Abstract
The efficacy and toxicity of cladribine (2-CdA) + prednisone (P) versus chlorambucil (Chl) + P were compared in previously untreated patients with progressive or symptomatic chronic lymphocytic leukemia (CLL) in a randomized, multicenter prospective trial. Eligible patients were assigned to either 2-CdA 0.12 mg/kg per day in 2-hour infusions and P 30 mg/m2 per day for 5 consecutive days or Chl 12 mg/m2 per day and P 30 mg/m2 per day for 7 consecutive days. Three courses were administered at 28-day intervals or longer if myelosuppression developed. The therapy was finished if complete response (CR) was achieved. Of 229 available patients 126 received 2-CdA+P and 103 received Chl+P as a first-line treatment. CR and overall response rates were significantly higher in the patients treated with 2-CdA+P (47% and 87%, respectively) than in the patients treated with Chl+P (12% and 57%, respectively) (P = .001). Progression-free survival was significantly longer in the 2-CdA–treated group (P = .01), but event-free survival was not statistically different. Thirteen percent of patients were refractory to 2-CdA+P and 43% to Chl+P (P = .001). Drug-induced neutropenia was more frequently observed during 2-CdA+P (23%) than Chl+P therapy (11%) (P = .02), but thrombocytopenia occurred with similar frequency in both groups (36% and 27%, respectively). Infections were seen more frequently in the 2-CdA+P-treated group (56%) than in the Chl+P-treated group (40%; P = .02). Death rates have so far been similar in patients treated with 2-CdA (20%) and with Chl (17%). The probability of overall survival calculated from Kaplan-Meier curves at 24 months was also similar for both groups (78% and 82%, respectively).
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is the most common type of leukemia in adults in Europe and North America.1 It is a disease for which there is no cure, and it mainly affects persons older than 50. The disease is characterized by a variable clinical course, and the overall median survival times range from a few months to the same survival times as in an age-matched normal population.2 Treatment is recommended in patients with progressive early-stage disease or advanced-stage disease, though the precise criteria required to identify patients at risk remain controversial.
For many years chlorambucil (Chl) has been considered the drug of choice for first-line therapy of CLL, and, when combined with prednisone (P), it gives the initial response rate of 60% to 80%, with a complete response (CR) in up to 20% of all patients.3-5 Many hematologists believe that such therapy should still be considered the standard therapy to which new treatment approaches are compared.6 Alternative treatment approaches, including combination chemotherapy and new purine nucleoside analogs, such as fludarabine (FAMP) and 2-chlorodeoxyadenosine (2-CdA), have also shown activity in CLL. FAMP has the highest therapeutic activity ever reported for a single agent in CLL, and it has been shown to induce an overall response rate of 80% in previously untreated patients in a study conducted at the MD Anderson Cancer Center.7 Encouraging results have also been achieved in previously treated CLL using FAMP alone or in combination with P.8 9
As yet, 2-CdA has been less extensively investigated than FAMP in patients with CLL. However, in a study reported by Saven et al10 on 90 patients with CLL previously treated with other regimens, an overall response rate of 44%, including 4% CR, was achieved after an average of 2 courses of 2-CdA given by 24-hour infusion for 7 days. No CR and 31% partial remission (PR) were achieved by Tallman et al11 in 26 heavily pretreated patients administered 2-CdA by continuous intravenous infusion for either 5 or 7 days. We have obtained a response rate of 48.4% in 184 patients with CLL refractory to conventional therapy, using 2-CdA in 2-hour infusions for 5 days.12 A higher response rate to 2-CdA was observed in previously untreated patients.13-15 In our previous study, the overall response (OR) rate to 2-CdA, with or without prednisone, in 194 untreated patients was 82.5%, including 45.4% CR.12
In this paper we present the results of a prospective, randomized, multicenter trial comparing the efficacy of 2-CdA+P versus Chl+P in previously untreated patients with B-CLL. We also evaluate the effectiveness of 2-CdA+P in patients refractory to Chl+P and the effect of Chl+P on patients who are resistant to, or who have had early relapses after, 2-CdA+P.
