Abstract
A novel nonmyeloablative conditioning regimen was investigated in 44 patients with hematologic malignancies. The median patient age was 41 years. Many of the patients had high-risk features, including 19 patients with a previous failed transplant. Recipient conditioning consisted of CAMPATH-1H, 20 mg/day on days −8 to −4; fludarabine, 30 mg/m2 on days −7 to −3; and melphalan, 140 mg/m2 on day −2. Thirty-six recipients received unmanipulated granculocyte colony-stimulating factor–mobilized peripheral blood stem cells from HLA-identical siblings, and 8 received unmanipulated marrow from matched unrelated donors. GVHD prophylaxis was with cyclosporine A alone for 38 patients and cyclosporine A plus methotrexate for 6 sibling recipients. Forty-two of the 43 evaluable patients had sustained engraftment. Results of chimerism analysis using microsatellite polymerase chain reaction indicate that 18 of 31 patients studied were full-donor chimeras while the other patients were mixed chimeras in one or more lineages. At a median follow-up of 9 months (range 3 to 29 months), 33 patients remain alive in complete remission or with no evidence of disease progression. Seven patients relapsed or progressed post-transplantation, and 4 of them subsequently died. Four patients died of regimen-related complications. There were no cases of grades III-IV acute GVHD. Only 2 patients developed grade II acute GVHD, and only 1 had chronic GVHD. The estimated probability of nonrelapse mortality was 11%. Although longer follow-up is needed to establish the long-term remission rates, this study demonstrates that this nonmyeloablative preparative regimen is associated with durable engraftment, minimal toxicity, and low incidence of GVHD.
Introduction
High-dose chemoradiotherapy followed by allogeneic stem cell transplantation (SCT) has been extensively used to treat patients with hematologic malignancies. This procedure is often limited to patients in good medical condition because of the increased risk of regimen-related toxicity and graft-versus-host disease (GVHD) that occurs with increasing age and poor performance status.1,2The curative potential of transplantation is not solely due to the conditioning regimen but also to the well-documented graft-versus-leukemia (GVL) effect.3 The most convincing evidence for this GVL effect is that donor leukocyte infusions (DLIs) can reinduce remissions in patients who have relapsed following allogeneic SCT.4,5 Patients with chronic myeloid leukemia are most likely to respond, but responses have also been documented in patients with acute leukemia, chronic lymphocytic leukemia, myeloma, and lymphoma.6 7
In an effort to reduce the transplant-related mortality (TRM) associated with allogeneic SCT, low-intensity fludarabine-based regimens have been developed.8-11 These have been designed to be immunosuppressive rather than myeloablative to facilitate donor engraftment and thereby limit systemic toxicity. There appears to be a spectrum of hematopoietic toxicity associated with these nonmyeloablative regimens—from minimally cytopenic regimens that use low-dose total body irradiation alone12 to regimens that combine fludarabine with melphalan or busulfan.10,13 While these studies have demonstrated impressive allogeneic engraftment with minimal nonhematologic toxicity, there is still a significant morbidity and mortality from acute and chronic GVHD.8-11
We have therefore developed a novel nonmyeloablative regimen for allogeneic SCT. Our regimen was designed to suppress the recipient immune system enough to allow allogeneic engraftment without excessive regimen toxicity or GVHD. The use of fludarabine as an immunosuppressant as part of the conditioning regimen was similar to previously published studies of nonmyeloablative SCT.8-11The combination of fludarabine with 180 mg/m2 of melphalan was originally described by the M. D. Anderson group.14 Our regimen used a different dose of melphalan, 140 mg/m2; however, the addition of in vivo CAMPATH-1H to the conditioning regimen was new and appears to have been crucial in limiting graft-versus-host reactions.
Patients and methods
Eligibility criteria
Patients with hematologic malignancies were enrolled at 6 hospitals in England. The study design was approved by the ethics committees at each participating site. All patients gave written informed consent to participate. Patients with lymphoma, acute leukemia, myelodysplasia, multiple myeloma, chronic lymphocytic leukemia, and chronic myeloid leukemia between ages 18 and 60 years were eligible to participate. Patients required an HLA-identical sibling or unrelated donor as determined by serologic typing for HLA A/B and molecular typing for HLA DR/DQ. Data were analyzed as of November 30, 1999.
