Abstract
The pathogenic effects of antiplatelet antibodies were investigated in mice. Monoclonal antibodies (mAbs) of different immunoglobulin G subclass directed against mouse GPIIbIIIa, GPIIIa, GPIbα, GPIb-IX, GPV, and CD31 were generated and characterized biochemically. MAbs against GPIb-IX, GPV, CD31, and linear epitopes on GPIIIa had mild and transient effects on platelet counts and induced no spontaneous bleeding. Anti-GPIbα mAbs induced profound irreversible thrombocytopenia (< 3% of normal) by Fc-independent mechanisms but only had minor effects on hematocrits. In contrast, injection of intact mAbs, but not F(ab)2 fragments, against conformational epitopes on GPIIbIIIa, induced irreversible thrombocytopenia, acute systemic reactions, hypothermia, decreased hematocrits, and a paradoxical loss of surface GPIIbIIIa on platelets in vivo, the latter suggesting the formation of platelet-derived microparticles. Blockage of platelet-activating factor receptors inhibited the acute reactions, but not thrombocytopenia, loss of GPIIbIIIa, and decreases in hematocrits. Repeated injections of low doses of anti-GPIIbIIIa antibodies resulted in profound thrombocytopenia and bleeding, whereas no acute systemic reactions were observed. These data strongly suggest that the identity of the target antigen recognized by antiplatelet antibodies determines the mechanisms of platelet destruction and the severity of bleeding in mice, the latter depending on previously unrecognized anti-GPIIbIIIa-specific inflammatory mechanisms.
Introduction
Autoimmune thrombocytopenic purpura (ITP) is an autoimmune bleeding disease in which autoantibodies are directed against the individual's own platelets, resulting in increased platelet destruction and frequently severe bleeding.1 It is well recognized that the vast majority of antiplatelet antibodies in patients with ITP are directed against the 2 most prominent membrane glycoprotein (GP) receptors GPIIbIIIa (αIIbβ3, fibrinogen receptor) and GPIb-IX-V [von Willebrand factor (vWF) receptor].2 Presently, it is not clear whether GPIIbIIIa and GPIb-IX-V are particularly immunogenic or, alternatively, antibodies against these receptor complexes develop with similar frequency as other antiplatelet antibodies but may be more pathogenic because of effects on platelet function. Studies on patients with ITP have suggested that antiplatelet antibodies that affect platelet function may not be uncommon.3-8 Nevertheless, the number of reports on well-characterized antiplatelet antibodies interfering with normal platelet function remains limited. This may be due to the fact that in most cases the thrombocytopenia is considered sufficient to explain the bleeding diathesis, and further studies are not performed. It is currently accepted that platelet destruction in ITP is mediated mainly by cells of the reticuloendothelial system (RES) and is Fc dependent.1,9 Therefore, the current concepts of treatment aim at inactivation of the RES and at increasing the platelet counts.9 However, many patients do not respond to such treatment, suggesting that the pathogenic mechanisms involved in platelet destruction may not be identical in all patients.10 11
(NZW × BXSB)F1 mice, which develop systemic autoimmunity including progressive thrombocytopenia, have served as a model for ITP, as antiplatelet antibodies are detectable in those mice.12The antigens recognized by these antibodies, however, have not been identified, making it difficult to determine the importance of the antigenic specificity of antiplatelet antibodies in the development of ITP. This may be of considerable importance, as platelet functions could be influenced by certain antibodies resulting in (antigen-specific) mechanisms of platelet destruction and, possibly, further inflammatory responses. We recently confirmed this hypothesis when we reported that the first defined monoclonal antibody (mAb) against mouse GPIIbIIIa [MWReg30, rat immunoglobulin G1 (IgG1)] specifically induced strong thrombocytopenia and acute systemic reactions in mice.13
Systematic in vivo studies on the relevance of the antigenic specificity of antiplatelet antibodies for their pathogenic effects have not been performed to date, because defined antibodies suited for in vivo studies were not available. Thus, it is presently not clear which parameters determine the pathogenic effects of antiplatelet antibodies: (1) antigenic specificity, (2) epitope-specific effects leading to platelet agglutination/aggregation, (3) abundance of the antigen on the membrane, or (4) IgG subclass of the antibody. To address these issues, we generated panels of novel mAbs of different isotype subclasses directed against nonoverlapping epitopes on mouse GPIIbIIIa, GPIb-IX, and GPV and examined their pathogenic effects in vivo.
Here we demonstrate Fc-dependent and -independent mechanisms of platelet destruction and provide evidence that the antigenic specificity of antiplatelet antibodies strictly determines their pathogenic potential. Furthermore, we show that antibodies directed against conformational epitopes on GPIIbIIIa induce previously unrecognized inflammatory responses that seem to be the major cause of the bleeding problems in those individuals.
Materials and methods
Animals
Specific-pathogen-free mice (NMRI) 6 to 10 weeks of age were obtained from Charles River (Sulzfeld, Germany) and kept in our animal facilities.
Reagents
EZ-Link sulfo-NHS-LC-biotin (Pierce, Rockford, IL), immobilized pepsin (Pierce), high molecular weight heparin (Sigma, Deisenhofen, Germany), thrombin (Boehringer Mannheim, Mannheim, Germany), and streptavidin-horseradish peroxidase (HRP; DAKO, Glostrup, Denmark) were purchased. WEB2170BS was kindly provided by Boehringer Ingelheim (Ingelheim, Germany).
Antibodies
The rat antimouse P-selectin mAb RB40.34 was kindly provided by D. Vestweber (Münster, Germany). Polyclonal rabbit antibodies to human fibrinogen and vWF were purchased from DAKO and were modified in our laboratories. Rabbit anti-fluorescein isothiocyanate (FITC)-HRP, rabbit anti-rat immunoglobulin-FITC were purchased from DAKO. All other antibodies were generated, produced, and modified in our laboratories.
Platelet preparation and counting
Mice were bled under ether anesthesia from the retro-orbital plexus. Blood was collected in a tube containing 10% (v/v) 0.1 mol/L sodium citrate or 7.5 U/mL heparin, and platelet-rich plasma was obtained by centrifugation at 300g for 10 minutes at room temperature (RT). The platelets were washed twice with phosphate-buffered saline (PBS) by centrifugation at 1300gfor 10 minutes and used immediately. Isolated platelets did not show any signs of activation as shown by flow cytometry (staining for P-selectin and surface-expressed fibrinogen). For determination of platelet counts, blood (20 μL) was obtained from the retro-orbital plexus of anesthetized mice by using siliconized microcapillaries and immediately diluted 1:100 in Unopette kits (Becton Dickinson, Heidelberg, Germany). The diluted blood sample was allowed to settle for 20 minutes in an Improved Neubauer hemocytometer (Superior, Bad Mergentheim, Germany), and platelets were counted under a phase contrast microscope at ×400 magnification.
