Abstract
Endovascular infection is a highly critical complication of invasive Staphylococcus aureus disease. For colonization, staphylococci must first adhere to adhesive endovascular foci. Von Willebrand factor (vWF) is a large, multimeric glycoprotein mediating platelet adhesion at sites of endothelial damage. Earlier it was demonstrated that vWF binds to and promotes the surface adhesion ofS. aureus, prompting this effort to identify the vWF adhesin. In Western ligand assays of S. aureus lysates, staphylococcal protein A (SPA) was recognized by purified vWF. Surface plasmon resonance demonstrated the binding of soluble vWF to immobilized recombinant protein A with a Kd of 1.49 × 10−8 mol/L. Using flow cytometry, the binding of fluorescein isothiocyanate–labeled vWF to S. aureus was found to be saturable and inhibitable by unlabeled vWF, antiprotein-A antibodies, or IgG. Isogenic Δspa::Tcr mutants were constructed by the insertion of a tetracycline resistance cassette intospa using allelic replacement, and it exhibited decreased binding of soluble vWF and decreased adhesion to vWF-adsorbed surfaces. The interaction was restored on complementation of the mutants withspa-containing plasmid pSPA7235. In conclusion, protein A confers interaction of S. aureus with soluble and immobilized vWF in a newly discovered function characterizing protein A as a novel member of the staphylococcal surface protein adhesin superfamily and suggesting its potential role in the pathogenesis of endovascular staphylococcal disease.
Introduction
The induction of endovascular infections involves complex interactions between surface components on the invading organism and various host determinants. Staphylococcus aureus is a major pathogen in endovascular infections, such as infective endocarditis, suppurative thrombophlebitis, or vascular or heart valve prosthetic infection.1 Among the host factors potentially contributing to endovascular infections, various plasma and extracellular matrix proteins—such as fibronectin, fibrinogen, thrombospondin, collagen, elastin, and vitronectin—have been found to bind to S. aureus, and several adhesins on the staphylococcal surface have been functionally or molecularly characterized.2 Experimental infections using defined adhesin deletion mutants have been suggestive of a role for these adhesins3 or for their pleiotropic regulatorssar and agr4,5 in invasive endovascular disease. However, in some instances, results from animal experimentation have been found to yield contrasting results.6,7 In addition to blood and matrix proteins, the interaction of S. aureus with platelets has been scrutinized for its role in the pathogenesis of infective endocarditis because platelets are considered to play a primary role in the early steps of staphylococcal adhesion to damaged endothelium and to spur the growth of the infective vegetation.8-10 Concomitantly, platelets have been shown to act as specialized inflammatory host defense cells exerting important antimicrobial activity through platelet microbicidal proteins.11
Among the factors released by endothelial cells and by platelets, constitutively or on stimulation, is von Willebrand factor (vWF), a large multifunctional glycoprotein characterized by high molecular weight multimers of up to 15 million daltons, consisting of subunits of 250 to 270 kd.12 After release, multimers are subject to plasma protease cleavage resulting in multimer heterogeneity, and they are subsequently bound to various subendothelial components, such as collagens, proteoglycans, and glucosaminoglycans.13Immobilized vWF is pivotal for platelet adhesion at sites of vascular injury in the presence of high shear stress, an adhesion reaction mediated through the GPIb-IX-V complex on resting and the GPIIb-IIIa complex on stimulated platelets.14 Previously, we described the interaction of S. aureus with vWF both in suspension and immobilized on the surface. Binding isotherms revealed a dose-dependent binding reaction to S. aureus Cowan 1 and other S. aureus isolates, adsorption kinetics showed saturable immobilization of vWF on solid substrates, and, consequently, solid-phase vWF could be demonstrated to promote adhesion of variousS. aureus isolates by 2 to 3 orders of magnitude.15
In this study, the promotion of adhesion was found to be sensitive to trypsin pretreatment of the bacteria. Furthermore, though the adhesion of all other clinical and laboratory isolates was promoted by surface-adsorbed vWF, the adhesion of S. aureus Wood 46, a protein A–negative strain, was not promoted on vWF-polymethyl methacrylate (PMMA). Therefore, we hypothesized that an adhesin, possibly of the MSCRAMM (microbial surface components recognizing adhesive matrix molecules) family of staphylococcal surface proteins,16 might be involved in the interaction ofS. aureus with vWF and therefore investigated the nature of the putative vWF adhesin by use of various biochemical and molecular experimental approaches.
Materials and methods
Bacteria
The microorganisms used in this study were S. aureuswild-type strains Cowan 1, Newman, and NCTC 8325-4.15 The following mutants were constructed: DU 5889, DU 5873, and DU 5875. These are Δspa::Tcr protein A deletion mutants of strains Cowan 1, Newman, and NCTC 8325-4, respectively. The mutants have been generated by allelic replacement mutagenesis performed as previously described.17,18 DU 5873 and DU 5875 were complemented with plasmid pSPA7235 as previously described,19 resulting in strains DUNew/253 and DU83/253. All strains were propagated on tryptic soy agar or in tryptic soy broth containing appropriate antibiotics for marker selection (tetracycline or chloramphenicol, 10 μg/mL, respectively). For the labeling of bacteria with [3H]-thymidine, bacteria were grown in Mueller-Hinton broth. For other binding or adhesion experiments, overnight cultures freshly grown in 5-mL brain–heart infusion were used. After washing and resuspension in phosphate-buffered saline (PBS), to prepare for binding and adhesion experiments, bacterial cells were subjected to brief sonication (10 cycles, each of 1 second, 50 W, Branson sonifier) for the separation of clumped microorganisms.
Preparation of bacterial lysates
Overnight cultures with 5 mL S. aureus cells (1 × 109/mL) were incubated with lysostaphin (Ambicin L; Applied Microbiology, New York, NY) (400 μg/g wet weight of bacteria) and DNase (Sigma, St Louis, MO) (100 μg/g). After centrifugation (3000g), the supernatant was heat-treated (10 minutes, 80°C) to inactivate bacterial proteases. Protein concentrations were determined using the BCA protein assay (Pierce, Rockford, IL).
Purification and labeling of vWF
vWF was purified from Haemate-HS500 (provided by Centeon Pharma, Marburg, Germany) as previously described.15,20 In brief, cryoprecipitates were subjected to size chromatography (using a column containing BioGel A-15m [Bio-Rad]) in a Tris-HCl buffer (50 mmol/L Tris, 150 mmol/L NaCl, 5 mmol/L Na+ citrate, pH 7.35) (TBS) containing a proteinase inhibitor cocktail consisting of phenylmethylsulfonyl fluoride (1 mmol/L; Sigma), leupeptin hemisulfate (5 μmol/L; Sigma), and aprotinin (10 μmol/L; Trasylol; Bayer, Leverkusen, Germany). Eluted fractions were analyzed for vWF content as previously described15 by use of the BCA protein assay, a vWF ELISA, and an assay for ristocetin cofactor activity. Only the first 3 fractions containing high-molecular-weight vWF and shown to be more than 99% pure were used.15
For labeling of vWF with fluorescein-isothiocyanate (FITC), a previously described method21 was modified as follows. TBS containing vWF was supplemented with 2 mmol/L Ca++, and the pH of the solution was adjusted to 9.5 by the addition of Na+ carbonate. FITC (10 mg/mL), isomer I, on Celite 10% (Calbiochem, La Jolla, CA) was solubilized in dimethyl sulfoxide, added to vWF-TBS in a 1:10 ratio, and incubated for 30 minutes at 21°C. Unbound label was separated using a Sephadex G25 PD10 column (Pharmacia, Freiburg, Germany) equilibrated with HEPES buffer (10 mmol/L HEPES, 140 mmol/L NaCl). The concentration of FITC-labeled vWF was calculated as follows: vWF (mg/mL) = [A280 − (0.350 × A495)/E] using an extinction coefficient E of 0.7 for vWF. For labeling of vWF with digoxigenin (DIG), a commercial kit (Digoxigenin Labeling Kit; Boehringer, Mannheim, Germany) was used according to the manufacturer's instructions. After labeling, all protein preparations were checked for degradation using SDS-PAGE.