Patient characteristics
Detailed characteristics are shown in Table1. Forty-four patients were enrolled in the study from June 1997 to September 1999. Twenty-eight were male, and 16 were female. Age range at the time of transplantation was 18 to 56 years (median, 41 years). Fourteen patients had non-Hodgkin lymphoma (NHL), 10 Hodgkin disease, 6 acute myeloid leukemia (AML), 7 multiple myeloma, 3 hypoplastic myelodysplastic syndrome, 1 acute lymphoblastic leukemia, 1 chronic lymphocytic leukemia, 1 chronic myeloid leukemia, and 1 plasma cell leukemia. This was a cohort of patients with high-risk features, including 19 patients with a previous failed autologous (18 patients) or allogeneic (1 patient) transplant, renal failure (2 patients), poor left ventricular function (2 patients), or refractory disease (9 patients). The median time interval from first to second transplant was 24 months (range, 8-79 months).
Monoclonal antibody
CAMPATH-1H is a humanized immunoglobulin (Ig) G1 monoclonal antibody against the CD52 antigen.15 It was prepared from the culture supernatant of Chinese hamster ovary cell transfectants cultured in a hollow fiber fermentor. It was purified by affinity chromatography on protein A-sepharose (Amersham Pharmacia Biotech, Little Chalfont, England) and size exclusion chromatography on Superdex 200 (Amersham Pharmacia Biotech) and formulated in phosphate-buffered saline. The half-life of CAMPATH-1H in humans is dependent on the amount of target CD52 antigen in the patient. Based on work in progress, there is persistence on CAMPATH-1H in vivo past day 0 sufficient to cause T-cell lysis by antibody-dependent cell-mediated cytotoxicity.
Conditioning regimen
Treatment consisted of the humanized monoclonal antibody CAMPATH-1H, 20 mg/day intravenous infusion over 8 hours on days −8 to −4; fludarabine, 30 mg/m2 intravenous infusion over 30 minutes on days −7 to −3; and melphalan, 140 mg/m2intravenous infusion over 30 minutes on day −2. Thirty-six recipients received unmanipulated peripheral blood stem cells (PBSCs) from their siblings, and 8 received unmanipulated marrow from matched unrelated donors.
Stem cell and bone marrow collection
Sibling donors received granulocyte colony-stimulating factor (G-CSF) at 10 μg/kg subcutaneously once daily on days −4 to 0. Leukaphereses were performed on days 0 and +1 using conventional techniques for PBSC collection. Unrelated donors had bone marrow collected on day 0 under general anesthesia using conventional techniques. In 2 sibling donors, we failed to collect more than 2 × 106/kg CD34+ cells from G-CSF–mobilized peripheral blood, and therefore bone marrow was also harvested. The total number of CD34+ cells collected from peripheral blood and the number of mononuclear cells collected from the bone marrow are shown in Table 1. Unmanipulated mobilized peripheral blood or bone marrow was infused through central venous catheters on days 0 and +1 and on day 0, respectively.
Supportive care
Patients were managed in reverse isolation in conventional or laminar airflow rooms. All patients received prophylaxis with cotrimoxazole or pentamidine against Pneumocystis cariniiinfection. Acyclovir and fluconazole or itraconazole prophylaxis were routinely used. Blood products were irradiated to 25 Gy. Red cell and platelet transfusions were given to maintain hemoglobin levels above 9 g/dL and platelet count above 10 to 15 × 109/L. The cytomegalovirus (CMV)-seronegative patients received only CMV-negative blood products; seropositive patients received CMV-unscreened blood products. Febrile neutropenic patients received broad-spectrum intravenous antibiotics according to each hospital's policy for the management of neutropenic sepsis. G-CSF at 5 μg/kg per day was administered subcutaneously at the discretion of the transplant physician to speed hematologic recovery in 38 patients until the patient's absolute neutrophil count was at least 1000/μL for 3 consecutive days (Table 2).
