclonal antibodies (mAb). We used a 51Cr assay to determine their lytic effects.14 As a marker of B-cell function, immunoglobulins were quantified with radial immunodiffusion.
Bone marrow progenitor cell assays
Mononuclear cells were isolated from the aspirates after hemolysis and centrifugation and were cultured in methylcellulose (MethoCult; StemCell Technologies, Vancouver, BC, Canada) supplemented with cytokines. After 2 weeks the number of colony-forming units (CFU) was scored. The growth of long-term culture-initiating cells (LTC-IC) was examined with the MyeloCult system (StemCell Technologies). We selectively promoted the short- or long-term growth of dendritic cell colonies, as described.15 16
Results
Neither phenotyping of blood or bone marrow cells using mAb nor morphologic examination revealed any differences in the cell concentrations among the 3 study groups (data not shown).
Reduced lymphocyte activity in paraplegia and tetraplegia
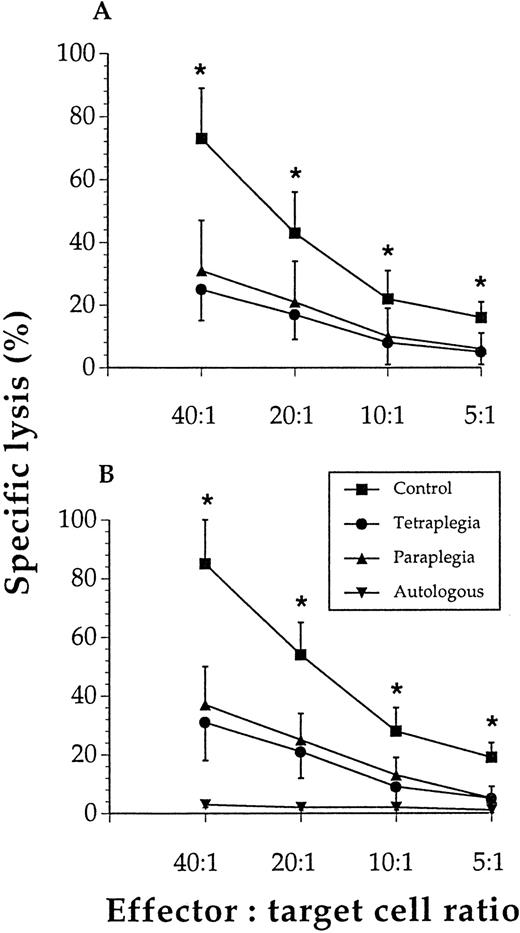
Figure 1A shows that though control NK cells exhibited marked cytotoxicity, the NK cells from patients had a profound loss of cytolytic capacity at all effector-to-target cell ratios tested. Figure 1B shows that T cells from the patients had a severely reduced ability to kill allogeneic lymphocytes. IgG levels were lower (P < .05) among the patients (mean ± SEM, n = 6): 5.2 ± 0.7 and 4.5 ± 0.7 g/L in the paraplegics and tetraplegics, respectively, versus 8.2 ± 0.8 g/L in the control subjects.
Lymphocyte cytotoxicity was reduced in subjects with spinal cord injury.
NK-cell lysis of K562 cells (A) and T-cell lysis of allogeneic lymphocytes (B) were significantly (*) reduced at all effector-to-target cell ratios tested when cells from paraplegics or tetraplegics were compared with those of controls. Values are mean and SEM, n = 6. The specific lysis of autologous lymphocytes was negligible in all 3 study groups, and these results were pooled for these groups (ie, n = 18).
Lymphocyte cytotoxicity was reduced in subjects with spinal cord injury.
NK-cell lysis of K562 cells (A) and T-cell lysis of allogeneic lymphocytes (B) were significantly (*) reduced at all effector-to-target cell ratios tested when cells from paraplegics or tetraplegics were compared with those of controls. Values are mean and SEM, n = 6. The specific lysis of autologous lymphocytes was negligible in all 3 study groups, and these results were pooled for these groups (ie, n = 18).
Decreased progenitor cell growth in decentralized bone marrow
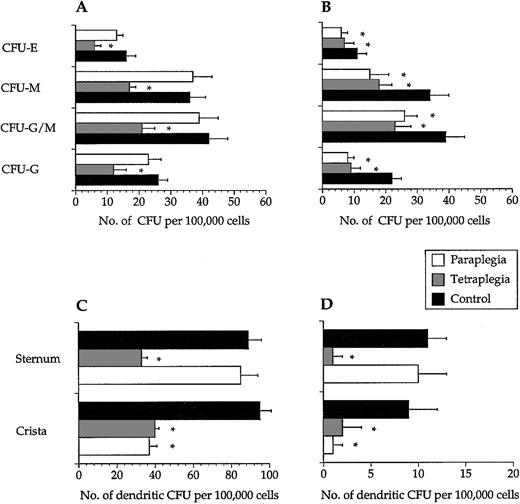
Colony numbers were not different among the 3 study groups when recorded after 2 weeks (data not shown). In long-term assays, colony numbers obtained from samples of decentralized bone marrow were lower than cells from intact bone marrow (Figure2A,B). In line with this, 12 ± 4 and 15 ± 4 LTC-IC per 106 input cells (mean ± SEM, n = 6) from crista aspirates were scored from the paraplegics and the tetraplegics, respectively, whereas the corresponding number for the control group was 27 ± 8 (P < .05). A similar reduction was found in LTC-IC numbers of cells sampled from the sternum of the tetraplegics but not from the paraplegics or the control subjects (data not shown). Dendritic cell colony formation was clearly reduced in decentralized bone marrow in short- and long-term assays (Figure 2C,D).