Western ligand assays and amino terminal amino acid sequencing of eluted proteins
Either purified vWF or whole bacterial lysate (40 μg/mL) was subjected to SDS-PAGE using 7.5% acrylamide slab gels. After migration, proteins were transferred to nitrocellulose membranes (membrane BA85; Schleicher & Schuell, Dassel, Germany) using a semidry trans-blot apparatus (Bio-Rad, Munich, Germany), and then the membranes were blocked using bovine serum albumin (BSA; 3% wt/vol) for 6 hours in PBS. Membranes were washed with PBS, incubated at gentle agitation with PBS containing DIG-labeled ligand (40 μg in 30 mL, 4 hours, room temperature), then washed 3 times in PBS/0.05% Tween 20 (10 minutes). For the detection of bound ligand, the digoxigenin detection kit (Boehringer) was used according to the manufacturer's instructions with alkaline phosphatase-coupled anti-DIG Fab fragments and nitroblue-tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate as color reagents. For N-terminal sequencing of blotted proteins, lysates were transferred after SDS-PAGE separation to polyvinylidene difluoride membranes. The vWF binding band was localized after detection by Western ligand analysis, eluted, and analyzed with an ABI 494-A automated sequencer (Applied Biosystems, Weiterstadt, Germany).
Detection of vWF binding by surface plasmon resonance
Surface plasmon resonance (SPR) was determined using a Biacore 2000 instrument (Biacore AB, Uppsala, Sweden). The detection principle of SPR is based on an optical phenomenon allowing the detection of changes in mass concentration on the surface of a sensor chip. For the determination of vWF binding to immobilized recombinant protein A (rSPA) (Sigma), 6150 relative response units (RU) rSPA (corresponding to 1.6 ng) was immobilized on sensor chip CM5 by amine coupling using 10 mmol/L NaAc buffer (pH 4.0). The design of the Biacore instrument allows the simultaneous detection of vWF binding to the immobilized rSPA and an uncoated control surface under continuous flow conditions in the detection chamber. HEPES buffer (25 mmol/L HEPES, 100 mmol/L NaCl, 1 mmol/L CaCl2, 1 mmol/L MgCl2, and 0.005% surfactant P20 [pH 7.4]) was used for instrument equilibration and protein injection, and regeneration after ligand binding was achieved using glycine buffer (100 mmol/L glycine, 500 mmol/L NaCl, pH 4.0). The flow rate used was 30 μL/minute. If not indicated otherwise, vWF was used at a concentration of 250 μg/mL. In some experiments, vWF was digested using S. aureus V8 (Sigma). For IgG binding studies, pooled human immunoglobulin (Venimmun; Sandoz, Nuremberg, Germany) was used.
Flow cytometric analysis of FITC-vWF binding to S. aureus
Bacteria from a fresh overnight culture were diluted at a concentration of 120 000 cells/μL in TBS buffer containing 2 mmol/L Ca++ (pH 7.4). FITC-labeled vWF (final concentration, 0-150 μg/mL) was incubated with the bacterial suspension (10 minutes at room temperature). After washing and sonication, bacteria (5000 cells/determination) were analyzed in a flow cytometer (FACScan; Becton Dickinson, Heidelberg, Germany) using an excitation wavelength of 488 nm at the FACScan standard configuration with a 530-nm bandpass filter. Data were obtained from fluorescence channels in a logarithmic mode.
Solid-phase adhesion assays
For radiometric analysis of S. aureus adhesion to solid surfaces, a previously described assay was used.22Briefly, solutions containing indicated concentrations of purified vWF were allowed to adsorb to PMMA coverslips (60 minutes at 37°C). Thereafter, coverslips were washed with PBS and incubated in a shaking water bath with [3H]-thymidine–labeled S. aureus cells (4 × 106 CFU in 1 mL PBS containing 0.5% human serum albumin [HSA]) (60 minutes at 37°C). After adhesion, unbound microorganisms were removed from coverslips by washing twice, and PMMA-adherent radioactivity was determined. For the determination of S. aureus binding to preadsorbed PMMA under flow conditions, slides (60 × 24 mm) were preincubated with 5 mL vWF (50 μg/mL) (60 minutes at 37°C), then placed in a parallel-plate flow chamber (CytoDyne, San Diego, CA). To maintain a well-defined laminar flow field between the parallel plates of the flow chamber, a syringe pump containing PBS/Ca++Mg2+/HSA buffer and either S. aureus NCTC 8325-4 or its Δspamutant, DU 5875, introduced the cell suspension into the chamber at a constant flow rate. The shear rate was calculated to be 110 s−1 according to the formula,S = 6Q/wh2 , where S is the shear rate (s−1), Q is the volumetric flow rate (0.008 cm3/s), w is chamber width (0.7 cm), andh is the chamber gap thickness (0.025 cm).23Analysis of the attached bacteria over time was performed using an inverted Axiovert 135 TV (Zeiss, Oberkochen, Germany) microscope, a motorized stage table (Märzhäuser, Wetzlar, Germany), and algorithms for an image analysis software package (Optimas 5.1; Media Cybernetics, Silver Spring, MD). A macro was written in the Optimas Analytical Language for Images (Media Cybernetics) to recursively capture and store digital images of 10 different microscopic fields at each time point. Counts of attached bacteria were subsequently performed by digital image analysis of the captured images (gray level thresholding and size discrimination) to distinguish bacteria from the background in the bright-field images.
Results
Detection of vWF binding to staphylococcal proteins by Western ligand analysis
Whole-cell lysates of S. aureus strains Cowan 1, NCTC 8325-4, and Newman were prepared using lysostaphin, resolved on 7.5% SDS-polyacrylamide gels, and transferred to nitrocellulose. Blot membranes were blocked and incubated with purified DIG-labeled vWF (37°C at 240 minutes). After washing, vWF bound to blot membranes was detected in a color reaction. As shown in Figure1A (left), a single band of 50 to 55 kd was identified in all 3 test strains. Parallel blots performed with DIG-labeled anti–protein A antibodies revealed a strong reaction of bands of identical size compared with those detected with DIG-vWF (Figure 1A, right). Control reactions prepared with nitrocellulose-transferred whole- cell staphylococcal lysates were developed using all reagents, with the exception of DIG-labeled vWF. These controls revealed no color reaction of the blot membrane (Figure1A, center). For further characterization, the blotted proteins ofS. aureus Cowan 1 and 8325-4 recognized by vWF were analyzed using automated N-terminal sequencing, and the following amino acid sequence was obtained: N-Ala-Gln-His-Asp-Glu-Ala-Gln-Gln-Asn-Ala-Phe-Tyr-Gln-Val-Leu-Asn-Met-Pro-Asn-Leu-Asn-Ala-Asp-Gln-Arg-Asn-Gly-Phe-Ile-Gln-(Ser)-Leu-Lys-Asp-Asp. This sequence is 100% identical to the published sequence of SPA.24 25 To confirm that SPA is the molecule recognized by vWF in the Western ligand assays, lysates of the Δspadeletion mutants DU 5889, DU 5873, and DU 5875 were separated by SDS-PAGE, blotted, and incubated with DIG-labeled vWF. These ligand-binding assays revealed no interaction of molecules contained in nitrocellulose-blotted S. aureus lysate with soluble vWF (Figure 1B, lane 1′-3′).
Interaction of vWF with soluble S. aureuscomponents or with SPA.
