Abstract
Terminal differentiation of erythroid cells results in terminal cell divisions followed by irreversible cell cycle withdrawal of hemoglobinized cells. The mechanisms leading to cell cycle withdrawal were assessed in stable transfectants of murine erythroleukemia cells, in which the activities of cyclin-dependent kinases (CDKs) and CDK inhibitors (CDKIs) could be tightly regulated during differentiation. Cell cycle withdrawal of differentiating cells is mediated by induction of several CDKIs, thereby leading to inhibition of CDK2 and CDK4. Manipulation of CDK activity in differentiating cells demonstrates that the onset of cell cycle withdrawal can be either greatly accelerated or greatly delayed without affecting hemoglobin levels. Extending the proliferation of differentiating cells requires the synergistic action of CDK2 and CDK4. Importantly, CDK6 cannot substitute for CDK4 in this role, which demonstrates that the 2 cyclin D–dependent kinases are functionally different. The results show that differentiating hemoglobinized cells can be made to proliferate far beyond their normal capacity to divide.
Introduction
The process of differentiation of primitive cells into more specialized cells involves both the expression of new sets of genes (phenotypic differentiation) and expansion of the differentiating cell population through cell division.1 2In recent years a great deal has been learned about the factors controlling gene expression during differentiation. Much less is known about the mechanisms that determine the number of cell divisions that a terminally differentiating cell can make and the mechanisms that maintain fully mature cells in the state of permanent cell cycle withdrawal. There is an equally important question to consider: Once triggered, are phenotypic differentiation and terminal cell division coupled, or alternatively, do they proceed independently?
The eukaryotic cell cycle is primarily regulated by a family of serine/threonine protein kinases that each consist of a catalytic subunit (cyclin-dependent kinase [CDK]) and a regulatory subunit (cyclin).3 In mammalian cells, progression through the G1 phase is controlled by the activities of CDK4 and CDK6, which associate with 1 of 3 D-cyclins (D1, D2, and D3), and CDK2, which associates first with cyclin E and then later in S phase with cyclin A.4 The enzymatic activities of the CDKs are controlled at several levels: cyclin binding,5 CDK-activating kinase (CAK) activation, CDK phosphorylation and dephosphorylation, and binding of cyclin-dependent kinase inhibitors (CDKIs).6,7To date 2 families of CDKIs that differ in their specificity and mechanism of inhibition have been identified. INK4 family members p16ink4a, p15ink4b, p18ink4c, and p19ink4dinhibit only CDK4 and CDK6 by interfering with cyclin D binding.8,9 The KIP family of inhibitors, p21CIP, p27KIP1, and p57kip2, is thought to inhibit primarily CDK2 in vivo.10 11 Whereas functional differences are known among the D-, E-, and A-dependent kinases, differences between the 2 cyclin D–dependent kinases, CDK4 and CDK6, have not been reported.
We have studied the mechanisms leading to terminal cell division and irreversible cell cycle withdrawal in differentiating red blood cells using murine erythroleukemia (MEL) cells.12 MEL cells are erythroid precursors that are blocked at the proerythroblast stage. Treatment of the cells with certain chemical inducers relieves the block to differentiation and allows the cells to resume erythroid differentiation. In vitro differentiation of MEL cells recapitulates many aspects of normal red blood cell differentiation including synthesis of hemoglobin and other erythrocyte-specific proteins as well as terminal cell division. After committing to terminal differentiation, the cells undergo a maximum of 5-6 cell divisions and then permanently withdraw from the cell cycle.13 This process closely resembles the terminal cell divisions of colony-forming unit–E (CFU-E), normal, committed erythroid precursors that also differentiate and divide 5-6 times before arresting.14 We found that MEL cell differentiation is accompanied by specific, temporally defined changes in the levels and activities of the CDKs and the CDK inhibitors including differences in the regulation of CDK4 and CDK6. To investigate the role of these changes in terminal cell division and cell cycle withdrawal, we generated and analyzed stable MEL cell transfectants expressing these cell cycle regulators under an inducible promoter. Our results show that cell cycle withdrawal of differentiating erythroid cells is mediated by the induction of CDKIs, which leads to inhibition of CDK2 and CDK4. The results also imply that once differentiation is triggered, phenotypic differentiation (hemoglobin synthesis) and terminal cell division need not be tightly coupled, and the 2 programs can proceed independently at different rates without affecting one another.