Production of mAbs
Female Wistar rats, 6 to 8 weeks of age, were immunized repeatedly with mouse platelets or with purified antigens. The rat spleen cells were then fused with mouse myeloma cells (Ag8.653), and hybridomas were selected in HAT medium. Hybridomas secreting mAbs directed against platelet receptors were identified by flow cytometry. Briefly, a 1:1 mixture of resting and thrombin-activated platelets (106) was incubated with 100 μL supernatant for 30 minutes at RT, washed with PBS (1300g, 10 minutes), and stained with FITC-labeled rabbit anti-rat immunoglobulin (DAKO) for 15 minutes. Samples were analyzed on a FACScan (Becton Dickinson) in the set-up mode. Platelets were gated by FSC/SSC characteristics. Positive hybridomas were subcloned twice before large-scale production. Isotype subclasses were determined by enzyme-linked immunosorbent assay with alkaline phosphatase-conjugated isotype-specific antibodies (Pharmingen, Hamburg, Germany).
Modification of antibodies
Affinity-purified antibodies were fluoresceinated to a fluorescein-to-protein ratio of approximately 3:1 by standard methods with FITC (Sigma) and separated from free FITC by gel filtration on a PD-10 column (Pharmacia, Uppsala, Sweden). F(ab)2 fragments were generated by 24-hour incubation of 10 mg/mL mAb with immobilized pepsin (Pierce). After peptic digest, the preparations were applied to an immobilized protein A column followed by an immobilized protein G column (Pharmacia) to remove Fc fragments and any undigested IgG. The purity of the F(ab)2 fragments was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining of the gel.
Immunoprecipitation and immunoblotting
Immunoprecipitation was performed as described previously.14 Briefly, 108 washed platelets were surface labeled with EZ-Link sulfo-NHS-LC-biotin (Pierce; 100 μg/mL in PBS) and subsequently solubilized in 1 mL lysis buffer (Tris-buffered saline containing 20 mmol/L Tris/HCl, pH 8, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L phenylmethylsulfonyl fluoride, 2 μg/mL aprotinin, 0.5 μg/mL leupeptin, and 0.5% Nonidet P-40; all from Boehringer Mannheim). Cell debris was removed by centrifugation (15 000g, 10 minutes). After preclearing (8 hours), 10 μg mAb was added together with 25 μL protein G-Sepharose (Pharmacia), and precipitation took place overnight at 4°C. Samples were separated on a 9% to 15% gradient SDS-PAGE along with a molecular weight marker and then transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was incubated with streptavidin-HRP (1 μg/mL; DAKO) for 1 hour after blocking. After extensive washing, biotinylated proteins were visualized by echochemiluminescence (ECL; Amersham).
For immunoblotting, platelets were not surface labeled. After lysis, the whole-cell extract was run on a SDS-PAGE gel and transferred to a PVDF membrane. The membrane was first incubated with 5 μg/mL FITC-labeled primary antibody followed by rabbit anti-FITC-HRP (1 μg/mL). Proteins were visualized by ECL.
Flow cytometry
Freshly isolated platelets were washed twice with PBS and then resuspended in platelet buffer (20 mmol/L Tris-HCl pH 7, 0.9% NaCl, 1 mmol/L CaCl2) at a concentration of 4 × 104/μL. Samples of 25 μL were preincubated with 50 μg/mL unlabeled mAb for 30 minutes at RT, followed by addition of saturating amounts of FITC-labeled antibodies. After 15 minutes incubation at RT, the samples were analyzed on a FACScan. Platelets were gated by FSC/SSC characteristics. For analysis of platelets from antibody-treated mice, whole blood was diluted 1:20 in platelet buffer and stained with the indicated fluorophore-labeled mAbs for 10 minutes at RT.
In vivo experiments
Ether-anesthetized mice received the indicated amounts of antibody intravenously. For the hypothermia measurement, body temperature was measured at the indicated times with a rectal probe. For the blockage of platelet-activating factor (PAF) receptors, mice received the indicated amounts of WEB2170 BS (in 200 μL sterile PBS) intravenously.
Results
Characterization of antiplatelet antibodies
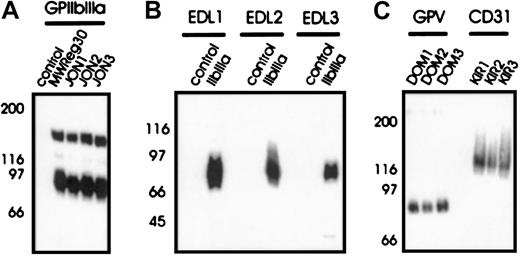
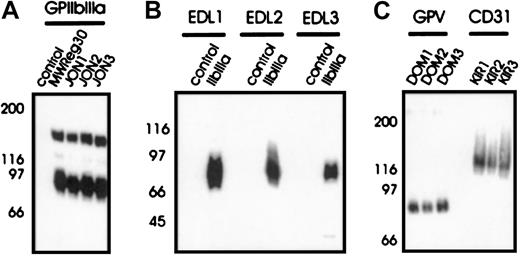
To investigate the mechanisms of immune thrombocytopenia, mAbs of different IgG subclass against different epitopes on the dominant surface receptors on mouse platelets were generated—GPIIbIIIa and GPIb-IX-V. The anti-GPIb-IX mAbs (p0p1-5) have been described recently.15 P0p3-5 are directed against different epitopes on the glycocalicin (GC) portion of GPIbα, whereas p0p1,2 recognize epitopes on the GPIb-IX complex distinct from the GC portion of GPIbα (furthermore referred to as GPIb-IX). P0p6 (IgG2b) is also directed against GPIb-IX (not shown). The mAbs against epitopes on GPIIbIIIa were generated as described.13 JON1-3 precipitated the platelet GPIIbIIIa complex (Figure 1A) but not integrin αVβ3 from surface-biotinylated bEnd.3 endothelioma cells (not shown), demonstrating that these mAbs are not directed against the GPIIIa subunit. Immunohistochemical staining of acetone-fixed frozen sections of various organs (lung, liver, spleen, kidney, heart, and intestine) confirmed earlier studies in which antibodies against GPIbα, GPIb-IX (p0p-mAbs), or GPIIbIIIa (JON-mAbs, MWReg30) exclusively bound to platelets/megakaryocytes13 15 (not shown). In contrast, EDL1-3 recognized the GPIIIa chain in a Western blot analysis under nonreducing conditions (Figure 1B) and precipitated integrin αVβ3 from surface-biotinylated bEnd.3 endothelioma cells (not shown). The anti-GPV mAbs (DOM mAbs) were obtained from fusions of spleen cells from rats immunized with affinity purified surface-cross-linked GPIb-IX-V complexes and identified by flow cytometric and biochemical methods (W.B. et al, manuscript in preparation). DOM1-3 precipitated an approximate 80-kD protein from platelet lysates (Figure 1C) and an approximate 68-kD band from the supernatant of thrombin-activated platelets (not shown). Three different mAbs against CD31 (PECAM; KIR1-3, Figure 1C) were used as controls in the current study, because this platelet antigen is not a common target for autoimmune antibodies in patients with ITP. Flow cytometric preincubation experiments demonstrated that mAbs directed against identical antigens did not block each other's binding (not shown). None of the mAbs used in the current study induced platelet activation (surface expression of P-selectin or fibrinogen). A summary of all mAbs is shown in Table 1.