Patients and methods
Patients
The current study was initiated in 1995 in 9 hematologic centers in Poland as an open prospective, randomized, multicenter trial. Only previously untreated patients with progressive or symptomatic B-CLL entered the study between May 1995 and May 1998. All fulfilled the National Cancer Institute (NCI)-sponsored Working Group diagnostic criteria for CLL.16
The clinical stage was determined before the randomization according to Rai classification.17 Patients in stage 0, I, and II were eligible if they had evidence of active disease, including progressive lymphocytosis, massive splenomegaly or bulky lymphadenopathy, recurrent disease-related infections, weight loss greater than 10% in a 6-month period, and temperature of 38°C related to disease or extreme fatigue. All patients in III and IV clinical stage disease were qualified for the treatment. Patients with poor performance status (World Health Organization scale 4), active infection, abnormal liver or renal function, and Richter syndrome were excluded from the study.
Assessment of the patient's history and physical examination results was performed as the initial diagnostic procedure. Laboratory tests included complete blood count, immunoglobulin level, liver and renal function tests, bone marrow aspiration for morphology, and immunophenotyping. Surface marker analysis was performed to confirm B-cell origin and monoclonal proliferation, including immunoglobulin heavy and light chains, CD5, CD10, CD19, CD20, and CD23. We used monoclonal antibodies manufactured by DAKO and 2-color flow cytometry (Coulter, Hialeah, FL). Characteristics of the patients with CLL included in the study are presented in Table1.
Randomization and treatment schedule
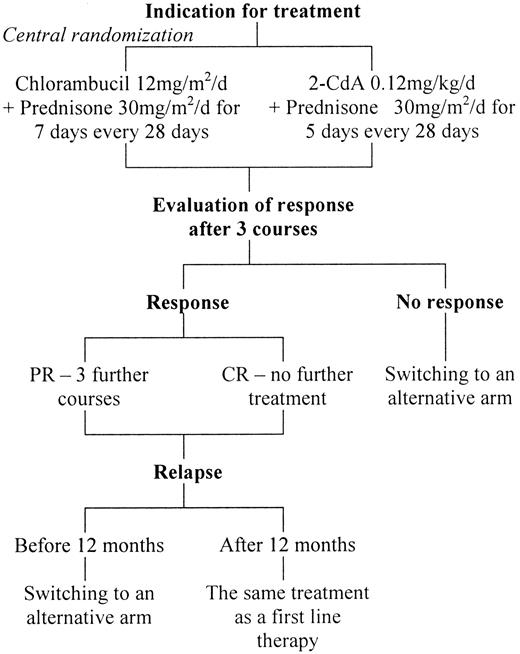
There were 9 participating centers in Poland, and eligible patients underwent a central randomization procedure for the assignment to either 2-CdA+P or Chl+P therapy by telephone, fax, or e-mail (Figure1). 2-CdA was synthesized according to the method of Kazimierczuk et al18 and was supplied by the Foundation for Development of Diagnostics and Therapy (Warsaw, Poland). More recently, the drug was commercially available from the Institute of Biotechnology and Antibiotics (Warsaw, Poland). It was administered at 0.12 mg/kg daily in 2-hour infusions for 5 consecutive days and was combined with oral prednisone 30 mg/m2 daily on days 1 to 5, starting with 2-CdA courses. Chlorambucil was given orally at 12 mg/m2 per day on 7 consecutive days, and prednisone was given at 30 mg/m2 per day on days 1 to 7. Both schedules were repeated over 28 days for 3 courses. If hematologic complications (thrombocytopenia less than 50 × 109/L; granulocytopenia less than 0.5 × 109/L) or severe infections developed, the drugs were readministered at time intervals longer than 28 days, ranging from 2 to 3 months, until the increase of hematologic parameters or recovery from infection was noted. Treatment was discontinued if CR was achieved. If there was partial but continuing response (PR), up to 3 additional courses were given. Patients with no response (NR) or progression after 3 courses of treatment or who had relapses earlier than 12 months after completing the treatment were switched to an alternative arm. Patients with late relapse (12 months from first remission) were retreated with the same schedule that induced the previous response. Criteria for retreatment were the same as the criteria for inclusion in the study. If there was no response to either regimen, the patients were treated with CHOP (cyclophosphamide, hydroxydaunomycin/doxorubicin, Oncovin, prednisone).