Materials and methods
Cell culture and DNA transfections
Clone DS19 MEL cells were grown, and differentiation was initiated as previously described.15,16 Benzidine staining and plasma clot assays were performed as previously described.15 Stable MEL cell transfectants expressing doxycycline (Dox)-inducible cell cycle regulators were generated by transfecting 1 of 2 MEL cell clones (B1 and C2) that stably express the reverse tetracycline–controlled transactivator (rtTA).17Clones B1 and C2 were prepared by cotransfecting DS19 MEL cells with pPGK-neo and pUHD 172-1.17 MEL cell transfectants p18 and p27 were generated by cotransfecting clone B1 with pPGK-puro and either pUHD 10-3 p18 or pUHD 10-3 p27. CDK2-HA (hemagglutinin), CDK4R24C-HA, and CDK6R31C-HA transfectants were generated by cotransfecting clone C2 with either pUHD 10-3 CDK2-HA, pUHD 10-3 CDK4R24C-HA, or pUHD 10-3 CDK6R31C-HA and with pPGK-puro. Transfectants were selected and maintained in growth medium containing 1 mg/mL G418 and 5 μg/mL puromycin. CDK(2 + 4R24C) and CDK(2 + 6R31C) MEL cell transfectants were generated by cotransfecting pUHD 10-3 CDK2-HA and pPGK-hygro into CDK4R24C.24 and CDK6R31C.34 transfectants, respectively. Transfectant clones were selected and maintained in medium containing 1 mg/mL G418, 5 μg/mL puromycin, and 1.1 mg/mL hygromycin. Antibiotic-resistant clones were expanded, and the cell extracts were analyzed by immunoblotting.
Plasmids
Complementary DNAs (cDNAs) encoding human p18 cDNA, CDK2-HA, CDK4R24C-HA, and CDK6R31C-HA (gifts of J. Koh, L. Zhu, D. Franklin, and Y. Xiong, respectively) were cloned into pUHD 10-3 (pUHD 10-3 p27, gift of R. Pestell).
Immunoblot and immunoprecipitation assays
Immunoblot assays were performed on 100 μg total protein extract as previously described.18 Immunoblotting for hemoglobin was performed similarly, except that sonication extracts were used and polyacrylamide gel electrophoresis (PAGE) gels were run under nondenaturing conditions. Immunoprecipitation assays were performed on 500 μg total protein extract from cells lysed by sonication, as previously described.18 19
Antibodies
Immunoblot analysis was performed with the following antibodies: polyclonal human-specific (nonmouse cross-reactive) α-CDK2 (AB-1, PC44; CalBiochem, Nottingham, England); polyclonal human α-CDK2, α-cyclin D1 and D3, α-p15, α-p18, and α-p27 (all gifts of Y. Xiong); polyclonal human α-CDK4 (C-22), α-CDK6 (C-21), α-cyclin D1 (R124), α-cyclin D1 (HD11), α-cyclin D2 (M-20), α-cyclin E (M-20), α-cyclin A (C-19), and α-p16 (M-156) (brand names in parentheses; all from Santa Cruz Biotechnology, Santa Cruz, CA); monoclonal human α-p21 (65951A; PharMingen, San Diego, CA); monoclonal α-hemagglutinin (HA) 12CA5 (Boehringer Mannheim, Mannheim, Germany); and polyclonal α-mouse hemoglobin (ICN-Koeppell). We also used HRP-conjugated antimouse immunoglobulin G (IgG), antirabbit IgG, and enhanced chemiluminescence (ECL) (Amersham); CDK2 blocking peptide (BP) (gift of Y. Xiong) and CDK4 bp (C-22) and CDK6 bp (C-21) (Santa Cruz Biotechnology); and for immunoprecipitation-kinase analysis, affinity-purified polyclonal human α-CDK2, α-CDK4, and α-CDK6 (gifts of Y. Xiong).