GVHD prophylaxis and grading
GVHD prophylaxis consisted of cyclosporine A (CsA), 3 mg/kg starting on day −1, and methotrexate (MTX) at a dose of 10 mg/m2 on days +1, +3, and +6 for 6 sibling recipients, and it consisted of CsA alone for the other 38 patients. Intravenous CsA was switched to an oral dose as soon as the patients would tolerate medications by mouth and was continued for a median of 4 months (range, 1 to 8 months). Patients who survived 100 days or longer were evaluable for chronic GVHD. Acute and chronic GVHD were graded according to the consensus criteria.16
Follow-up
Patients had regular follow-up at 3 monthly intervals post-transplantation to assess disease response and remission status. These evaluations varied depending on the underlying diagnosis but included bone marrow aspirates or biopsies, cytogenetics, computed tomography scans, paraprotein levels, and skeletal surveys.
Chimerism analysis
DNA was prepared from pretransplantation recipient blood and donor blood. Following transplantation, either buffy coat or granulocyte T-cell and B-cell preparations were obtained from peripheral blood as previously described.17 We used 4 different primer sets each flanking highly polymorphic short tandem repeat units on different human chromosomes. With primers VWA31 (Perkin-Elmer) and THO1 (Perkin-Elmer), polymerase chain reaction (PCR) volumes were 50 μL containing Genamp PCR buffer II (Perkin-Elmer), 1.5-mmol/L MgCl, 0.2 mmol/L of each deoxyribonucleoside triphosphate (dNTP), 1 mmol/L of each primer, 0.5 U of AmpliTaq DNA polymerase (Perkin-Elmer, Foster City, CA), and 5 μL of DNA. Cycling conditions were 95°C for 45 seconds, 54°C for 30 seconds, and 72°C for 1 minute for 30 cycles. With primers ACPP (forward: ACTGTGCCTAGCCTATACTT, backward: AGTGAGCCAAGAGTGCACTA) and HUMSTRX1 (forward: CTCCTTGTGGCCTTCCTTAAATGG, backward: CTTCTCCAGCACCCAAGGAAGTCA), PCR volumes were 50 μL containing VNTR buffer (45-mmol/L Tris-HCl, 11-mmol/L NH4SO4, 6.7-mmol/L 2-mercaptoethanol, 4.5-μmol/L ethylenediaminetetraacetic acid, 110-μg/mL bovine serum albumin), 5 mmol/L of each dNTP, 1 mmol/L of each primer, 0.5 U of AmpliTaq polymerase (Perkin-Elmer), and 5 μL of DNA. Cycling conditions were 95°C for 30 seconds, 58°C for 30 seconds, and 95°C for 30 seconds for 30 cycles. The forward primer of each pair was labeled with either JOE or FAM fluorescent dyes. One microliter of PCR product was denatured in 12 μL of formamide and electrophoresed through Performance Optimized Polymer 4 (Perkin-Elmer) on an ABI 110 automated sequencer (Perkin-Elmer) in the presence of Rox 500 size standard (Perkin-Elmer). Genescan software 2.1 (Perkin-Elmer) was used to analyze the data. Primers that gave rise to recipient/donor–specific peaks were identified and used for post-transplantation determination of chimeric status in the various cell populations.
Study endpoints
The primary study endpoint was the successful durable hemopoietic engraftment and TRM. There were secondary endpoints, including regimen-related toxicity, incidence and severity of GVHD, and progression-free survival.
Statistical methods
Actuarial curves were estimated according to the Kaplan-Meier method. Surviving patients were censored on the last day of follow-up. The significance of differences between the curves was estimated by the log-rank test. Cox multivariate regression analysis was performed to calculate the independent effects of various risk factors influencing nonrelapse mortality, overall survival, and disease-free survival. The proportional hazards assumption was tested using a time-dependent covariant approach.
Results
Toxicities
All patients were assessable for toxicity. The conditioning regimen was generally well tolerated in patients who only received CsA as GVHD prophylaxis. The use of MTX in addition to CsA was associated with severe mucositis and delayed engraftment (Table 2). The original intention was to give MTX to all patients, but its use was abandoned because of toxicity. There were no cases of veno-occlusive disease. Four patients died of regimen-related toxicity. One patient died on day +21 of gram-negative septicemia while still aplastic. The second patient died on day +24 of idiopathic pneumonitis after engrafting on day +14. The third patient died of MRSA pneumonia on day +153. The fourth patient with myeloma, whose creatinine clearance prior to transplantation was 23 mL/min, died of renal failure on day +148.