Response criteria
Physical examination and peripheral blood analysis were performed before and after every course of treatment, and the response to the drug was estimated. Bone marrow biopsy and immunophenotype studies were performed after 3 or 6 courses of treatment to confirm CR. Creatinine and bilirubin levels were measured; GOT, GPT, electrocardiography, and urinalyses were performed before each cycle, and results were recorded. Response criteria were those recommended by the NCI-sponsored Working Group.16 CR required the absence of symptoms and organomegaly and the return to normal blood count, with granulocyte count greater than 1.5 × 109/L, thrombocyte count greater than 100 × 109/L, and hemoglobin concentration greater than 11.0 g/dL, and bone marrow lymphocyte percentage less than 30% in aspiration and biopsy material for at least 2 months. PR was indicated by greater than 50% decrease in the size of lymph nodes, liver, and spleen, and peripheral blood findings either identical to those of CR or improved over pretherapy values by at least 50%. Patients who did not achieve CR or PR were classified as nonresponders (NR). Clinical relapse was assessed according to Robertson et al19 as an increase in the absolute lymphocyte count above 10 × 109/L, more than 50% lymphocytes on marrow differential analysis, more than 50% increase in the sum of the sizes of at least 2 lymph nodes, appearance of new lymph nodes, more than 50% increase in the liver or spleen span below the costal margin, new appearance of palpable hepatosplenomegaly, or development of aggressive lymphoma.
Toxicity monitoring
Hematologic toxicity was evaluated according to the criteria developed by the NCI-sponsored Working Group.16Drug-induced anemia, thrombocytopenia, and neutropenia were considered if, after the treatment course, a further decrease in hemoglobin level or platelet and neutrophil numbers were observed. Other side effects were monitored and assessed according to the Eastern Cooperative Oncology Group criteria.20
Statistical analysis
The trial was designed as a 2-arm comparison of 2-CdA+P with Chl+P. We used the results of the earlier phase II studies as the base and designed this randomized trial to test the assumption that 2-CdA+P might increase the overall response rate of 40% expected for Chl+P by 20%. A sample size of 100 patients per group was calculated to prove the study hypothesis at a significance level of 5%, with a detection power of 80%. Both first-line treatments were compared for CR, PR, and OR (defined as the sum of CR and PR) rates after the completion of at least 3 initial courses. Overall survival (OS), progression-free survival (PFS), and event-free survival (EFS) were calculated according to the method of Kaplan and Meier21 and compared between groups by the log-rank test. OS was calculated from the first day of treatment to the last day of follow-up or death. PFS was the time from the end of first-ine therapy to disease progression or death. EFS was defined as the time from the beginning of first-ine treatment to a first adverse event: death, progression requiring a change in therapy, infections or thrombocytopenic hemorrhage requiring hospitalization, and autoimmune hemolytic anemia. The Mann-Whitney U test was used to compare differences in continuous variables between groups, and the χ2 test or Fisher exact test were used to compare percentages. P < .05 was considered statistically significant.
Study approval
The study was conducted in accordance with the updated Declaration of Helsinki. It was approved by the local ethics committees at the participating institutions, and all patients signed consent forms before entering the study.