Antigenic specificity of the mAbs.
Immunoprecipitation from surface-biotinylated resting platelets (A, C). NP-40 lysates were incubated with nonimmune rat IgG1 (control) or the indicated mAbs, followed by protein G-Sepharose. Proteins were separated on a 9%-15% gradient SDS-PAGE gel under nonreducing conditions, transferred onto a PVDF membrane and detected by streptavidin-HRP and ECL. (B) Western blot analysis of immunoprecipitated GPIIbIIIa with EDL1-3. Unlabeled platelet proteins were immunoprecipitated with nonimmune rat IgG1 (control) or MWReg30 (IIbIIIa), followed by SDS-PAGE and immunoblotting with FITC-labeled EDL1-3. Bound antibody was detected by HRP-labeled rabbit anti-FITC.
Antigenic specificity of the mAbs.
Immunoprecipitation from surface-biotinylated resting platelets (A, C). NP-40 lysates were incubated with nonimmune rat IgG1 (control) or the indicated mAbs, followed by protein G-Sepharose. Proteins were separated on a 9%-15% gradient SDS-PAGE gel under nonreducing conditions, transferred onto a PVDF membrane and detected by streptavidin-HRP and ECL. (B) Western blot analysis of immunoprecipitated GPIIbIIIa with EDL1-3. Unlabeled platelet proteins were immunoprecipitated with nonimmune rat IgG1 (control) or MWReg30 (IIbIIIa), followed by SDS-PAGE and immunoblotting with FITC-labeled EDL1-3. Bound antibody was detected by HRP-labeled rabbit anti-FITC.
Intensity of thrombocytopenia depends on the antigenic specificity but not on the IgG subclass of the injected antibody
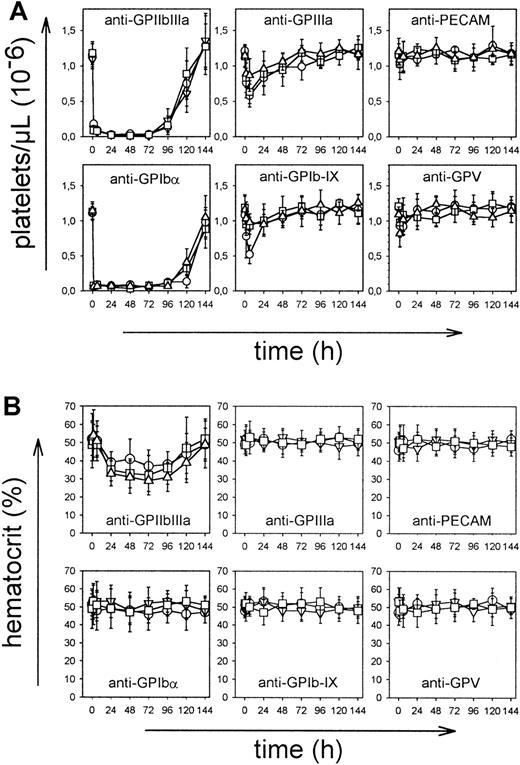
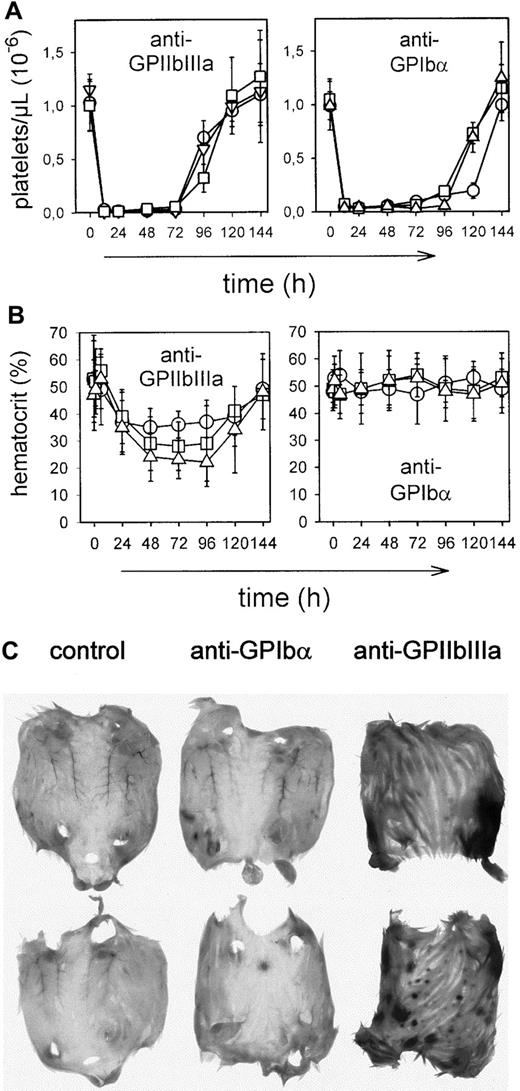
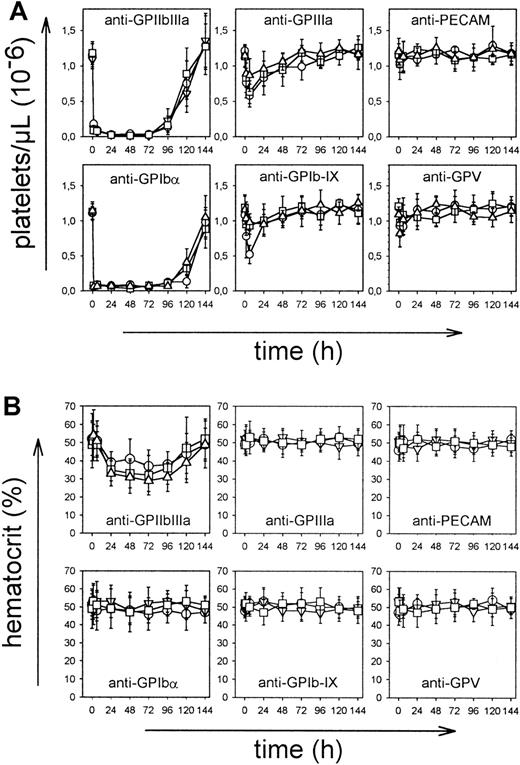
To examine the pathogenic effects of the mAbs in vivo, mice received 100 μg purified mAb intravenously, and platelet counts were monitored for 6 days at the indicated time intervals. As shown in Figure 2A, most mAbs induced thrombocytopenia, but intensity and time course differed significantly between the mAb panels. Injection of JON mAbs (anti-GPIIbIIIa) of either IgG subclass resulted in a drop of platelet count to below 15% of control within 1 hour that further decreased to less than 3% at 24 hours and remained at this level for 3-4 days. Comparable results were obtained with the anti-GC mAbs p0p3-5, confirming earlier results.15 After 24 hours, intestinal hemorrhages and subcutaneous bleeding were evident in anti-GPIIbIIIa-treated mice (not shown). Consequently, hematocrits in those animals decreased and reached a minimum on day 3-4 (Figure 2B). Although anti-GPIbα antibodies displayed virtually identical cytotoxic effects as anti-GPIIbIIIa antibodies, they induced neither subcutaneous nor intestinal hemorrhages. As a result, the hematocrits were not significantly affected (Figure 2B). In contrast to anti-GPIIbIIIa and anti-GPIbα antibodies, injection of mAbs directed against GPIIIa, GPV, or GPIb-IX of either isotype resulted in delayed platelet clearing and a maximum drop of platelet counts to approximately 50% of control for some mAbs after 24 hours and in increasing counts after 48 hours. The anti-CD31 mAbs had virtually no effect on platelet counts. Bleeding or decreased hematocrits were not detectable in any of these mice (Figure 2B).