Engraftment
One patient was not evaluable for engraftment because of early death on day +21. Of the 43 patients eligible for assessment of engraftment, 42 had sustained engraftment as defined by neutrophil counts above 0.5 × 109/L and an untransfused platelet count of above 20 × 109/L for at least 3 consecutive days. Details of the neutrophil and platelet reconstitution are shown in Table 2. The median time to recover an absolute neutrophil count of 0.5 × 109/L was 13 days (range, 8-23 days) and of 1.0 × 109/L was 17 days (range, 8-47 days). The median time to achieve platelets above 20 × 109/L was 13 days (range, 3-96 days) and above 50 × 109/L was 17 days (range, 8-118 days). Patient No. 7 developed graft rejection. After initial engraftment on day +11, the patient became cytopenic on day +20 and reconstituted recipient hemopoiesis on day +31 without autologous stem cell support.
Chimerism
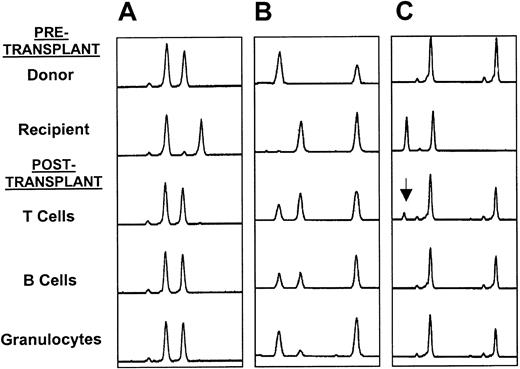
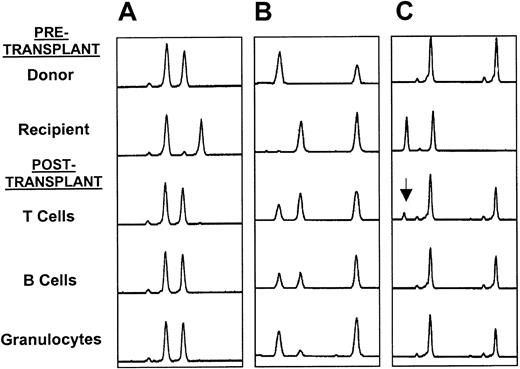
Thirty-one patients had chimerism studies performed using microsatellite PCR or fluorescent in situ hybridization for X and Y chromosomes on peripheral blood. Detailed results are shown in Table3. Patient No. 7, who rejected his graft, had only recipient myeloid and lymphoid cells present. Of the other 30 patients, 18 had only donor cells present. Of the 12 patients with mixed chimerism, 5 had detailed lineage-specific studies performed using microsatellite PCR. Three of these patients were full-donor chimeras in the myeloid and B-cell lineages but were mixed T-cell chimeras. Two patients were mixed chimeras in all lineages tested (Figure 1). Six of the 8 unrelated recipients had chimerism studies performed, and all 6 were found to have only donor cells present following transplantation (Table 3). Two patients (Nos. 1 and 11) who were mixed chimeras post-transplantation became full-donor chimeras following DLI therapy.
Microsatellite analyses.
(A) Full-donor chimerism in all 3 lineages; (B) mixed chimerism in all 3 lineages; and (C) mixed T-cell chimerism (arrow shows recipient T-cell marker) with full-donor chimerism and B cells and granulocytes.
Microsatellite analyses.
(A) Full-donor chimerism in all 3 lineages; (B) mixed chimerism in all 3 lineages; and (C) mixed T-cell chimerism (arrow shows recipient T-cell marker) with full-donor chimerism and B cells and granulocytes.
Graft-versus-host disease
No grade III-IV acute GVHD was observed post-transplantation. Three patients developed grade I skin, 1 patient grade II gastrointestinal, and 1 patient grade II skin and gastrointestinal (Table 2) acute GVHD. Only 1 patient has developed chronic GVHD, limited to skin and liver involvement. Two patients developed GVHD following DLI to treat relapse of disease. One of these patients had steroid-resistant grade IV acute GVHD, and the other had limited chronic GVHD.