Immunoprecipitation-kinase assay
We immunoprecipitated 100 μg (for CDK2) or 500 μg (for CDK4 or CDK6) of total cellular protein extract with either a mouse-specific anti-CDK antibody (nonreactive with HA-tagged human CDKs) for endogenous CDKs or with an anti-HA antibody for exogenous CDKs. Kinase assays were performed as previously published18 with either histone H1 (CDK2; Sigma Chemical Co., St Louis, MO) or a glutathione S-transferase (GST)–tagged carboxy-terminal fragment of Rb (gift of R. Pestell) as a substrate. For CDK(2 + 4R24C) and CDK(2 + 6R31C) transfectants containing 2 exogenous kinases, the assays were carried out as described above except that human-specific α-CDK2 antibody (nonreactive with mouse CDK2) was used first to completely immunoprecipitate exogenous CDK2 activity. Extracts found to be free of exogenous CDK2 were then immunoprecipitated for exogenous CDK4R24C-HA and CDK6R31C-HA as described above.
Results
Resumption of terminal differentiation by erythroleukemia cells is accompanied by specific temporal changes in cell cycle regulators
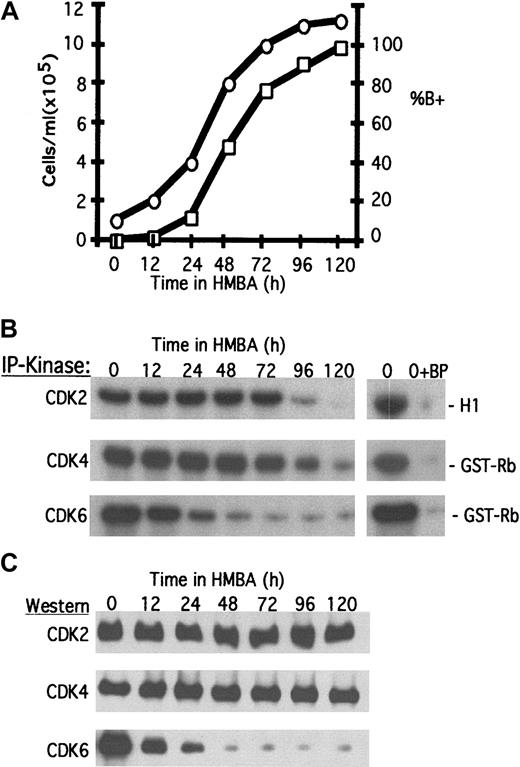
Treatment of MEL cells with inducers of differentiation, such as hexamethylene bisacetimide (HMBA), causes the cells to become irreversibly committed to reinitiate erythroid differentiation.12 By 48 hours most cells are committed and no longer require the presence of the inducer to complete differentiation.13 As cells differentiate they continue to undergo cell division and accumulate hemoglobin (Figure1A, percent of B+) and other red cell markers. By 96-120 hours nearly all cells have undergone terminal cell division (Figure 1A) and enter permanent growth arrest in phase G1/G0.20
Changes in CDK activities during erythroleukemia cell terminal differentiation.
Logarithmically growing MEL cells were induced to differentiate with 5 mmol/L HMBA as described in “Materials and methods.” (A) At the indicated times the cell number (○) and the percentage of benzidine-positive hemoglobinized cells (percent of B+, □) were determined. (B) Total cellular protein extracts were prepared and analyzed for specific CDK activities by immunoprecipitation (IP)-kinase assays, and (C) CDK protein levels were determined by immunoblotting as described in “Materials and methods.” BP indicates blocking peptide.
Changes in CDK activities during erythroleukemia cell terminal differentiation.
Logarithmically growing MEL cells were induced to differentiate with 5 mmol/L HMBA as described in “Materials and methods.” (A) At the indicated times the cell number (○) and the percentage of benzidine-positive hemoglobinized cells (percent of B+, □) were determined. (B) Total cellular protein extracts were prepared and analyzed for specific CDK activities by immunoprecipitation (IP)-kinase assays, and (C) CDK protein levels were determined by immunoblotting as described in “Materials and methods.” BP indicates blocking peptide.
To ascertain the relationship between cell cycle regulatory proteins and permanent cell cycle withdrawal, we assayed the levels and activities of the 3 G1/S-phase CDKs (CDK2, CDK4, and CDK6) throughout differentiation (Figure 1B,C). Both CDK6 activity and CDK6 protein levels are markedly reduced within 24-48 hours after initiating HMBA treatment. The early decline of CDK6 occurs as cells are committing to differentiation and suggests that the loss of CDK6 may be important for this first step in the reprogramming of the tumor cells (see “Discussion”). However, the late decline in CDK2 and CDK4 activities, occurring as the cells undergo terminal cell division, suggests that these 2 CDKs drive cell division in differentiating cells and that loss of their activities is responsible for withdrawal of the cells from the cell cycle. An earlier study21 indicated that CDK4 protein levels decline rapidly after HMBA treatment. Although contrary to expectation but in agreement with the data of Figure 1A, this study also showed that CDK4 activity declined at later times as cells complete differentiation and undergo cell cycle arrest. It is not clear from this work how CDK4 protein levels could decline while enzyme activity is maintained. CDK6 was not investigated in this earlier work.