In vivo effect of the mAbs.
Mice received 100 μg purified mAb intravenously in 200 μL sterile PBS. (A) Platelet counts were determined at the indicated times, using an improved Neubauer hemocytometer. Injection of a nonimmune IgG1 had no significant effect on the platelet count (not shown). Results are shown as mean ± SD for groups of 6 mice each (○: IgG1; ■: IgG2a; ▿: IgG2b). (B) Hematocrits were determined at the indicated times.
In vivo effect of the mAbs.
Mice received 100 μg purified mAb intravenously in 200 μL sterile PBS. (A) Platelet counts were determined at the indicated times, using an improved Neubauer hemocytometer. Injection of a nonimmune IgG1 had no significant effect on the platelet count (not shown). Results are shown as mean ± SD for groups of 6 mice each (○: IgG1; ■: IgG2a; ▿: IgG2b). (B) Hematocrits were determined at the indicated times.
Phenotypes of circulating platelets in antiplatelet-treated mice
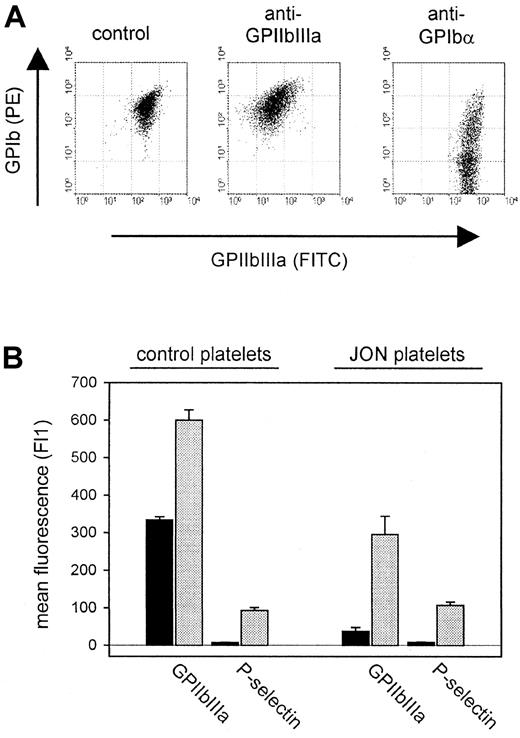
In all mice treated with antibodies against GPIb-IX, GPIIIa, GPV, or CD31, more than 99% of the circulating platelets were opsonized with the respective mAb 6 hours after injection as demonstrated by flow cytometric detection of surface-bound rat IgG. Furthermore, the surface expression of GPIIbIIIa, GPIbα, GPV, and CD9 was unchanged compared with control platelets (Table2). In contrast, the remaining platelets circulating in mice on injection of anti-GPIbα or anti-GPIIbIIIa antibodies displayed different phenotypes. While p0p3-5 had induced shedding of GPIbα on some but not all remaining platelets, injection of JON1-3 had resulted in a more than 90% loss of surface-expressed GPIIbIIIa (Figure 3A). These GPIIbIIIa-negative platelets, however, expressed virtually normal amounts of GPIbα, GPIb-IX, and GPV, whereas the CD9 levels were decreased (Table 2). The internal pools of GPIIbIIIa had not been affected, as shown by the translocation of these receptors (along with P-selectin) to the surface on thrombin activation (Figure 3B). Western blot analysis of a whole cell lysate of these platelets confirmed that the total amount of GPIIIa per platelet was reduced to approximately half of normal (not shown). The membrane GP composition on the circulating platelets in both anti-GPIbα- and anti-GPIIbIIIa-treated mice remained unchanged as long as they were detectable in the circulation (approximately 7 days).
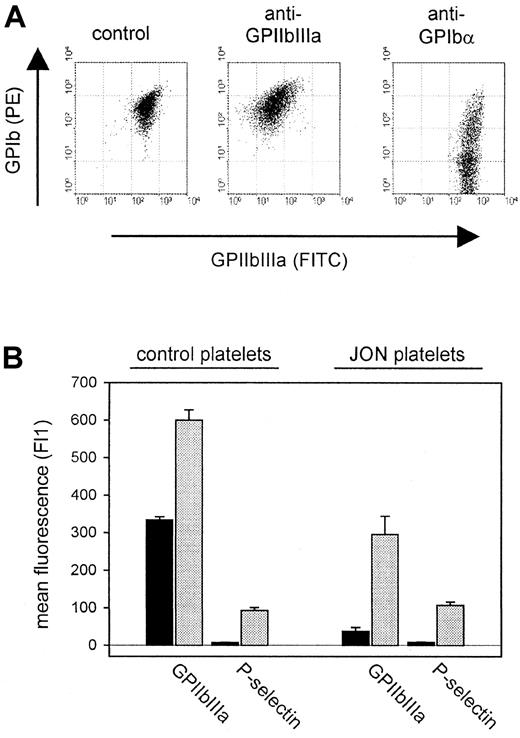
Loss of surface-expressed antigen induced by anti-GPIbα and anti-GPIIbIIIa mAbs.