Results
First-line treatment
Of 250 patients enrolled in the study, 229 were available for the estimation of treatment response; data are presented in Table2. The remaining 21 patients (7 during 2-CdA+P and 14 during Chl+P) discontinued the treatment before its completion and were lost from observation. Of the 126 patients who received 2-CdA+P as the first-line treatment, 59 (47%) achieved CR and 50 (40%) achieved PR, which gives an overall response rate of 87%. In the group of 103 patients who received Chl+P, CR was achieved in 12 (12%) and PR in 46 (45%), for an overall response rate of 57%. The median number of courses inducing CR was 3 in the 2-CdA+P-treated group and 4 in the Chl+P–treated group. The CR rate was statistically higher in 2-CdA+P– than in Chl+P–treated patients (P = .0001). The number of NR was statistically higher in the Chl+P- than in the 2-CdA+P–treated group: 45 (43%) versus 17 (13%) patients, respectively (P = .0001). Analysis in relation to Rai classification, evaluated before the beginning of therapy, indicated that the overall response rates for 2-CdA+P-treated patients in less advanced (I and II) and in more advanced (III and IV) clinical stages of disease were 97% and 73%, respectively, and were statistically higher than in the Chl+P–treated group—61% and 51%, respectively (P = .0005). The response rate to 2-CdA+P was higher than to Chl+P in the subgroups analyzed according to age, but the biggest difference was noted in patients younger than 55 (P = .006; Table 3).
Response duration and relapse
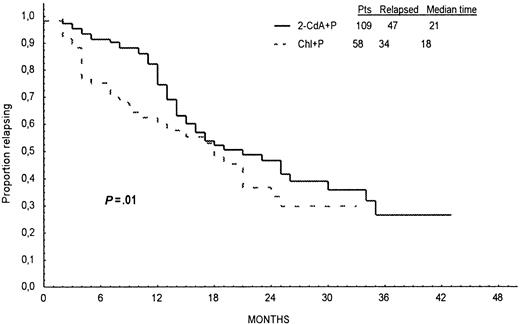
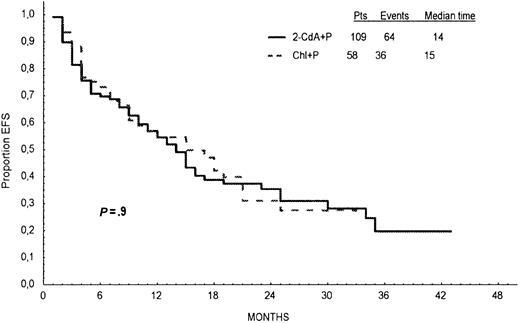
Response duration, survival, and incidence of relapse are presented in Table 4. In 2-CdA+P–treated patients, the median PFS was 21 months, and in Chl+P–treated patients it was 18 months. Probabilities of PFS calculated from Kaplan-Meier curves at 24 months for all patients treated initially with 2-CdA+P or Chl+P were 46% and 33%, respectively, and the difference was statistically significant (log-rank test, P = .01; Figure2). Of the 109 patients who responded to 2-CdA+P, early relapse—within 12 months of the start of therapy—was noted in 17 (16%) patients, and late relapse—after 12 months from the start of therapy—occurred in 30 (27%) patients. Corresponding values in 58 patients who responded to Chl+P were 22 (38%) and 12 (21%), respectively. Early relapses occurred more frequently after Chl+P than after 2-CdA+P (P = .004), but there was no statistical difference in the incidence of late relapses between the groups (P = .9). EFS was similar in both groups of patients (log-rank test, P = .9; Figure3).
Progression-free survival.
Progression-free survival defined as time from the end of first-line therapy to disease progression or death for patients in CR or PR after treatment with 2-CdA+P (continuous line) and Chl+P (dotted line).
Progression-free survival.
Progression-free survival defined as time from the end of first-line therapy to disease progression or death for patients in CR or PR after treatment with 2-CdA+P (continuous line) and Chl+P (dotted line).
Event-free survival.