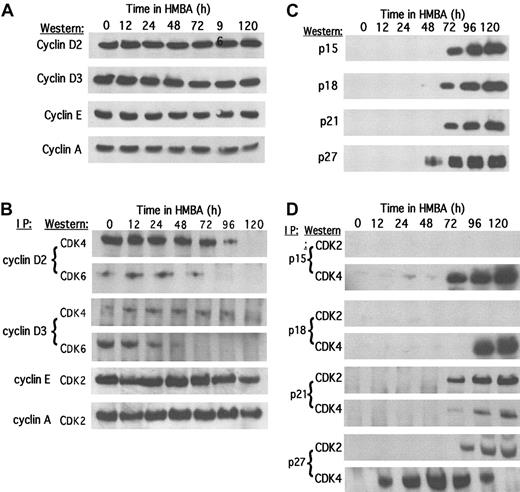
Given the constant levels of the CDK2 and CDK4 proteins (Figure 1C), the loss of CDK2 and CDK4 activities at 96-120 hours could be due to either a decline in the levels of cyclins or an induction of the CDK inhibitors. The cyclin D2, D3, E, and A levels were observed to be mostly constant throughout differentiation (Figure2A), although cyclin D1 could not be detected in MEL cells even with the use of several antisera. Interestingly, we observed that in undifferentiated cells, CDK4 associates primarily with cyclin D2, whereas CDK6 associates primarily with cyclin D3 (Figure 2B). The loss of CDK6–cyclin D3 complexes was observed early in the differentiation program and probably reflects the rapid decline in CDK6 levels induced by HMBA. On the other hand, CDK4–cyclin D2 complexes dissociate much later, during terminal cell division. This dissociation occurs despite the fact that CDK4 (Figure1C) and cyclin D2 (Figure 2A) protein levels remain constant during this period. Furthermore, we observed only a minor reduction in CDK2–cyclin E/A complexes despite the complete inhibition of CDK2 activity during this late period.
Levels of cyclins and CDK inhibitors and their associations with CDKs during MEL cell differentiation.
Total cellular protein extracts were prepared at the indicated times of HMBA treatment, and the levels of (A) specific cyclins and (C) CDK inhibitors were determined by immunoblotting. Extracts were immunoprecipitated (IP) with antibodies specific for the indicated (B) cyclin or (D) CDK inhibitor as described in “Materials and methods.” The immunoprecipitates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted for the indicated CDK.
Levels of cyclins and CDK inhibitors and their associations with CDKs during MEL cell differentiation.
Total cellular protein extracts were prepared at the indicated times of HMBA treatment, and the levels of (A) specific cyclins and (C) CDK inhibitors were determined by immunoblotting. Extracts were immunoprecipitated (IP) with antibodies specific for the indicated (B) cyclin or (D) CDK inhibitor as described in “Materials and methods.” The immunoprecipitates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted for the indicated CDK.
The foregoing observations suggest that inhibition of CDK2 and CDK4 activities is more likely due to induction of CDK inhibitors. We observed that p15, p18, p21, and p27 levels increase dramatically during the period of terminal cell division, with the increase in p27 slightly preceding that of p15, p18, and p21 (Figure 2C). Undifferentiated MEL cells express p16 and p19; however, their levels decline early in the differentiation program (data not shown), suggesting that they are not involved in terminal cell division. Using 2 different commercial antibodies, p57 was not detected in MEL cells at any stage. We found that during the late stages of differentiation, the induced p15 and p18 associate with CDK4, whereas p21 associates primarily with CDK2 (Figure 2D). However, the induced p27, which as noted above increases slightly earlier than the other CDKIs, is found initially in complexes with CDK4, and later, as cells cease dividing, it associates with CDK2. Note that the earlier increase in p27 compared with the other inhibitors is accentuated when assayed in complexes with CDK4. These results suggest that during terminal cell division, p15 and p18 block cyclin binding to CDK4, which results in the inhibition of CDK4 activity, whereas p21 and p27 associate with and inhibit dimeric CDK2–cyclin E/A complexes.