Hematopoietic progenitor cells from decentralized bone marrow showed decreased long-term colony formation.
In long-term assays, progenitor cells collected from bone marrow below the site of the spinal cord injury had a significantly (*) reduced ability to form all types of colonies from bone marrow sampled from the sternum (A) and the iliac crest (B). CFU-E–erythroid, CFU-M–macrophage, CFU-G/M–granulocyte/macrophage, and CFU-G–granulocyte colonies. Progenitor cells collected from bone marrow below the site of the spinal cord injury had a significantly reduced ability to form dendritic cell colonies in both short- (C) and long-term (D) assays. Values are mean + SEM, n = 6. Note the different values on the x-axes.
Hematopoietic progenitor cells from decentralized bone marrow showed decreased long-term colony formation.
In long-term assays, progenitor cells collected from bone marrow below the site of the spinal cord injury had a significantly (*) reduced ability to form all types of colonies from bone marrow sampled from the sternum (A) and the iliac crest (B). CFU-E–erythroid, CFU-M–macrophage, CFU-G/M–granulocyte/macrophage, and CFU-G–granulocyte colonies. Progenitor cells collected from bone marrow below the site of the spinal cord injury had a significantly reduced ability to form dendritic cell colonies in both short- (C) and long-term (D) assays. Values are mean + SEM, n = 6. Note the different values on the x-axes.
Discussion
Lymphocyte-mediated nonspecific (NK cell) and adaptive (B and T cell) immunity were markedly impaired in patients with stable spinal cord injury. Neither the decreased immunity nor the hampered hematopoiesis correlated with the time of the injuries. Malfunctioning lymphocyte-mediated immunity among spinal cord-injured patients might be translated into a clinical context because these patients are prone to various diseases.17-19
Reduced lymphocyte activity most likely reflected a qualitative defect because leukocyte numbers in blood and bone marrow were similar in patients and controls. In the tetraplegics, substantial fractions of NK and naive B cells must have been formed in decentralized bone marrow. However, the defects in lymphocyte activity were similar in paraplegics and tetraplegics. The reduced activities were likely caused by suboptimal stimulation of the cells or the qualitative cellular defects, such as, for example, a reduced expression of the Fas ligand.20 Both NK- and T-cell functions were found to be reduced after spinal cord injury and remained low for the first year.21 However, these data were from stressed subjects (high urine cortisol levels) with recent and probably unstable injuries that were not further described.
A major and novel finding was the consistent decrease in bone marrow levels of LTC-IC and dendritic-CFU, whereas most types of short-term colony formation of decentralized bone marrow cells were normal. Because only the early noncommitted progenitor cells survive the long-term assay, a growth defect probably occurred in these cells. Given that dendritic cells play a pivotal role in regulating T- and B-cell function, impaired dendritic cell growth might cause or further aggravate the insufficient lymphocyte-mediated immunity found in the patients.22
In paraplegics and tetraplegics, the decentralized part of the spinal cord and the sympathetic ganglia function as reflex centers for neuronal activity below the injury level. Possibly the decreased lymphocyte functions and dendritic progenitor cell levels, as well as the reduced LTC-IC values in decentralized bone marrow, reflect a lack of centrally coordinated neuronal control of hematopoiesis. However, in rodent studies, no functional role of the bone marrow innervation was detected.6 7 Our findings may be explained by the inactivity characterizing these patients, possibly by impaired blood flow through decentralized bone marrow. The addition of another experimental group consisting of bedridden patients would be desirable. However, such patients are not easily studied because they are often old or suffer from other diseases affecting the nervous system. Finally, inactivity is a less likely explanation for our results because the differences between decentralized and intact bone marrow were also found in the paraplegics. It will, therefore, be important to investigate further the properties of innervated and denervated bone marrow.
Acknowledgment
We thank the staff at the Hormone Laboratory at Aker University Hospital for analyzing cortisol and testosterone.
Supported in part by the Norwegian Cancer Society, the Research Council of Norway, and Anders Jahre's Foundation for the Promotion of Science.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Per Ole Iversen, Department of Hematology, Ullevaal University Hospital, Kirkeveien 166, 0407 Oslo, Norway; e-mail: poiversen@hotmail.com.