Event-free survival defined as the time from the beginning of first-line treatment to a first adverse event: death, progression requiring a change in therapy, infections or thrombocytopenic hemorrhage requiring hospitalization, and autoimmune hemolytic anemia for the patients who responded to the first-line treatment with 2-CdA+P (continuous line) and Chl+P (dotted line).
Event-free survival.
Event-free survival defined as the time from the beginning of first-line treatment to a first adverse event: death, progression requiring a change in therapy, infections or thrombocytopenic hemorrhage requiring hospitalization, and autoimmune hemolytic anemia for the patients who responded to the first-line treatment with 2-CdA+P (continuous line) and Chl+P (dotted line).
Second-line treatment and retreatment
In 43 patients resistant to first-line therapy with Chl+P or with PFS shorter than 12 months, second-line therapy with at least 3 courses of 2-CdA+P was given. CR was achieved in 10 patients, and PR was achieved in 19 patients (Table 5). Chl+P as second-line treatment was administered to 26 patients, and PR was noted in 7. The overall response (CR+PR) rate to second-line therapy was higher after 2-CdA+P (67%) than after Chl+P (27%), and the difference was statistically significant (P = .03). Retreatment with at least 3 cycles of 2-CdA+P was given to 12 patients with PFS longer than 12 months, and 6 (50%) of them responded. Five patients who had relapses later than 12 months from first remission obtained with Chl+P were retreated with the same schedule, and 2 of them responded.
Fifteen patients from the 2-CdA group and 11 from the Chl group who did not respond to first- and second-line therapy were treated with the CHOP regimen. Overall response rates to this third-line therapy were 20% and 27%, respectively.
Overall survival
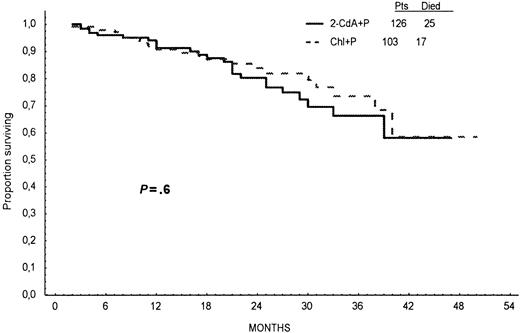
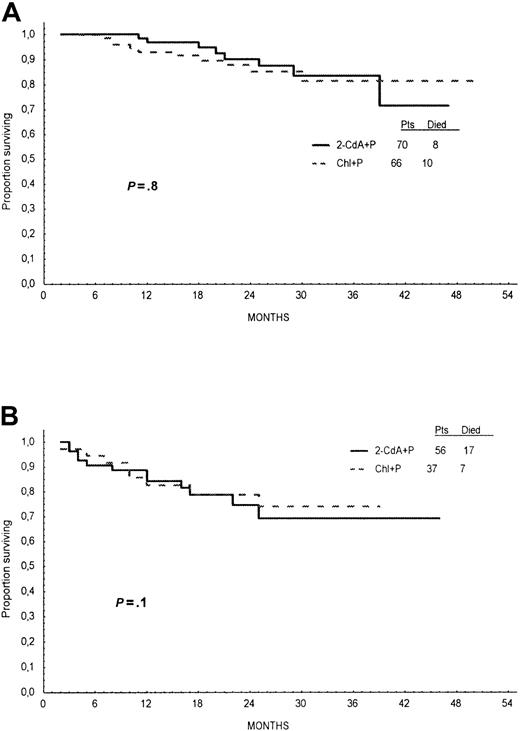
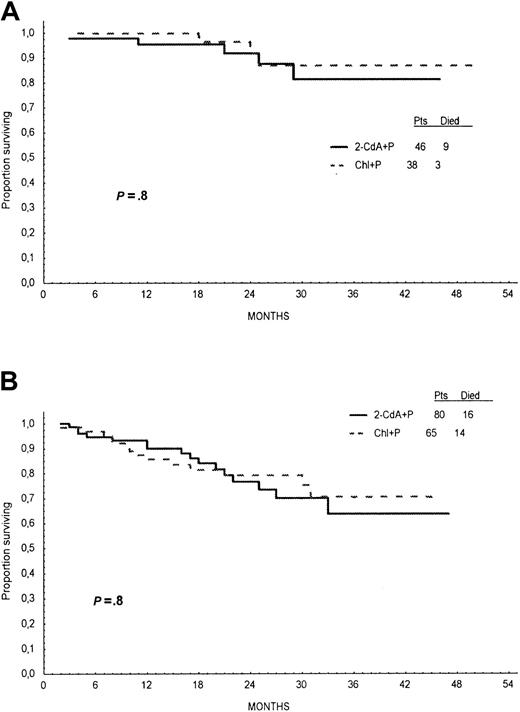
Probabilities of OS for 2-CdA+P– and Chl+P–treated patients, calculated from Kaplan-Meier curves at 24 months, were similar—78% and 82%, respectively (log-rank test, P = .6; Figure4). Analysis of OS stratified for Rai stage and age did not show any significant difference between treatment groups (Figures 5,6).