Disease response and relapses
Current disease status is shown in detail in Table 2. The conditioning regimen induced remissions in 2 of 2 patients with refractory AML and 2 of 2 evaluable patients with myelodysplasia. Of the 6 patients with Hodgkin disease in partial remission or with refractory disease at transplantation, 2 achieved a complete remission (CR) following transplantation, and 4 are progression-free. Seven patients with NHL in partial remission or with refractory disease prior to transplantation were evaluable for disease response (Table 2). Two of these patients achieved a CR, and 3 remain progression-free; however, 2 patients with transformed low-grade NHL had early post-transplantation disease progression (Table 2). None of the 6 patients with myeloma achieved a CR following the transplantation procedure alone, although patient No. 11 achieved a CR following post-transplantation DLI.
Seven patients relapsed or progressed following transplantation (Table2). Patient No. 1, with mantle cell lymphoma, relapsed 9 months post-transplantation and was treated with DLI. He died 11 months post-transplantation with steroid-resistant grade IV GVHD. Patient No. 12, with relapsed AML, progressed 3 months post-transplantation and was treated with DLI but died 3 months later of disease progression. Patient No. 13, with refractory AML, relapsed 6 months post-transplantation, was treated with DLI, and achieved transient remission but relapsed and died 14 months post-transplantation. Patient No. 14, with Hodgkin disease, relapsed 15 months post-transplantation and was treated with DLI and has not yet responded to this therapy. Patient No. 25, with refractory transformed low-grade NHL, progressed shortly after engraftment and, despite DLI, died a month later. Another patient with transformed low-grade NHL, patient No. 35, progressed 3 months post-transplantation. Patient No. 43 had progression of myeloma following transplantation.
Survival analyses
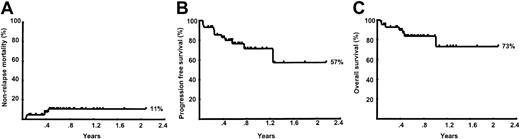
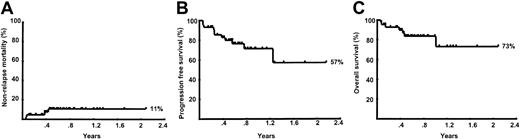
The median follow-up of the patients is only 9 months. The Kaplan-Meier estimated probabilities of nonrelapse mortality, progression-free survival, and overall survival for all 44 patients are shown in Figure 2. The estimated probability of nonrelapse mortality at 12 months was 11% (95% confidence interval [CI], 4%-26%). The estimated probability of progression-free survival at 12 months was 71% (95% CI, 53.6%-84.2%). The estimated probability of overall survival at 12 months was 73.2% (95% CI, 44.3%-88.7%).
Kaplan-Meier curves.
(A) Nonrelapse mortality; (B) progression-free mortality; and (C) overall survival for the entire group of 44 patients.
Kaplan-Meier curves.
(A) Nonrelapse mortality; (B) progression-free mortality; and (C) overall survival for the entire group of 44 patients.
Discussion
TRM remains a major obstacle to successful allogeneic SCT. The introduction of nonmyeloablative purine analogue conditioning regimens has facilitated allogeneic engraftment while limiting regimen-related mortality.8-11,13 Despite this, GVHD remains a significant cause of mortality and morbidity following nonmyeloablative conditioning. Previously published results reported using other nonmyeloablative conditioning regimens have shown a 38% to 60% incidence of grade II-IV acute GVHD.8-11 13 This was the primary cause of death in some patients.