(A) Western ligand and Western blot analyses of whole-cell staphylococcal lysates. S. aureus Cowan 1, Newman, and NCTC 8325-4 lysates (2 μL) (lanes 1, 2, and 3, respectively) were separated on 7.5% SDS-PAGE, blotted on nitrocellulose, and incubated either with [DIG]-vWF in PBS (left), with polyclonal [DIG]-anti-SPA-Abs in PBS (right), or with PBS alone (center). Binding was detected using anti-DIG-Fab fragments and subsequent exposure with a chromogenic substrate (5 minutes). (B) Western ligand analysis of vWF binding to lysates of S. aureus wild-type (lanes 1, 2, and 3, respectively) and their respective Δspa deletion mutants (lanes 1′-3′). Experimental conditions identical to those in A (left). For enhanced detection sensitivity, gels were intentionally overloaded (20 μL lysate/lane), and blots were overexposed (30 minutes). (C) SDS-PAGE of rhvWF and Western ligand analysis of SPA binding. rhvWF expressed from Chinese hamster ovary cells (40 μg) was subjected to SDS-PAGE (7.5% gels), and gels were either stained with Coomassie blue (left) or used for blotting of vWF onto nitrocellulose (right). Membranes were subsequently blocked and incubated with [DIG]-rSPA, and rSPA binding was demonstrated in a color reaction described in A.
Interaction of vWF with soluble S. aureuscomponents or with SPA.
(A) Western ligand and Western blot analyses of whole-cell staphylococcal lysates. S. aureus Cowan 1, Newman, and NCTC 8325-4 lysates (2 μL) (lanes 1, 2, and 3, respectively) were separated on 7.5% SDS-PAGE, blotted on nitrocellulose, and incubated either with [DIG]-vWF in PBS (left), with polyclonal [DIG]-anti-SPA-Abs in PBS (right), or with PBS alone (center). Binding was detected using anti-DIG-Fab fragments and subsequent exposure with a chromogenic substrate (5 minutes). (B) Western ligand analysis of vWF binding to lysates of S. aureus wild-type (lanes 1, 2, and 3, respectively) and their respective Δspa deletion mutants (lanes 1′-3′). Experimental conditions identical to those in A (left). For enhanced detection sensitivity, gels were intentionally overloaded (20 μL lysate/lane), and blots were overexposed (30 minutes). (C) SDS-PAGE of rhvWF and Western ligand analysis of SPA binding. rhvWF expressed from Chinese hamster ovary cells (40 μg) was subjected to SDS-PAGE (7.5% gels), and gels were either stained with Coomassie blue (left) or used for blotting of vWF onto nitrocellulose (right). Membranes were subsequently blocked and incubated with [DIG]-rSPA, and rSPA binding was demonstrated in a color reaction described in A.
Protein A interacts with vWF
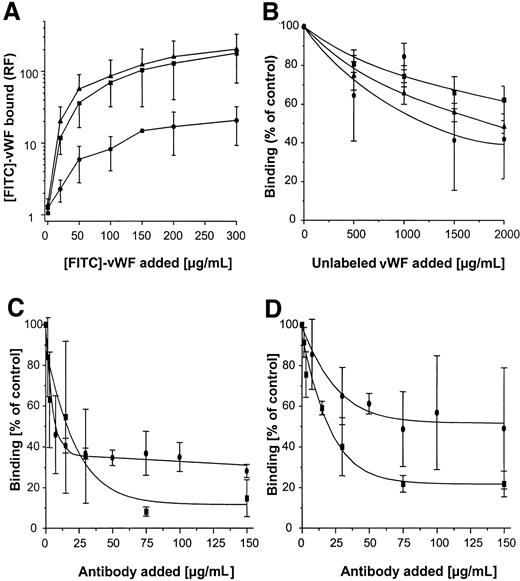
To confirm further the interaction of vWF with SPA, in a reciprocal binding reaction recombinant human (rh) vWF was subjected to SDS-PAGE, blotted onto nitrocellulose, and incubated with DIG-labeled rSPA. After development of the color reaction, a single band recognized by SPA and corresponding to a protein with an apparent molecular weight of 250 kd was detected (Figure 1C, right lane). This protein was the same size as rhvWF in Coomassie-stained SDS-PAGE (Figure 1C, left lane). In addition to Western ligand analysis, the interaction of vWF with SPA was evaluated using SPR. For this purpose, rSPA was immobilized on a CM5 sensor chip and exposed to purified vWF, and binding was analyzed using a Biacore 2000 instrument (Biacore AB) (Figure 2A). After the injection of vWF, a linear increase in RU was measured on the rSPA-coupled surface, whereas the control surface showed no response increase. Assuming a mean of 10 vWF monomers per multimeric molecule,15 the kon was calculated to be 3.07 × 103mol/L-1 s-1. At the end of the injection, the response signal on the rSPA surface remained elevated, and it decreased at a koff rate of 4.58 × 10-5s-1. The resultant Kd was calculated as 1.49 × 10−8 mol/L. After surface regeneration, the RU value on the rSPA surface was identical to that on the control surface. The binding of vWF was found to be dose dependent when vWF concentrations ranging from 200 to 500 μg/mL were tested (Figure 2A, insert). HSA or BSA (3 μmol/L) did not bind to rSPA surfaces (Figure2B). As expected, injections of IgG (1060 nmol/L) resulted in a response increase on rSPA surfaces as a result of a saturable binding reaction (Figure 2C). After the saturation of binding sites, no further increase in response could be observed with the injection of vWF. However, regeneration of the surface restored the ability of rSPA to recognize the vWF ligand (Figure 2C). Taken together, these findings clearly demonstrate the dose-dependent, reversible, and specific character of the interaction of vWF with SPA. Because S. aureusexpresses a serine protease (V8 protease), the effect of vWF pretreatment with V8 protease on the vWF-SPA interaction was further determined. Digestion of vWF with V8 protease (4 U/mL) was found to be complete after 10 minutes, as demonstrated by SDS-PAGE (not shown). As shown in Figure 2D, binding of vWF fragments to SPA yielded an increase in RU similar to that observed using undigested vWF.
SPR analysis of vWF interaction with rSPA-coupled CM5 sensor surfaces (solid lines) or uncoupled surfaces (dashed lines).
(A) After instrument equilibration, vWF (250 μg/mL in HEPES buffer) was injected (➁) followed by HEPES buffer (➀) and surface regeneration with glycine buffer (③) as described in “Materials and methods,” and RU was determined. (inset) Response measured as a function of time using different vWF concentrations (250/300/350/400/450/500 μg vWF/mL, lower through upper line) calculated by subtracting RUvWF-CM5-RUCM5. (B) Response curves after the injection of HSA (upper 2 lines) and BSA (lower 2 lines) followed by the injection of HEPES buffer. (C) Response after 4 injections of pooled human IgG (1060 nmol/L) (➁) followed by the injection of vWF (250 μg/mL) (➀). After regeneration of the surfaces, vWF (250 μg/mL) was injected (③), and surfaces were equilibrated and regenerated as in A. (D) Interaction of vWF and V8 protease-treated vWF with SPA. After immobilization of SPA (9300 RU), either vWF (100 μg/mL, 30 μL) (➁) or vWF digested with protease V8 (4 IU, 30 minutes at 37°C) (➀) was injected. After binding, surfaces were regenerated as described in A. Shown is the difference plot of signal obtained on [RUvWF-CM5 − RUCM5].
SPR analysis of vWF interaction with rSPA-coupled CM5 sensor surfaces (solid lines) or uncoupled surfaces (dashed lines).