Overall survival.
Overall survival time calculated from the first day of treatment to the last day of follow-up or death for patients treated with 2-CdA+P (continuous line) or Chl+P (dotted line) as first-line therapy.
Overall survival.
Overall survival time calculated from the first day of treatment to the last day of follow-up or death for patients treated with 2-CdA+P (continuous line) or Chl+P (dotted line) as first-line therapy.
Overall survival according to Rai stage.
Overall survival time of patients treated with first line 2-CdA+P (continuous line) or Chl+P (dotted line) analyzed for patients with Rai stages 0, I, and II (A) and for Rai stages III and IV (B).
Overall survival according to Rai stage.
Overall survival time of patients treated with first line 2-CdA+P (continuous line) or Chl+P (dotted line) analyzed for patients with Rai stages 0, I, and II (A) and for Rai stages III and IV (B).
Overall survival according to age.
Overall survival time of patients treated with first-line 2-CdA+P (continuous line) or Chl+P (dotted line) analyzed for patients 55 years or younger (A) and older than 55 years (B).
Overall survival according to age.
Overall survival time of patients treated with first-line 2-CdA+P (continuous line) or Chl+P (dotted line) analyzed for patients 55 years or younger (A) and older than 55 years (B).
Drug toxicity
Drug-related side effects are presented in Table6. Thrombocytopenia was the most frequent undesirable effect observed after treatment with 2-CdA (36%) and Chl+P (27%; P = .2), but it was usually mild or moderate. However, granulocytopenia and infections or fever of unknown origin were more frequent after treatment with 2-CdA+P (23%) than with Chl+P (11%; P < .02). The most frequent side effects were pneumonia and upper respiratory infections (sinusitis and bronchitis), which occurred during first-line treatment in 38 (30%) patients treated with 2-CdA+P and in 25 (24%) patients treated with Chl+P. Herpes zoster reactivation and herpes simplex infections were observed in 26 (21%) patients and in 11 (11%) patients, respectively. Opportunistic infections were not observed in the patients treated with 2-CdA+P or Chl+P. Autoimmune hemolytic anemia (AIHA) during treatment was observed in 7 patients treated with 2-CdA+P and in 2 treated with Chl+P. Other side effects were observed sporadically, with similar incidences in both groups. The most frequent causes of death were pneumonia and disease progression (Table7). AIHA caused the fatal outcomes for 4 (16%) patients treated with 2-CdA+P and 1 patient treated with Chl+P. Richter syndrome was observed in 3 (2.4%) patients treated initially with 2-CdA+P and in 4 (3.9%) patients treated with Chl+P. Secondary cancers included brain glioma and pulmonary cancer in the 2-CdA–treated group (one patient each) and hepatic cancer in the Chl-treated group (one patient). Secondary MDS/AML has not been seen thus far in either group.