(A) Two-color flow cytometric analysis of platelets from mice 6 hours after antibody injection. The data shown are representative of 6 mice per group. (B) Surface expression of GPIIbIIIa and P-selectin on platelets from mice 6 hours after injection of anti-GPIIbIIIa mAb (JON platelets). Resting (black bars) or thrombin-activated (gray bars) platelets were incubated with the indicated FITC-labeled antibody for 10 minutes at RT and analyzed directly.
Loss of surface-expressed antigen induced by anti-GPIbα and anti-GPIIbIIIa mAbs.
(A) Two-color flow cytometric analysis of platelets from mice 6 hours after antibody injection. The data shown are representative of 6 mice per group. (B) Surface expression of GPIIbIIIa and P-selectin on platelets from mice 6 hours after injection of anti-GPIIbIIIa mAb (JON platelets). Resting (black bars) or thrombin-activated (gray bars) platelets were incubated with the indicated FITC-labeled antibody for 10 minutes at RT and analyzed directly.
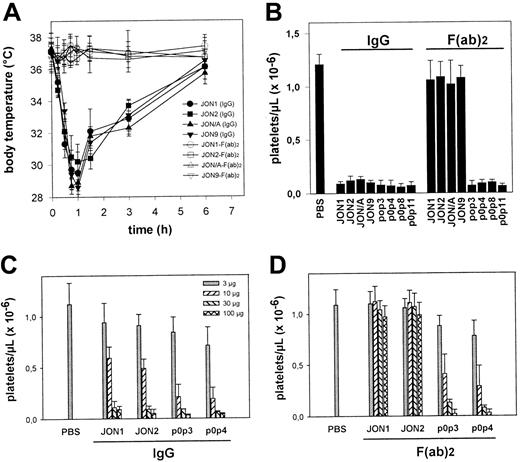
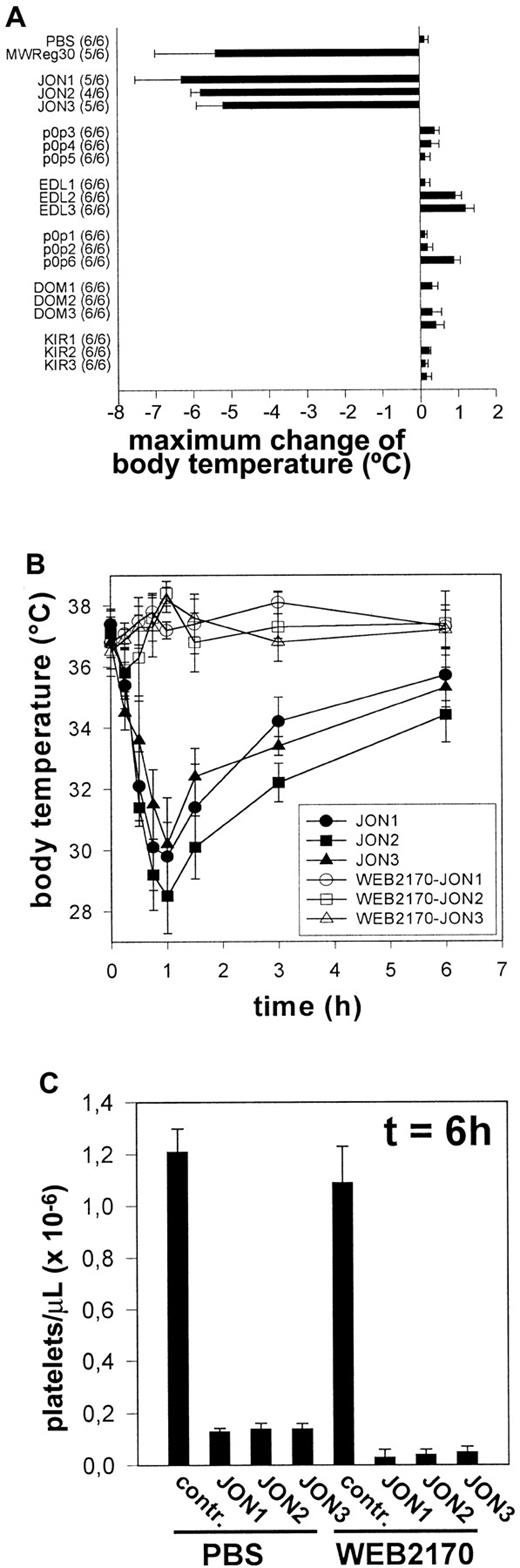
All anti-GPIIbIIIa mAbs induce PAF-dependent acute systemic reactions
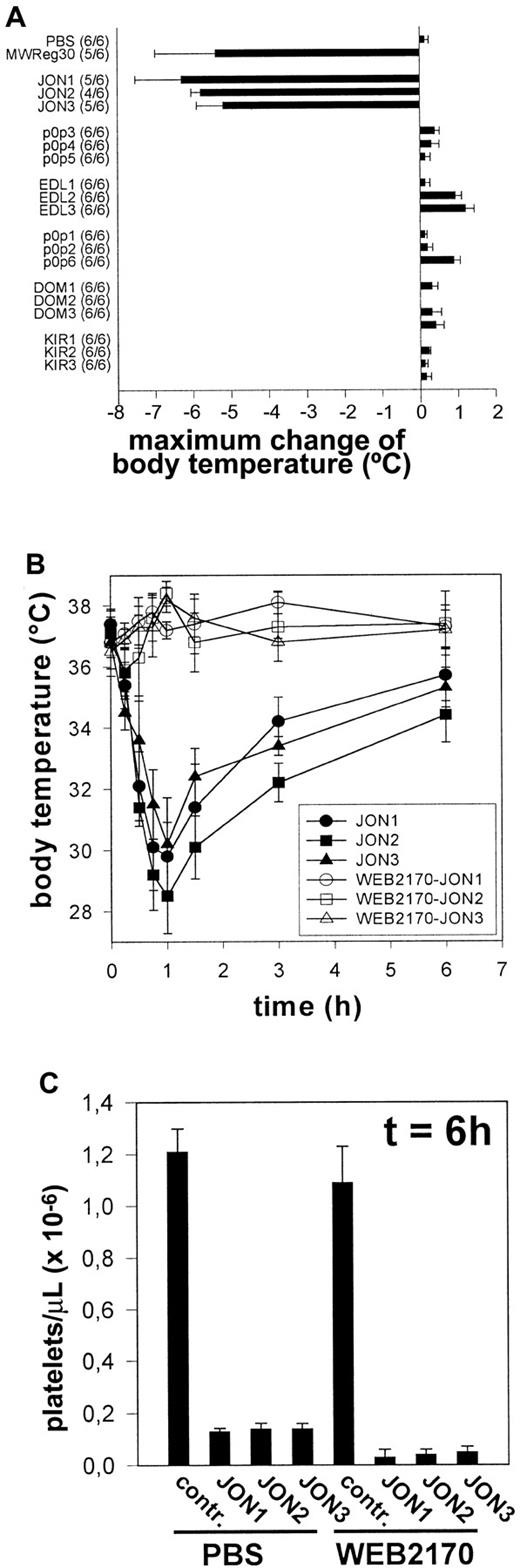
A bolus injection of either JON1-3 (n = 6 per group) induced severe hypothermia in mice. This condition was accompanied by peripheral vasodilation and uncoordinated movements as described to occur on injection of MWReg30.13 In contrast, injection of EDL-, p0p-, DOM-, or KIR-mAbs (n = 6 for each mAb) induced neither hypothermia nor any other signs of acute reactions (Figure4A). PAF was suspected to be a critical mediator of the observed pathology, as this potent inflammatory phospholipid is known to induce similar effects in mice.16To test this hypothesis, mice were treated with different doses (1 mg/kg, 3mg/kg, or 10 mg/kg) of the potent PAF receptor antagonist WEB2170 BS17 60 minutes before injection of anti-GPIIbIIIa (JON1-3). Pretreatment with 1mg/kg (as with the other doses) WEB2170 BS abolished hypothermia induced by either mAb (Figure 4B). The body temperature was monitored for 6 hours, and blood was subsequently drawn from the retro-orbital plexus for determination of platelet counts. WEB2170 BS did not prevent JON-induced thrombocytopenia. In contrast, platelet counts were even lower in WEB2170 BS-pretreated mice (Figure4C). Thus, PAF is a mediator of anti-GPIIbIIIa-induced systemic reactions, but it is clearly not involved in the development of thrombocytopenia. Importantly, the WEB2170 BS-treated mice, like the control mice, developed subcutaneous bleeding and significantly decreased hematocrits, indicating that PAF was not responsible for these complications (not shown).
Induction of PAF-dependent hypothermia by anti-GPIIbIIIa but not other mAbs.
(A) Mice received 100 μg purified mAb. The mean values of the maximum change of body temperatures of 6 mice per group within 2 hours after injection of the antibodies are given ± SD. The number of survivors per group is shown on the left. (B) Mice received 1 mg/kg WEB2170 BS or PBS and 1 hour later 100 μg purified mAb intravenously in 200 μL sterile PBS. The mean values of the body temperatures of 6 mice per group at the indicated times after injection of the antibodies are given ± SD. (C) Platelet counts were determined at t = 6 hour, using an improved Neubauer hemocytometer. Results are expressed as the mean platelet count ± SD for groups of 6 mice each.