(A) After instrument equilibration, vWF (250 μg/mL in HEPES buffer) was injected (➁) followed by HEPES buffer (➀) and surface regeneration with glycine buffer (③) as described in “Materials and methods,” and RU was determined. (inset) Response measured as a function of time using different vWF concentrations (250/300/350/400/450/500 μg vWF/mL, lower through upper line) calculated by subtracting RUvWF-CM5-RUCM5. (B) Response curves after the injection of HSA (upper 2 lines) and BSA (lower 2 lines) followed by the injection of HEPES buffer. (C) Response after 4 injections of pooled human IgG (1060 nmol/L) (➁) followed by the injection of vWF (250 μg/mL) (➀). After regeneration of the surfaces, vWF (250 μg/mL) was injected (③), and surfaces were equilibrated and regenerated as in A. (D) Interaction of vWF and V8 protease-treated vWF with SPA. After immobilization of SPA (9300 RU), either vWF (100 μg/mL, 30 μL) (➁) or vWF digested with protease V8 (4 IU, 30 minutes at 37°C) (➀) was injected. After binding, surfaces were regenerated as described in A. Shown is the difference plot of signal obtained on [RUvWF-CM5 − RUCM5].
Binding of soluble vWF to S. aureus wild-type strains, to Δspa S. aureus mutants, and to complemented mutants
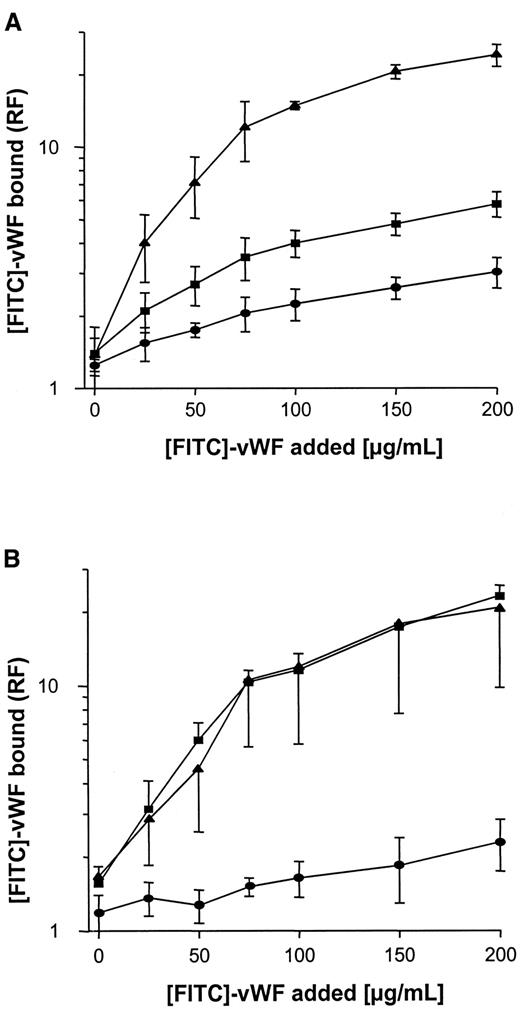
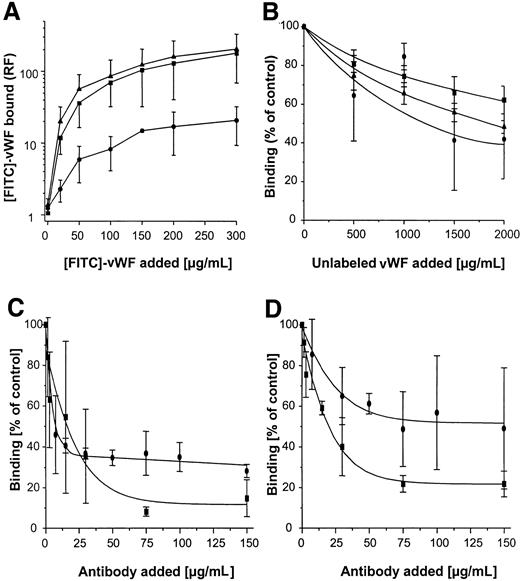
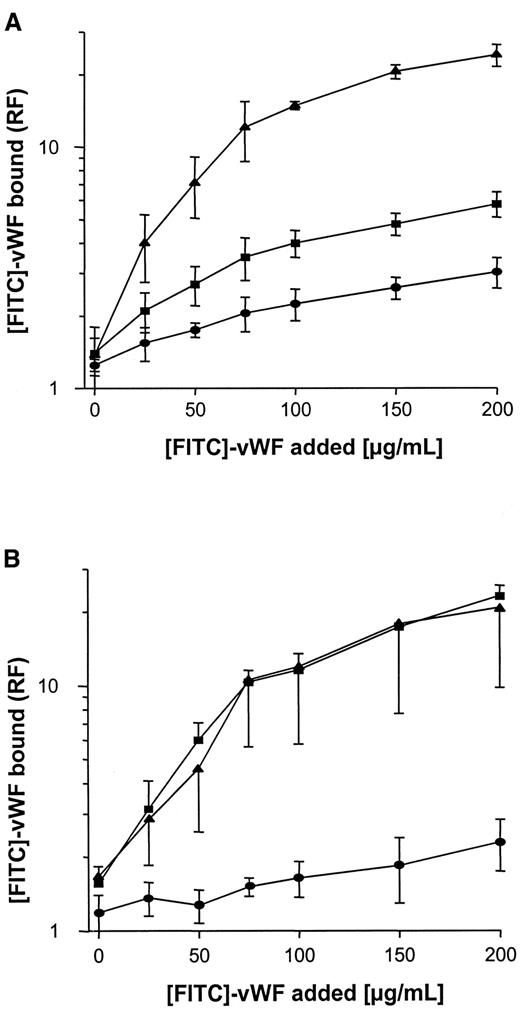
Binding of FITC-labeled vWF to S. aureus was determined using flow cytometry. As shown in Figure3A, binding was found to be dose dependent, with saturable binding achieved at concentrations of 150 to 200 μg/mL and half-maximal binding achieved at concentrations of approximately 50 μg/mL. Although binding of vWF to S. aureus Cowan 1 and Newman was found to be similar, maximal binding to NCTC 8325-4 was approximately one tenth that of S. aureusCowan 1 and Newman. Binding of FITC-labeled vWF to NCTC 8325-4 was inhibited by the addition of unlabeled vWF; up to 58% binding inhibition was observed with the addition of 10-fold binding excess to the incubation mixture (Figure 3B). These findings suggest specificity of the interaction, possibly by an adhesin-mediated reaction. To test the role of SPA in this binding reaction, anti-SPA polyclonal antibodies were used in inhibition assays and were found to inhibit vWF-binding by 85% and 78% for S. aureus Cowan 1 and Newman, respectively. Binding was also inhibited, albeit to a lesser extent, by IgG (Figure 3C,D). Two isogenic site-directed Δspa mutants were further explored. Both mutants ofS. aureus Newman and NCTC 8325-4 showed greatly reduced binding compared with the wild-type strain (Figure4). Although relative fluorescence values of vWF bound to DU 5873 were approximately one tenth of those observed with wild-type S. aureus Newman, thespa-deficient strain DU 5875 exhibited approximately one tenth of the binding values observed with wild-type strain NCTC 8325-4. Complementation of the deletion mutants fully restored binding. Although binding isotherms of the wild-type and complemented mutants ofS. aureus Newman were almost superimposable, vWF bound to the complemented strain DU83/256 to a larger extent than to the wild-type strain S. aureus NCTC 8325-4 (Figure 4).
Flow cytometric analysis of binding of vWF to S. aureus.
Microorganisms (120 000 cells/mL) were incubated with FITC-vWF (150 μg/mL, 10 minutes at 21°C), then analyzed for binding by flow cytometry as described in “Materials and methods.” (A) Binding of vWF to 3 S. aureus strains (▴, Cowan 1; ▪, Newman; ●, 8325-4). (B) Inhibition of vWF binding to S. aureus strains by the addition of excess unlabeled vWF over FITC-vWF (200 μg/mL) (symbol denotation as in A). (C, D) Inhibition of vWF binding toS. aureus Cowan 1 (C) and Newman (D) by the addition of polyclonal anti-SPA (▪) and IgG (●) antibodies. Shown are means ± SD (n = 3). RF indicates relative fluorescence.
Flow cytometric analysis of binding of vWF to S. aureus.