Discussion
The aim of our prospective, randomized, multicenter study was to compare the efficacy and toxicity of 2-CdA+P and Chl+P in previously untreated patients with progressive B-CLL. Moreover, patients who were resistant to first-line therapy or who had early relapses (PFS less than 12 months) were switched to an alternative arm. This design allowed us to determine whether 2-CdA is more efficient than chlorambucil as a second-line treatment. It is worth emphasizing that until now the results of prospective, randomized studies comparing 2-CdA with standard treatment in patients with CLL have not been published.
Data obtained from our trial on 229 untreated patients with CLL indicate that the OR rate of 87% after 2-CdA+P was significantly higher than after Chl+P (57%; P < .0001), and the percentage of nonresponders was significantly lower in 2-CdA+P–treated group (13%) than in the Chl+P–treated group (43%;P < .0001). We want to emphasize that in our study, the CR rate after 2-CdA+P treatment was significantly higher (47%) than after Chl treatment (12%), with a similar proportion of PR in each group. Moreover, it is worth noting that CR and OR rates to 2-CdA are comparable to those reported for FAMP. In a large, randomized study comparing FAMP and Chl in 233 previously untreated patients with CLL, the CR rate after FAMP was 33% and was significantly higher than after Chl treatment (8%).22 Better results in terms of CR rate after FAMP than after CAP (cyclophosphamide, doxorubicin, and prednisone) were also reported by a French Co-operative Group in untreated and in previously treated CLL patients with more advanced clinical stages (B and C according to Binet's classification).23 In a recently reported Italian randomized, multicenter trial of FAMP versus Chl+P in 128 previously untreated CLL patients, the CR rate after FAMP was 43%, which is similar to our results after treatment with 2-CdA+P.24
Responses to the first-line treatment of patients with CLL, as seen on the PFS and EFS plots, are characterized by longer survival times without disease progression in patients treated with 2-CdA. EFS is similar in both groups, reflecting the toxicity associated with purine analog administration that outweighs the theoretical advantage of longer response.
We have not shown any significant difference in the overall survival in patients treated with 2-CdA+P or Chl+P as a first-line regimen, even when the analysis was stratified for age and Rai stage. In 3 recently published trials22-24 comparing the efficacy of FAMP with Chl or CAP, longer survival times of patients treated with a purine analog as a first-line drug were not observed. However, it should be kept in mind that our trial was designed in such a way that patients resistant to Chl+P or who had early relapses after this treatment were subsequently given 2-CdA+P, and most of them responded to second-line therapy. Finally, they received similar dosages and quality of treatment, which may explain the lack of difference in OS rates.
Response rates similar to those seen after FAMP were obtained by others using high-dose Chl regimens with or without prednisone.24,25 The question arises whether dose escalation increases the effectiveness of Chl or 2-CdA in inducing durable remission in CLL. Such studies were undertaken by Jaksic et al,26 who administered high-dose Chl (15 mg daily) to patients with CLL and obtained an OR rate of 89.5%, comparable to that seen after therapy with purine analogs. The toxicity of such treatment was mild or moderate. In our study patients received Chl at a similar dose (12 mg/m2), but the drug was given intermittently only for 7 days per month, and its effectiveness was lower. Taking into account that the myelotoxicity and immunotoxicity of 2-CdA may often be severe or even life-threatening, we are not in favor of increasing the 2-CdA dose to improve the results of treatment. Similar conclusions may be drawn from the study by Saven et al,27 who defined the maximal tolerated 2-CdA dose as 0.1 mg/kg per day administered intravenously for 7 days.
In our patients whose disease became progressive after 12 months from the start of treatment, retreatment with 2-CdA+P induced 50% OR, whereas retreatment with Chl+P was successful in 24% of patients. This observation confirms the results published by Juliusson and Liliemark,28 who achieved second remission with 2-CdA in all 6 patients retreated with this drug. In the study published recently by Keating et al,29 67% of patients responded to retreatment with FAMP.