Induction of PAF-dependent hypothermia by anti-GPIIbIIIa but not other mAbs.
(A) Mice received 100 μg purified mAb. The mean values of the maximum change of body temperatures of 6 mice per group within 2 hours after injection of the antibodies are given ± SD. The number of survivors per group is shown on the left. (B) Mice received 1 mg/kg WEB2170 BS or PBS and 1 hour later 100 μg purified mAb intravenously in 200 μL sterile PBS. The mean values of the body temperatures of 6 mice per group at the indicated times after injection of the antibodies are given ± SD. (C) Platelet counts were determined at t = 6 hour, using an improved Neubauer hemocytometer. Results are expressed as the mean platelet count ± SD for groups of 6 mice each.
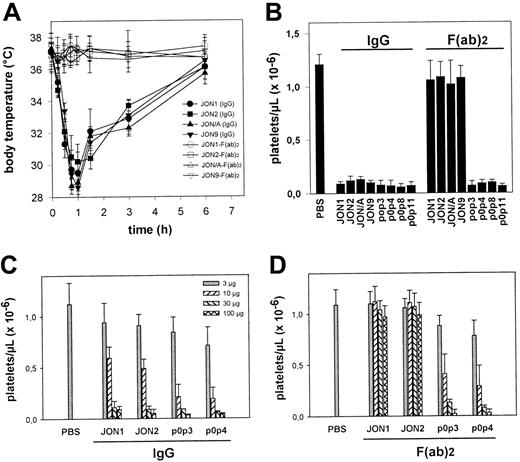
Importance of the Fc part of anti-GPIIbIIIa and anti-GPIbα antibodies
It is currently accepted that antibody-mediated platelet destruction is Fc dependent.1 To test this hypothesis, F(ab)2 fragments from various anti-GPIIbIIIa and anti-GPIbα were generated. Only IgG2a and IgG2b antibodies were used as F(ab)2 fragments because rat IgG1 was resistant against pepsin digest. Therefore, further antibodies against GPIIbIIIa (JON/A and JON9) and GPIbα (p0p8, p0p11) were used in this part of the study. The F(ab)2 fragments of anti-GPIIbIIIa mAbs (JON1, JON2, JON/A, and JON9) neither induced hypothermia (Figure5A), peripheral vasodilation, nor significant bleeding (n = 6 per group) and only had mild effects on platelet counts (Figure 5B). Membrane-bound F(ab)2fragments were demonstrated by staining platelets ex vivo with rabbit anti-rat IgG-FITC. The surface levels of GPIIbIIIa on these platelets remained virtually unchanged for at least 3 days (not shown). Thus, anti-GPIIbIIIa-induced hypothermia, thrombocytopenia, loss of GPIIbIIIa, and the resulting bleeding problems depended on the Fc part of the mAbs. In contrast, F(ab)2 fragments of anti-GPIbα mAbs (p0p3, p0p4, p0p8, and p0p11) induced thrombocytopenia with the same intensity as the intact antibodies, confirming earlier results15 and demonstrating that anti-GPIbα-mediated thrombocytopenia is Fc independent. To compare the cytotoxic effects of anti-GPIIbIIIa and anti-GPIbα antibodies, we performed dose-response studies with intact IgG and F(ab)2 fragments of JON1, JON2, p0p3, and p0p4. As shown in Figure 5C, low doses (3 and 10 μg) of intact anti-GPIbα mAbs had slightly stronger effects on platelet counts than anti-GPIIbIIIa mAbs, whereas injection of higher doses resulted in a more than 90% drop of platelet counts in all mice. The F(ab)2 fragments of p0p3 and p0p4 induced thrombocytopenia at similar doses as the intact antibodies, whereas the F(ab)2 fragments of JON1 and JON2 had no significant effects on platelets counts.