Microorganisms (120 000 cells/mL) were incubated with FITC-vWF (150 μg/mL, 10 minutes at 21°C), then analyzed for binding by flow cytometry as described in “Materials and methods.” (A) Binding of vWF to 3 S. aureus strains (▴, Cowan 1; ▪, Newman; ●, 8325-4). (B) Inhibition of vWF binding to S. aureus strains by the addition of excess unlabeled vWF over FITC-vWF (200 μg/mL) (symbol denotation as in A). (C, D) Inhibition of vWF binding toS. aureus Cowan 1 (C) and Newman (D) by the addition of polyclonal anti-SPA (▪) and IgG (●) antibodies. Shown are means ± SD (n = 3). RF indicates relative fluorescence.
Binding of vWF to Δspa mutants and pSPA7235-complemented Δspa mutants.
Experimental conditions as described in Figure 3. (A) S. aureus NCTC 8325-4. (B) S. aureus Newman. ▪, wild-type strains; ●, Δspa mutants; ▴, complemented mutants. RF indicates relative fluorescence.
Binding of vWF to Δspa mutants and pSPA7235-complemented Δspa mutants.
Experimental conditions as described in Figure 3. (A) S. aureus NCTC 8325-4. (B) S. aureus Newman. ▪, wild-type strains; ●, Δspa mutants; ▴, complemented mutants. RF indicates relative fluorescence.
Adhesion of S. aureus wild-type strains, Δspa mutants, and complemented mutants to vWF-adsorbed surfaces
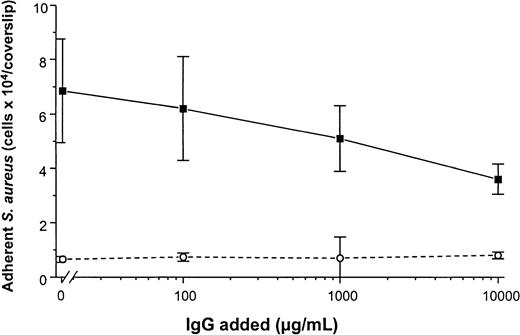
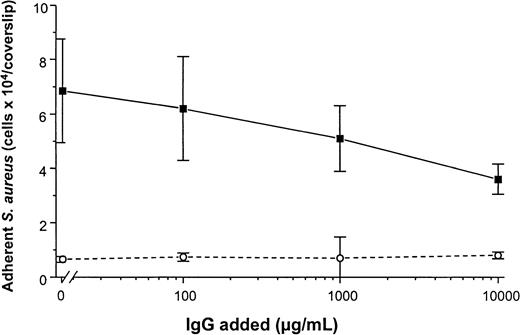
To determine the promotion of staphylococcal adhesion to vWF surfaces, a radiometric adhesion assay and an assay for the determination of adhesion under defined flow conditions was used. For the radiometric adhesion assay, [3H]-thymidine–labeledS. aureus NCTC 8325-4 cells (4 × 106 CFU/mL) were incubated with PMMA coverslips preadsorbed with vWF (30 μg/mL) (HSA-PBS, 60 minutes at 37°C). Although adhesion of the wild-type strain NCTC 8325-4 was promoted by surface-bound vWF (adhesion, 4.9 ± 0.9 × 104 CFU/PMMA coverslip), adhesion of the Δspa mutant DU5875 was significantly less (adhesion, 1.2 ± 0.5 × 104; 24% of wild type;P = .003 [unpaired t test]). With the complementation of DU5875, adhesion could not only be restored but was found to be higher when compared with the wild-type strain (adhesion, 6.1 ± 1.3 × 104; 124% with wild type) (Figure5A). Similar results were observed when Cowan 1 and its SPA-deficient mutant were tested (Figure 5B). Although human IgG interacts with SPA, S. aureus adheres to immobilized vWF even at physiological concentrations of IgG. Adhesion to vWF-adsorbed surfaces was inhibitable only to 48% in the presence of excess amounts (10 000 μg/mL) of IgG (Figure6).
Adhesion of S. aureus to vWF surfaces.
PMMA coverslips were preadsorbed with indicated concentrations of vWF, then incubated with [3H]-thymidine–labeled cells (4 × 106 CFU/mL) of either S. aureus 8325-4 (A), Cowan 1 (B), the respective Δspa mutants, or the pSPA7235-complemented Δspa mutant in PBS/HSA (60 minutes at 37°C). ▪, wild-type strains; ●, Δspa mutants; ♦, complemented mutant. Shown are mean values ± SD of 3 determinations performed in quintuplicate.
Adhesion of S. aureus to vWF surfaces.
PMMA coverslips were preadsorbed with indicated concentrations of vWF, then incubated with [3H]-thymidine–labeled cells (4 × 106 CFU/mL) of either S. aureus 8325-4 (A), Cowan 1 (B), the respective Δspa mutants, or the pSPA7235-complemented Δspa mutant in PBS/HSA (60 minutes at 37°C). ▪, wild-type strains; ●, Δspa mutants; ♦, complemented mutant. Shown are mean values ± SD of 3 determinations performed in quintuplicate.
Adhesion of S. aureus Cowan 1 to vWF-PMMA in the presence of IgG.
Experimental conditions as in Figure 5. After adsorption of vWF (50 μg/mL), adhesion assays were performed in the presence of increasing concentrations of human pooled IgG (Venimmun) (solid line). Control assays were performed with PMMA pretreated with PBS alone (dashed line). Shown are mean values ± SD of a quintuplicate determination.
Adhesion of S. aureus Cowan 1 to vWF-PMMA in the presence of IgG.
Experimental conditions as in Figure 5. After adsorption of vWF (50 μg/mL), adhesion assays were performed in the presence of increasing concentrations of human pooled IgG (Venimmun) (solid line). Control assays were performed with PMMA pretreated with PBS alone (dashed line). Shown are mean values ± SD of a quintuplicate determination.
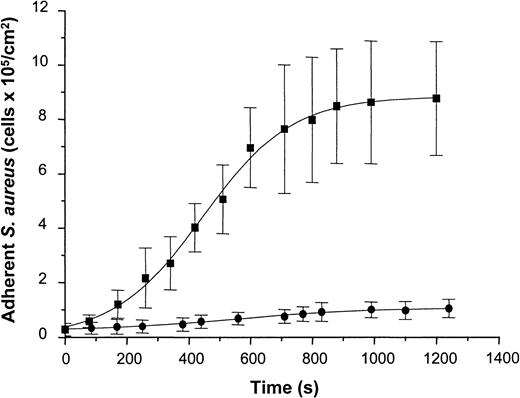
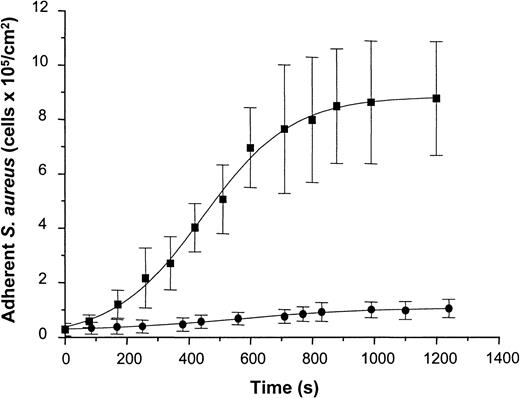
To determine staphylococcal adhesion to vWF-adsorbed surfaces under well-defined flow conditions, a suspension containing S. aureus NCTC 8325-4 cells (5 × 107 CFU/mL in PBS-HSA) was introduced into the parallel-plate flow chamber at a shear rate of 110/s. Attaching cells were detected by video-enhanced light microscopy and analyzed using image analysis. Adhesion of the wild-type strain increased as a function of time and exhibited an almost linear increase in the number of adherent cells during the first 10 minutes of perfusion and saturation of adhesion after prolonged times of perfusion. Cell density values of 8.78 ± 2.09 × 105 cells/cm2 were achieved after 20 minutes of perfusion (Figure 7). In contrast to the wild-type strain, the Δspa mutant of NCTC 8325-4 showed only a slight linear increase in adhesion with 1.04 ± 0.32 × 105 cells/cm2 adhering after 20 minutes of perfusion. This difference was found to be highly significant (t test; P < .001). Based on these data, the average rate constant of attachment was calculated from the rate of attachment divided by the concentration of bacteria in suspension. This yielded values of 1.3 ± 0.1 × 10−3 cm/min for the wild-type strain and 8.1 ± 1.2 × 10−5 cm/min for the mutant strain. Based on an analysis of convective–diffusive transport of bacteria in the flow chamber described elsewhere,26 27 the rate constant for the wild-type corresponded to the transport-limited rate of attachment in the flow cell (ie, the attachment probability of cells reaching the surface was close to 100%).