Our study has shown that 2-CdA+P is an effective treatment for patients resistant to Chl+P and for patients who had early relapses after treatment with an alkylating agent. The OR rate in this group was 67%, whereas in patients primarily resistant to 2-CdA+P, the OR rate to Chl+P as an alternative regimen was only 33% (P = .03). Multidrug regimens such as CHOP, applied as rescue treatment for patients who did not respond to previous therapy with 2-CdA and Chl, offered little in terms of clinical effectiveness.
Another important aim of our study was to compare the toxicity of 2-CdA+P and Chl+P. Analysis of drug-induced toxicity confirms the strong myelosuppressive effect of 2-CdA that resulted in a high incidence of neutropenia and infections in patients treated with this purine analog in comparison with Chl, which is in accordance with previous reports.30,31 Our data do not show, however, a higher risk for opportunistic infections in patients treated with 2-CdA+P in contrast to the observations performed in the patients treated with FAMP and prednisone.32 Thrombocytopenia was the most frequent side effect observed with equal frequency after 2-CdA and Chl. Other side effects were mild or moderate and were acceptable.
Earlier reports33,34 suggested that purine analogs may induce AIHA in patients with CLL. In our study, AIHA was noted in 7 patients treated with 2-CdA+P and in 2 patients treated with Chl+P, but this difference was not significant statistically (P = .3). This is in accordance with the findings of DiRaimondo et al,33 who observed AIHA in 5 of 12 patients with CLL who were treated with FAMP. We emphasize that the response of AIHA to steroids was poor; fatal outcomes occurred in 5 patients (4 after 2-CdA+P treatment and 1 after Chl+P treatment).
Secondary malignancy was observed in 2 patients treated with 2-CdA and in 1 patient treated with Chl. We have not yet observed an incidence of secondary MDS/AML. These complications may become more evident with longer observations of the patients.35 36
In conclusion, the results of our prospective, randomized trial confirm significantly higher CR and OR rates after 2-CdA+P than after Chl+P in untreated patients with CLL. In addition, 2-CdA+P was shown to be effective as the second-line treatment in patients resistant to Chl+P. Moreover, 2-CdA was effective in the retreatment of patients who had relapses after 2-CdA–induced remission. It has been shown that the relapse rate after 2-CdA+P did not differ significantly from that noted after Chl+P. There was also no difference in overall survival time and event-free survival time in patients treated with 2-CdA+P or Chl+P as the first-line therapy. The results of the current study do not identify definitively which treatment is preferred as first-line therapy for CLL. The similarity in overall survival times in both groups implies that Chl might still be regarded as a safe first-line therapy for older patients. If there is resistance, 2-CdA may be used as a second-line treatment. However, the higher CR rates after 2-CdA treatment may make this a more favorable approach as first-line therapy for younger patients, who are likely to benefit from more aggressive therapy, including stem cell transplantation.37 38
Acknowledgments
We thank Professor Emili Montserrat (Postgraduate School of Hematology, Barcelona, Spain) and Professor Gunnar Juliusson (University Hospital, Linkoping, Sweden) for their critical reading of the manuscript. We also thank Dr Richard Szydlo (Leukemia Unit, Hammersmith Hospital, London, UK) for statistical advice.
Supported in part by grants 4PO5BO271 and 4PO5BO619 from KBN Warsaw, Poland.
Preliminary results of this study were presented at the 39th Annual Meeting of the American Society of Hematology, San Diego, CA, December 5-9, 1997; at the 40th Annual Meeting of the American Society of Hematology, Miami Beach, FL, December 4-8, 1998; and at the VIII International Workshop on CLL, Paris, France, October 29-31, 1999.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tadeusz Robak, Department of Hematology, Medical University of Lodz, Copernicus Memorial Hospital, ul.Pabianicka 62, 93-513 Lodz, Poland; e-mail: robaktad@psk2.am.lodz.pl.