Importance of the Fc part of anti-GPIIbIIIa and anti-GPIbα.
(A) Mice received 100 μg purified mAb (or F(ab)2fragments) intravenously in 200 μL sterile PBS. The mean values of the body temperatures of 6 mice per group at the indicated times after injection of the antibodies are given ± SD. (B) Platelet counts were determined at t = 6 hours, using an improved Neubauer hemocytometer. Results are shown as the mean platelet count ± SD for groups of 6 mice each. (C, D) Mice (3 per group) received the indicated amounts of purified mAb or F(ab)2 fragments intravenously. Platelet counts were determined at t = 6 hours, using an improved Neubauer hemocytometer.
Importance of the Fc part of anti-GPIIbIIIa and anti-GPIbα.
(A) Mice received 100 μg purified mAb (or F(ab)2fragments) intravenously in 200 μL sterile PBS. The mean values of the body temperatures of 6 mice per group at the indicated times after injection of the antibodies are given ± SD. (B) Platelet counts were determined at t = 6 hours, using an improved Neubauer hemocytometer. Results are shown as the mean platelet count ± SD for groups of 6 mice each. (C, D) Mice (3 per group) received the indicated amounts of purified mAb or F(ab)2 fragments intravenously. Platelet counts were determined at t = 6 hours, using an improved Neubauer hemocytometer.
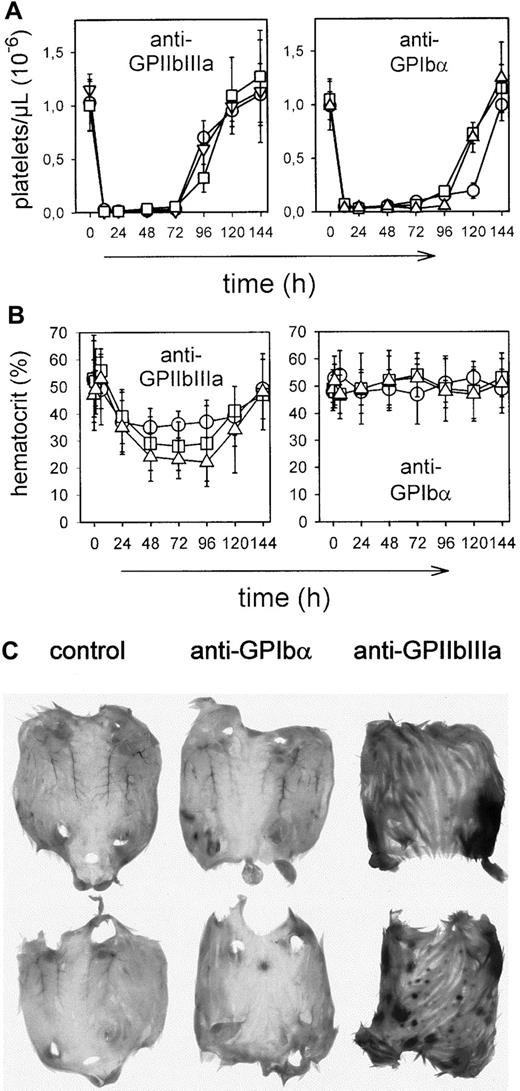
Repeated injections of low doses of anti-GPIIbIIIa antibodies induce severe bleeding
A bolus injection of antiplatelet antibody does not reflect the situation in patients in which ITP is often a chronic disease, suggesting that antiplatelet antibodies reach the circulation continuously. To mimic this situation, mice were repeatedly injected with low amounts of anti-GPIIbIIIa or anti-GPIbα antibodies (7 × 7.5 μg intraperitoneally within 8 hours). The acute systemic responses seen on a bolus injection of more than 10 μg anti-GPIIbIIIa were not detectable in these mice, confirming that this process requires a threshold dose of antibody to occur.13 The number of circulating platelets, however, decreased to less than 3% of normal within 24 hours in all mice (Figure6A), and the remaining cells displayed the same phenotype as those found in bolus-injected mice (see above). All JON-treated mice developed marked intestinal and subcutaneous hemorrhages and markedly decreased hematocrits, whereas these effects were not observed in p0p-treated mice (Figure 6B-C). As with the bolus injection, WEB2170 BS had no protective effect on thrombocytopenia and bleeding (not shown).
Effects of repeated injections.
Mice received 7 injections of 7.5 μg mAb each intraperitoneally. (A) Platelet counts were determined at the indicated times, using an improved Neubauer hemocytometer. Results are shown as mean ± SD for groups of each 6 mice (○: IgG1; ■: IgG2a; ▿: IgG2b). (B) Hematocrits were determined at the indicated times. (C) Subcutaneous bleeding 48 hours after antibody injections.
Effects of repeated injections.
Mice received 7 injections of 7.5 μg mAb each intraperitoneally. (A) Platelet counts were determined at the indicated times, using an improved Neubauer hemocytometer. Results are shown as mean ± SD for groups of each 6 mice (○: IgG1; ■: IgG2a; ▿: IgG2b). (B) Hematocrits were determined at the indicated times. (C) Subcutaneous bleeding 48 hours after antibody injections.
Discussion
In this study, we investigated the pathogenic effects of antibodies directed against different epitopes on the major platelet antigens GPIIbIIIa and GPIb-IX-V systematically for the first time. It is well recognized that the majority of autoimmune antiplatelet antibodies in patients with ITP are directed against epitopes on GPIIbIIIa and/or GPIb-IX-V.2 However, in most patients with ITP, autoantibodies are directed against different antigens, making it difficult to determine potentially existing antigen-specific mechanisms of platelet destruction. Our results strongly suggest that antibodies directed against GPIIbIIIa and GPIbα are by far the most pathogenic antiplatelet antibodies, at least in mice. This hypothesis is supported by the observation that antibodies against CD31 (Figure 2) and a variety of other platelet antigens, including integrin α2β1 and unidentified surface GPs (unpublished, not shown), had no significant influence on platelet counts and hematocrits. Therefore, a more detailed knowledge of the pathogenic effects of antibodies directed against the clinically relevant antigens, namely GPIIbIIIa and GPIb-IX-V, is of particular interest. As shown in Figure 2, the extent and the time course of thrombocytopenia induced by those antibodies depended on the antigenic specificity of the injected mAb, but not on its IgG subclass. The surprising result that antibodies of identical IgG subclass recognizing antigens with similar expression rates on the platelet membrane exerted completely different pathogenic effects in vivo strongly suggests that antigen-specific (but not epitope-specific) platelet-mediated mechanisms must be involved. In the case of p0p3-5 (anti-GPIbα), the rapidly occurring and irreversible platelet depletion may be based on their in vitro platelet-aggregating activity15 (without platelet activation), but the underlying mechanisms still need to be established. The finding that antibodies against GPIbα (IgG and F(ab)2 fragments) induce the destruction of circulating platelets by Fc-independent mechanisms in mice (Figure 5) and baboons18 indicates related mechanisms and suggests that the Fc gamma receptor (FcγR)-bearing cells in the RES are not involved in anti-GPIbα-induced platelet destruction. According to the assumption that anti-GPIbα antibodies also act Fc independently in humans, it may be concluded that therapeutic strategies directed primarily at the inactivation (infusion of intravenous immunoglobulin, or anti-D19) or removal of the spleen, the major site of Fc-mediated platelet destruction, would not be appropriate in ITP patients with anti-GPIbα-induced thrombocytopenia.