Adhesion of S. aureus 8325-4 and of DU 5875 to vWF adsorbed PMMA under defined flow conditions.
PMMA slides were preincubated with vWF (50 μg/mL), mounted on a flow chamber, and perfused with S. aureus cells (5 × 107 CFU) (shear rate, 110/s). Adherent microorganisms visualized by light microscopy were determined over indicated time periods using automated image analysis. Shown are mean values of 10 fields ± SD. ▪, S. aureus 8325-4; ●, DU 5875.
Adhesion of S. aureus 8325-4 and of DU 5875 to vWF adsorbed PMMA under defined flow conditions.
PMMA slides were preincubated with vWF (50 μg/mL), mounted on a flow chamber, and perfused with S. aureus cells (5 × 107 CFU) (shear rate, 110/s). Adherent microorganisms visualized by light microscopy were determined over indicated time periods using automated image analysis. Shown are mean values of 10 fields ± SD. ▪, S. aureus 8325-4; ●, DU 5875.
Discussion
In this report, we describe the identification of a novel function of SPA as an adhesin for a platelet and extracellular matrix protein, vWF. SPA recognition in Western ligand assays of staphylococcal whole-cell lysates as the putative vWF-binding adhesin prompted the detailed analysis of the role of SPA in the interaction of S. aureus with vWF by evaluating spa-deficient allelic replacement mutants and spa-complemented Δspadeletion mutants in various binding and adhesion assays. The results provide clear evidence for a role of SPA in the interaction with vWF.
Seven surface proteins from S. aureus have been characterized so far at the molecular level. Five proteins—the collagen-binding protein Cna, the fibronectin-binding proteins FnBPA and FnBPB, and the fibrinogen-binding proteins ClfA and ClfB—share common structural features, leading to a characterization of these molecules as adhesins of the MSCRAMM family.2 Although the extracellular portion of the various MSCRAMMs is unique and consists of different domains, the wall-spanning regions share homology in consisting of proline- or glycine-rich residues or of Ser-Asp dipeptide repeats and an LPXTG motif. This motif provides the anchoring of the protein by an amide bond between the carboxyl of threonine and the amino of the pentaglycine cross-bridge attached to the ε-amino group of lysyl.28,29 A sixth MSCRAMM, the elastin-binding protein EbpS, does not contain the LPXTG motif.30 The seventh protein is the archetypical LPXTG-anchored, wall-associated protein SPA. However, SPA is not considered an MSCRAMM because it has never been demonstrated to contain binding functions for adhesive matrix molecules.
SPA is an exoprotein that binds to the Fc region of immunoglobulins of most mammalian species.31 It consists of 5 extracellular domains (designated E, D, A, B, and C), cell wall–spanning regions (Xr and Xc), and an 18- to 20-residue hydrophobic membrane spanning domain distal to LPXTG.24,25Each extracellular domain can bind 1 IgG molecule through its Fcγ binding sites (the classical site).32,33 The Fc-binding function of SPA has traditionally been viewed as a major contributor to its role in bacterial virulence31 because it competes with phagocytic cells for available IgG-Fc sites, resulting in diminished IgG-mediated opsonization.34 In addition, an alternative site on SPA that recognizes the Fab fragments of immunoglobulins independent of the heavy-chain isotype has been identified,35 with domains D and E binding Fab fragments through variable (V) regions.36 These Fv-binding sites endow SPA with the ability to cross-link membrane IgM on B cells,37 hence inducing their activation and conferring a superantigen function to SPA.38,39 In addition to the immunoglobulin-binding functions, SPA has been shown to activate complement40; recently, it has been shown that this SPA-induced activation of the classical complement pathway depends on the binding of a VH3+ IgM molecule to SPA.32 By the generation of an inflammatory reaction, in addition to its anti-opsonic function, SPA-induced complement activation may contribute to staphylococcal virulence.41These in vitro findings have been supported by evidence from experimental models. Although studies determining the virulence of chemically mutagenized staphylococcal strains have yielded ambiguous results with respect to SPA because of multiple phenotypic changes of the mutants,42,43 direct evidence of the role of SPA has resulted from studies with a site-specific mutant in mouse infection models. Using these defined SPA− mutants, significant differences between the virulence of the mutant and the wild-type models in peritonitis and subcutaneous infections17 and in the mouse mastitis model44 were observed, whereas virulence in a rabbit keratitis model was attributable to α-toxin but not to SPA.41
For identification of the vWF adhesin, we selected a strategy that has already been successfully used for other staphylococcal adhesins.45,46 In Western ligand assays, we could identify a single protein band recognized by soluble vWF that weighed 50 to 55 kd. Because the reported molecular weights of SPA ranged from 42 kd47 to 57 kd,25,48 it was suggested that the vWF-recognized protein could be SPA. This could be confirmed by Western immunoblot experiments and N-terminal amino-acid sequencing and by the observation that Western ligand experiments using soluble vWF exposed to immobilized lysates of Δspa deletion mutants failed to identify any vWF-interacting S. aureus proteins in these mutants. Western ligand assays using solubilized purified proteins, however, do not provide proof of the nature of these proteins as adhesins. Therefore, we used SPR for the determination of direct interaction of SPA and vWF. SPR has been successfully used for label-free, real-time binding studies.49 Lately, this technique has been adapted for the evaluation of adhesins of Gram-positive cells.50 Using SPR, we analyzed the interaction of soluble vWF with immobilized rSPA and could demonstrate binding, thus further suggesting the specificity of the vWF-SPA interaction. When it is exposed to S. aureus, vWF may be digested by secreted proteases such as the S. aureus serine protease V8.51 Despite proteolytic degradation, however, we found that vWF fragments retain their ability to bind to immobilized protein A. This suggests that even after proteolytic degradation, the adhesive function of vWF may be operational.
To establish its role clearly as staphylococcal adhesin, a putative MSCRAMM molecule must be demonstrated to mediate the interaction of whole bacterial cells with extracellular matrix molecules. In previous reports, flow cytometry has been successfully used to monitor the interaction of staphylococci with whole cells.8 Thus, we modified and adapted this technique, which we have established for the determination of vWF binding to platelets52 for staphylococci. Our findings confirm and extend the previously published data on vWF–S. aureus interaction by demonstrating both the saturation ability of binding and binding inhibition with the addition of unlabeled ligand. Half-maximal binding concentrations were found to approximate 50 μg/mL. Given this value to correspond to an estimate of Kd, the apparent dissociation constant would be in the order of nanomolar considering the multimeric size of the molecule, suggesting binding affinities similar to those of fibronectin53 or thrombospondin.54 To evaluate further the role of SPA, we tested Δspa deletion mutants and spa-complemented mutants in this system. Our demonstration of decreased binding of the mutants and restored binding of the complemented strains further corroborates the role of SPA in the staphylococcal interaction with vWF.