In our study, the incidence of GVHD was exceptionally low. No patients had grade III-IV acute GVHD, and only 2 patients (5%) developed grade II acute GVHD. The incidence of chronic GVHD was also low, with only 1 patient developing limited skin GVHD. While the incidence of chronic GVHD cannot yet be fully assessed in some of the patients because of relatively short follow-up, given the fact that only 1 patient has experienced this complication and that all but 3 of the patients are off all immunosuppression, we anticipate a very low rate of chronic GVHD. Because the use of post-transplantation CsA was similar to other nonmyeloablative regimens, the differences in the incidence and severity of GVHD may in part reflect the in vivo use of the humanized monoclonal antibody CAMPATH-1H as part of the conditioning regimen.18 This was administered to the patients on days −8 to −4 prior to transplantation. Because CAMPATH-1H has a prolonged half-life in humans, there was significant circulating antibody when the unmanipulated donor PBSCs or bone marrow was infused into the recipient, resulting in a degree of in vivo T-cell depletion. This combination of in vivo CAMPATH-1H together with CsA appears to have been very effective in preventing GVHD in both sibling and unrelated donor allograft recipients. A number of these patients have had their CsA discontinued between 1 and 3 months post-transplantation without the development of GVHD.
While our nonmyeloablative conditioning regimen facilitated engraftment in all but one of the evaluable patients, many of the patients were mixed chimeras. Some patients were mixed chimeras in all lineages tested, while others were only mixed chimeras in the T-cell lineage. It has been demonstrated that patients who are mixed chimeras may experience less GVHD than full-donor chimeras.19,20 On the other hand, mixed chimerism may diminish the potential benefit of the GVL effect seen in the allograft setting.21,22 While mixed chimeras can be converted to full-donor chimeras following DLI,5 this was not attempted as part of this pilot study. The primary end points of this study were to explore the incidence of durable engraftment and acute and chronic GVHD. DLIs were only given for overt relapse of disease and were not given prophylactically or pre-emptively because we wished to assess the impact of the conditioning regimen on disease control and relapse. In the present study, we were generally not able to show the benefit of DLI in the setting of post-transplantation relapse. DLIs were either ineffective or led to toxicity from GVHD in all but one of the patients treated. This is not surprising, because the response rate in relapsed AML is low and there are few data to show that DLIs are effective in inducing remissions in patients with aggressive lymphomas.23 24Only a single patient with multiple myeloma has achieved CR following DLI therapy.
The use of this conditioning regimen has been relatively safe in a group of patients that had many high-risk features: patients who had prior high-dose therapy, patients with renal or cardiac impairment, or patients with high-risk diagnoses for allogeneic SCT such as Hodgkin disease or multiple myeloma. Indeed, allogeneic transplantation using myeloablative conditioning following failed autologous transplantation has been associated with a treatment mortality ranging between 50% and 80%.25 26 Undoubtedly, such a high mortality rate may offset a potential for cure, and therefore conventional transplants have generally been avoided in such patients. In our study, 19 patients received a second transplant, and only 3 patients (17%) died of transplantation-related complications, demonstrating that this nonmyeloablative approach could be attempted if a second transplant has to be considered.
While a conditioning regimen containing fludarabine and melphalan appears to have been active in tumor control, particularly in patients with Hodgkin disease and NHL, the follow-up period is still very limited and all patients remain at risk of relapse. In such a high-risk group of patients, any conditioning regimen is likely to be associated with a significant relapse risk, and therefore the survival curves shown in Figure 2 should be interpreted with caution. This is particularly so in some hematologic malignancies, such as acute leukemia, where the GVL effect of DLI for the treatment of relapse is of limited efficacy.23 However, the antitumor responses seen with the conditioning regimen might allow the use of DLI to be delayed until 6 to 12 months post-transplantation, when this intervention might be associated with less GVHD.5,27 28
In summary, our results show that our nonmyeloablative regimen facililitates allogeneic engraftment, with a low incidence of GVHD and TRM. The long-term antitumor activity of this regimen remains unknown; however, if used in combination with the prophylactic or pre-emptive use of DLI, prolonged remissions might be obtained in some types of hematologic malignancies.
Acknowledgments
We thank the staff members of the Therapeutic Antibody Centre, University of Oxford, for their contributions to the production of CAMPATH-1H antibody.
Supported by the United Kingdom Medical Research Council, Leukosite Inc, and the E.P. Abraham's Trust.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stephen Mackinnon, Department of Hematology, University College Hospital, 98 Chenies Mews, London WC1E 6H, England; e-mail: s.mackinnon@ucl.ac.uk.