In contrast, all antibodies against the GPIIbIIIa complex induced thrombocytopenia with similar efficiency as anti-GPIbα mAbs, but this process was clearly Fc dependent (Figure 5). Additionally, these antibodies induced PAF-mediated acute systemic reactions that also required the Fc part of the antibody (Figure 4). These processes occurred independently of the exact binding epitope on the GPIIbIIIa complex, but linear epitopes on GPIIIa were not sufficient as shown by the experiments with EDL1-3 (anti-GPIIIa). This finding strongly indicates that antibodies against the GPIIbIIIa complex are more pathogenic than those directed against GPIIIa alone, at least in mice. A variety of studies suggest that the usual ITP epitopes are “conformational,” depending on the 3-dimensional (folded) structure of an intact GPIIbIIIa complex to allow antibody binding.20-22 Furthermore, it has been demonstrated that sequence-specific external antigens are unusual targets of eluted ITP antibodies (only 1 of 33 positive),23 but these results are not commonly accepted.2 This discrepancy may be explained by different techniques of antibody detection and by the fact that most patients with ITP have multiple antiplatelet antibodies circulating simultaneously.2 Therefore, antibodies directed against both conformational and linear epitopes may be present in many patients.
The anti-GPIIbIIIa-induced acute reactions only occurred when antibody amounts of more than 10 μg were given as a bolus injection (which does not reflect the situation in patients with ITP). Probably, lower amounts of antibody also induced PAF production, but the concentrations may not have been high enough to affect the systemic circulation. The very short half-life of PAF in vivo may explain why the critical concentration was not reached by repeated injections of anti-GPIIbIIIa antibodies. The source of PAF could not be identified in the present study, but it seems likely that FcγR-bearing cells of the RES release this phospholipid during the pathology, as the Fc part of the antibody was strictly required for the induction of PAF. But why should only antibodies against GPIIbIIIa induce such an inflammatory reaction? It is well accepted that soluble immune complexes (sIC) induce the release of a variety of inflammatory mediators (including PAF) from FcγR-bearing immune cells, resulting in anaphylactic reactions and chronic inflammatory diseases.24,25According to the data presented here, we speculate that anti-GPIIbIIIa antibodies induce the formation of microparticles (MPs) that carry most of the surface GPIIbIIIa densely opsonized with antibody. These MPs may then function as sIC to elicit the observed anaphylactic reactions and further inflammatory responses that contribute to the increased bleeding tendency. The formation of platelet-derived MPs has been reported to be a GPIIbIIIa-dependent process.26JON1-3 most likely induced MP formation in vivo as platelets lost their surface GPIIbIIIa and a subpopulation of CD9 (which is associated with GPIIbIIIa27) on injection of either anti-GPIIbIIIa antibody. In contrast, other membrane receptors, including GPIb, were not affected (Table 2). We were not able, however, to induce significant MP formation with JON1-3 in vitro, suggesting that a second signal is required for this process to occur. This signal may be provided by cells of the RES13 as the loss of GPIIbIIIa/CD9 was Fc dependent. This hypothesis is supported by immunohistochemical examinations of the major organs of anti-GPIIbIIIa-treated mice, demonstrating that platelets became trapped in the spleen, liver, and lung within 5 minutes of antibody injection, thereby, probably interacting with FcγR-bearing cells (not shown). Some investigators have described the appearance of platelets with “unusual” membrane GP composition (reduced GPIIbIIIa and CD9, increased GPIb-IX) in ITP patients with anti-GPIIbIIIa antibodies.3 Such platelets, however, are not commonly observed, but this could be explained by the presence of other antiplatelet antibodies mediating their progressive clearing.
We found that PAF itself is not involved in the development of thrombocytopenia and the increased bleeding tendency in anti-GPIIbIIIa-treated mice (Figure 4). This finding confirms results from studies in patients with ITP in which PAF-receptor antagonists had no beneficial effects.28 However, the lack of circulating platelets alone cannot account for the marked blood loss as no such effect was observed in anti-GPIbα-treated animals (Figure 6). Therefore, other mediators released from sIC-stimulated leukocytes (simultaneously with PAF) might have induced inflammatory processes that finally lead to severe bleeding problems in the absence of functional platelets. Further studies will be required to identify these processes.
Taken together, the results presented here provide the first evidence that antibodies directed against GPIbα or conformational epitopes on GPIIbIIIa are far more pathogenic than other antiplatelet antibodies in mice. The mechanisms of platelet destruction, however, differed significantly between the 2 antibody clusters. Anti-GPIbα antibodies did not require the Fc part for their cytotoxic activity, strongly suggesting that the RES was not involved. In contrast, anti-GPIIbIIIa antibodies clearly acted by Fc-dependent mechanisms and thereby specifically induced previously unrecognized inflammatory responses that significantly contributed to the bleeding problems in those mice. In most patients with ITP, autoantibodies against different autoantigens circulate simultaneously, making it difficult to correlate the severity of the disease with particular autoantigens. However, the demonstration of antigen-specific mechanisms underlying antibody-mediated platelet destruction and bleeding in mice strongly suggests that such studies are needed. The identification of antigen-specific mechanisms in the development of ITP in humans might be of considerable importance as different pathogenic mechanisms would require distinct therapeutic strategies to reduce the risk of life-threatening bleeding events in those patients.
Acknowledgments
The authors would like to thank N. Huss for critically reading the manuscript and U. Barnfred for constant support.
Supported in part by grant Ni 556/2-1 (to B.N. and J.E.G.) from the Deutsche Forschungsgemeinschaft and the Bayer ag, Germany.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bernhard Nieswandt, IMMI, Klinikum Wuppertal, Universität Witten-Herdecke, Heusnerstr. 40, D-42283 Wuppertal, Germany; e-mail: nieswand@klinikum-wuppertal.de.