SPA not only confers binding of soluble ligand, it promotes the adhesion of staphylococcal cells to vWF-adsorbed surfaces. A first indication for the role of SPA in the interaction with surface-bound vWF was given by our previous observation, demonstrating the lack of adhesion promotion of S. aureus Wood 46.15 In this study, the role of SPA in surface adhesion assays was evidenced by the demonstration of significantly decreased adhesion of the Δspa deletion mutants of NCTC 8325-4 (DU 5875) and ofS. aureus Cowan (DU 5889) to vWF-adsorbed PMMA, whereas the complementation of DU 5875 with spa resulted in adhesion to vWF comparable to that of wild type. In addition, when the binding of NCTC 8325-4 and DU 5875 under defined shear forces as a function of time was evaluated, a significant difference was observed.
Interaction of S. aureus with vWF occurs in vivo in an IgG-containing milieu. Our observations demonstrating the IgG-binding SPA as the adhesin for vWF and the inhibition of vWF interaction with immobilized SPA on pretreatment with IgG may suggest that this interaction does not take place in vivo. On the other hand, when investigating other S. aureus–binding plasma proteins, such as fibronectin or fibrinogen, it has been clearly demonstrated that attachment to these adhesive molecules occurs even in the presence of plasma concentrations of the ligand,55 that adhesion to ex vivo catheter material is promoted by these ligands,56,57and, most important, that staphylococcal deletion mutants deficient in adhesins for fibronectin- or fibrinogen-binding proteins are less virulent in endovascular infection models irrespective of large plasma concentrations of the ligand.3,7 In line with these observations, our experiments revealed the inhibition of adherence to immobilized vWF by maximally 50% in the presence of large (10 000 μg/mL) concentrations of IgG. For fibrinogen, it has been suggested that high local concentration58 and conformational changes59 confer the effect of the surface-immobilized ligand on platelet binding and activation. Although we have previously shown that vWF is significantly adsorbed on PMMA,15 it remains an open issue whether similar mechanisms contribute to the retained adhesion of S. aureus to immobilized vWF, even in the presence of large concentrations of IgG.
In conclusion, in experiments using site-directed S. aureus mutants for the evaluation of binding with and adhesion to vWF, we provide compelling evidence that SPA confers interaction with this adhesive extracellular matrix protein. The molecular architecture of SPA fulfills all characteristics of most staphylococcal adhesins of the MSCRAMM family (all except EbpS, which is not LPXTG anchored). SPA expression has been demonstrated to be coregulated by 2 repressor systems, agr and sar,19,60 resulting in decreased expression in stationary growth phases, a characteristic that SPA shares with other MSCRAMMs. Most recently, S. aureusisolates from patients with Kawasaki disease, an acute vasculitis of young children complicated by coronary artery abnormalities, have been shown to express high levels of SPA.61 Together with our findings, this observation attributes to SPA a hitherto unknown function in staphylococcal pathogenesis. Further investigations will focus on the binding domains on both molecules involved in the SPA-vWF interaction and on the application of this in vitro evidence to adequate experimental models, such as endovascular infection. These issues are the subjects of current investigations in our laboratories.
Acknowledgments
We thank Susanne Weber and Marion Schiphorst for excellent technical help, Dorothea Voss and Centeon Pharma for providing Haemate HS, and M. A. Schmidt for helpful discussions.
Supported by Deutsche Forschungsgemeinschaft, Collaborative Research Center 293, Project A6, and the German Minister for Education, Science, Research, and Technology (grant 01 KI 9750/9). Supported in part by The Wellcome Trust (T. J. F.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mathias Herrmann, Department of Medical Microbiology, University of Münster, Domagkstrasse 10, 48129 Münster, Germany; e-mail: mathias.herrmann@uni-muenster.de.

![Fig. 1. Interaction of vWF with soluble S. aureuscomponents or with SPA. / (A) Western ligand and Western blot analyses of whole-cell staphylococcal lysates. S. aureus Cowan 1, Newman, and NCTC 8325-4 lysates (2 μL) (lanes 1, 2, and 3, respectively) were separated on 7.5% SDS-PAGE, blotted on nitrocellulose, and incubated either with [DIG]-vWF in PBS (left), with polyclonal [DIG]-anti-SPA-Abs in PBS (right), or with PBS alone (center). Binding was detected using anti-DIG-Fab fragments and subsequent exposure with a chromogenic substrate (5 minutes). (B) Western ligand analysis of vWF binding to lysates of S. aureus wild-type (lanes 1, 2, and 3, respectively) and their respective Δspa deletion mutants (lanes 1′-3′). Experimental conditions identical to those in A (left). For enhanced detection sensitivity, gels were intentionally overloaded (20 μL lysate/lane), and blots were overexposed (30 minutes). (C) SDS-PAGE of rhvWF and Western ligand analysis of SPA binding. rhvWF expressed from Chinese hamster ovary cells (40 μg) was subjected to SDS-PAGE (7.5% gels), and gels were either stained with Coomassie blue (left) or used for blotting of vWF onto nitrocellulose (right). Membranes were subsequently blocked and incubated with [DIG]-rSPA, and rSPA binding was demonstrated in a color reaction described in A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/6/10.1182_blood.v96.6.2149/5/m_h81800139001.jpeg?Expires=1767727131&Signature=GuOt42-pmyttOaA86gy5~0AV5LCOMkXSF2GqWzKRaUFMnM-Dn5a4u555~ouplSLlw0AcMQQrbq76WkztxXcWJqjtn4IJl0axRFggsZy07AG5hcUjpyBpKDG75grq1OLqp3T57OH7Nc21tYVbTPzUpg3xG4xUEYwIHfSCNQGnv7yJdALfbDkV163hPfoylzlnVt43tviHJHrH-pnj4bnTRZMkoSprM32F2nmyoVTqxykan0DnQe5Nr0vv0uHEuQ7UCMRvAUAvHWeObKLCuGO7XrV9wAsn2HQ8tRdCgTqOcU0pyJYH4Q4ZnLnU9HRumLmksusacj3KfRAq1LWnRNyNsQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. SPR analysis of vWF interaction with rSPA-coupled CM5 sensor surfaces (solid lines) or uncoupled surfaces (dashed lines). / (A) After instrument equilibration, vWF (250 μg/mL in HEPES buffer) was injected (➁) followed by HEPES buffer (➀) and surface regeneration with glycine buffer (③) as described in “Materials and methods,” and RU was determined. (inset) Response measured as a function of time using different vWF concentrations (250/300/350/400/450/500 μg vWF/mL, lower through upper line) calculated by subtracting RUvWF-CM5-RUCM5. (B) Response curves after the injection of HSA (upper 2 lines) and BSA (lower 2 lines) followed by the injection of HEPES buffer. (C) Response after 4 injections of pooled human IgG (1060 nmol/L) (➁) followed by the injection of vWF (250 μg/mL) (➀). After regeneration of the surfaces, vWF (250 μg/mL) was injected (③), and surfaces were equilibrated and regenerated as in A. (D) Interaction of vWF and V8 protease-treated vWF with SPA. After immobilization of SPA (9300 RU), either vWF (100 μg/mL, 30 μL) (➁) or vWF digested with protease V8 (4 IU, 30 minutes at 37°C) (➀) was injected. After binding, surfaces were regenerated as described in A. Shown is the difference plot of signal obtained on [RUvWF-CM5 − RUCM5].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/6/10.1182_blood.v96.6.2149/5/m_h81800139002.jpeg?Expires=1767727131&Signature=cj7w~ZJewnWkzVnfJmdXNTMLjx4mtRzuFS-FEc-NH8o~ewyZZi~cp4T79ZK3vPd7JLPy51Dlcp0OJiDf6u957JRSH47ojIwpCZ28JrtcDqn05Gn2Ho4Mr5iXSqjmRjIcEMWe5Zjrb7zoB~TAoCFWWt7hrLvvnqMh2lMTaYuurGTWfvd0WpPtbP0dLO-6qB3q9J1uZvvTJRHfJkzdpa6hQUdAyp20JLzGeQwmwu1uS7HgPENuHeiHmW3vX8AD3I2sN9~NYTsS4k6usnB9k2~6g1hAXWbijNx~Cg2mBvBlmZpaMmZlbssB1~qO~2-X16MCR6A5VxetNP5LpH-5W9feuw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Adhesion of S. aureus to vWF surfaces. / PMMA coverslips were preadsorbed with indicated concentrations of vWF, then incubated with [3H]-thymidine–labeled cells (4 × 106 CFU/mL) of either S. aureus 8325-4 (A), Cowan 1 (B), the respective Δspa mutants, or the pSPA7235-complemented Δspa mutant in PBS/HSA (60 minutes at 37°C). ▪, wild-type strains; ●, Δspa mutants; ♦, complemented mutant. Shown are mean values ± SD of 3 determinations performed in quintuplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/6/10.1182_blood.v96.6.2149/5/m_h81800139005.jpeg?Expires=1767727131&Signature=mUw8WKL-hfs3b0bJaPk9CBnhgK-DtksyeddCRr8BUAFqwcZyrjic~9n2EzsqaeDQbYiM41nnx4RsS8jatCY3NhvRZo-nWdJC7lMU8cLBM8PLDHDnqBJe~gFI2lCwwvD~AIPaS~i3PuwJRpaBViwejBDtKPAdt0L4awdEx3U2I4ck-0yq1MdNJW6asQ4pyx-mBxuWQECOhBIuWZt5NXR1vZvhUza97U~-pxhbs1eYxe7JD9crs1eaMAfnreGZxKzICaDIN87Re9GyDu8gzi-mWAkZWXTtGWiMzBYi9yO2eiAfVDidXzw-uniBwuu-PyRcKAvX1Y3QvbSsE0m3GvBkyA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 1. Interaction of vWF with soluble S. aureuscomponents or with SPA. / (A) Western ligand and Western blot analyses of whole-cell staphylococcal lysates. S. aureus Cowan 1, Newman, and NCTC 8325-4 lysates (2 μL) (lanes 1, 2, and 3, respectively) were separated on 7.5% SDS-PAGE, blotted on nitrocellulose, and incubated either with [DIG]-vWF in PBS (left), with polyclonal [DIG]-anti-SPA-Abs in PBS (right), or with PBS alone (center). Binding was detected using anti-DIG-Fab fragments and subsequent exposure with a chromogenic substrate (5 minutes). (B) Western ligand analysis of vWF binding to lysates of S. aureus wild-type (lanes 1, 2, and 3, respectively) and their respective Δspa deletion mutants (lanes 1′-3′). Experimental conditions identical to those in A (left). For enhanced detection sensitivity, gels were intentionally overloaded (20 μL lysate/lane), and blots were overexposed (30 minutes). (C) SDS-PAGE of rhvWF and Western ligand analysis of SPA binding. rhvWF expressed from Chinese hamster ovary cells (40 μg) was subjected to SDS-PAGE (7.5% gels), and gels were either stained with Coomassie blue (left) or used for blotting of vWF onto nitrocellulose (right). Membranes were subsequently blocked and incubated with [DIG]-rSPA, and rSPA binding was demonstrated in a color reaction described in A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/6/10.1182_blood.v96.6.2149/5/m_h81800139001.jpeg?Expires=1767839135&Signature=4k6kg7yJymcpuhvyACkjRSeaDxgU17KN99Vr5cHajrUfBQ3yiCPoALG70w~WDrIVomOAJMrjW1LdJcOiCwXMMKfjn0lNIa2GPlx2tCUhQAZz2CdfQiQItSUkI1ebbzFTXOb1ZGsD1G8ylJb6DRFhYMX5zkfIM1DtrjSPwgQqDhX~u1RvWrUY2sN4xqdng5iKwHjmSOdkuImKUokwHCi2Amlls3AnVvcZ7yBOeXeQ~tSyKR~5QCyNvXfJcxgvBSuPhwQKzSLLHOGgL3~KrVntHCC-S-zhE5Cs7xR7IvEjE9WC6GUwrnD52HLfA2IfeBvYcQsZqlooVDIPyXK4IQnqaw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. SPR analysis of vWF interaction with rSPA-coupled CM5 sensor surfaces (solid lines) or uncoupled surfaces (dashed lines). / (A) After instrument equilibration, vWF (250 μg/mL in HEPES buffer) was injected (➁) followed by HEPES buffer (➀) and surface regeneration with glycine buffer (③) as described in “Materials and methods,” and RU was determined. (inset) Response measured as a function of time using different vWF concentrations (250/300/350/400/450/500 μg vWF/mL, lower through upper line) calculated by subtracting RUvWF-CM5-RUCM5. (B) Response curves after the injection of HSA (upper 2 lines) and BSA (lower 2 lines) followed by the injection of HEPES buffer. (C) Response after 4 injections of pooled human IgG (1060 nmol/L) (➁) followed by the injection of vWF (250 μg/mL) (➀). After regeneration of the surfaces, vWF (250 μg/mL) was injected (③), and surfaces were equilibrated and regenerated as in A. (D) Interaction of vWF and V8 protease-treated vWF with SPA. After immobilization of SPA (9300 RU), either vWF (100 μg/mL, 30 μL) (➁) or vWF digested with protease V8 (4 IU, 30 minutes at 37°C) (➀) was injected. After binding, surfaces were regenerated as described in A. Shown is the difference plot of signal obtained on [RUvWF-CM5 − RUCM5].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/6/10.1182_blood.v96.6.2149/5/m_h81800139002.jpeg?Expires=1767839135&Signature=4L3eqa4KmfVvzoqh0kLa2iIRKilmJcwndTErFQBTYA~DjIpNA0b9~3W4IYSKHyA0CR6cdUtfv~VVDzUMm1ENW-cVfrTS4yUnNJIuNUSlbPBzrJuRsKAIFB8sWZcNeztHbtDIpb1isRkc6rrS9dF2QDlp6nX-IQKctJxnt7UI5O2kE-wIHQSDfg6MqwW9PQf0yUJf6EUGCQBpaByeFTQtDpv3zMoM0SGv76VTomX58IFKuHvqA6vv4qBarZVGLYXUYZ6L4vctZxroGk2iMICK89I4ifgIVP68UVDdaW4vktk7iOI0mt2oL8M0I1fWJHvV8kHSg5oOqbH4ch83RY9qrQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Adhesion of S. aureus to vWF surfaces. / PMMA coverslips were preadsorbed with indicated concentrations of vWF, then incubated with [3H]-thymidine–labeled cells (4 × 106 CFU/mL) of either S. aureus 8325-4 (A), Cowan 1 (B), the respective Δspa mutants, or the pSPA7235-complemented Δspa mutant in PBS/HSA (60 minutes at 37°C). ▪, wild-type strains; ●, Δspa mutants; ♦, complemented mutant. Shown are mean values ± SD of 3 determinations performed in quintuplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/6/10.1182_blood.v96.6.2149/5/m_h81800139005.jpeg?Expires=1767839135&Signature=pAKOvLFjkJrL-D-Gmk5ZpDMrZ8FLsYY7ymIuZ~1h7IVmPVMg6Un-~B6sgoK3NOTkkostD1DSffiJu~TiTpb6IpmnEAuMVv8fk~6tOHd1VK~MSMN9b4FeeFZoDzNB8lY52hRAW1mVejvFpYZFpTC7hmlmFtBTHQ6fcAz9DtpcIdEv4SRxy9jVeY2KVCbkaJubnpaGiLMM885dLn3XigWVpoFdtypMCQNaIqKdrFYjik9YwFcHwDWhlRmi~lOVUanPZS2OLPsyx~Gb6QdD0HgMwz5PuQPZ-2KuNrZBx0yqFg2PFC4DC6bNigP4e2TNuEka7UexkPCXuV7A9bbN2Xu2lw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)