Premature inhibition of CDK2 and CDK4 in differentiating cells causes early and permanent cell cycle withdrawal without affecting the formation of hemoglobinized cells
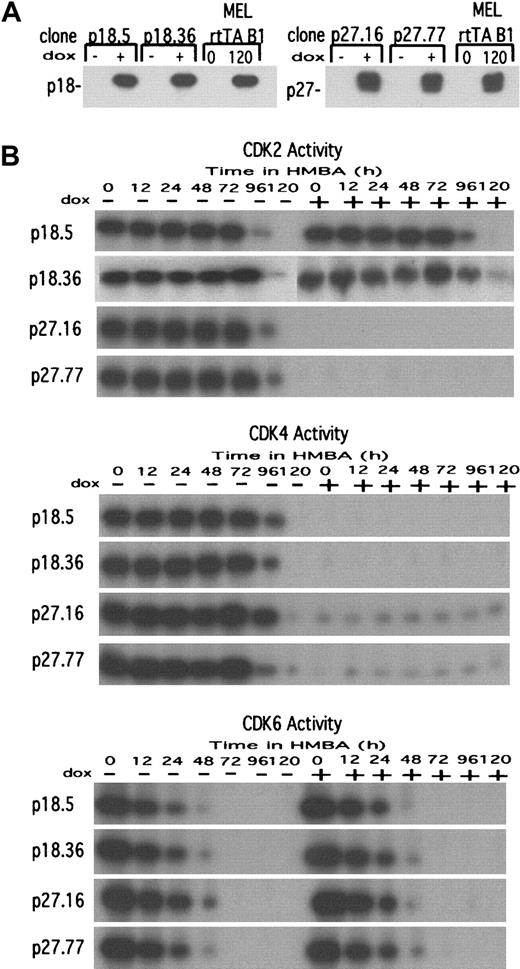
The observed induction of several CDKIs, their association with CDK2 and CDK4, and the concomitant inhibition of the 2 kinase activities, all occurring as cells undergo terminal cell division, suggest that these events may cause the cells to withdraw permanently from the cell cycle. To test this hypothesis we sought to cause premature inhibition of CDK activities in differentiating cells by generating stable MEL cell transfectants containing tetracycline-controlled expression vectors17 driving the synthesis of human p18 or p27. For each inhibitor, many transfectants were screened. Two transfectant clones were chosen that (1) did not exhibit detectable expression of the exogenous inhibitors in the absence of Dox and (2) had Dox-induced levels of the exogenous inhibitors equivalent to the levels of endogenous inhibitors present in fully differentiated parental MEL cells (Figure3A).
Characterization of p18 and p27 MEL cell transfectants.
(A) MEL cell transfectant clones expressing either p18 or p27 under control of the tetracycline inducible promoter in pUHD 10-3 were cultured either in the absence (−) or presence (+) of 1 μg/mL Dox for 36 hours. Total cellular protein extracts were prepared, and the levels of p18 (left panel) and p27 (right panel) were determined by immunoblotting. The parental MEL cells (clone B1) containing only the rtTA regulator were cultured in the presence of 5 mmol/L HMBA for 120 hours to indicate the levels of endogenous p18 and p27 present in fully differentiated MEL cells. For further details see “Materials and methods.” (B) The effect of exogenous p18 and p27 on CDK activities in undifferentiated and differentiated MEL cell transfectants. Extracts prepared from the indicated transfectant clones cultured in the presence of 5 mmol/L HMBA and either in the absence (−) or presence (+) of Dox were prepared at the indicated times and assayed for the indicated CDK activity by IP-kinase assays as described in “Materials and methods.”
Characterization of p18 and p27 MEL cell transfectants.
(A) MEL cell transfectant clones expressing either p18 or p27 under control of the tetracycline inducible promoter in pUHD 10-3 were cultured either in the absence (−) or presence (+) of 1 μg/mL Dox for 36 hours. Total cellular protein extracts were prepared, and the levels of p18 (left panel) and p27 (right panel) were determined by immunoblotting. The parental MEL cells (clone B1) containing only the rtTA regulator were cultured in the presence of 5 mmol/L HMBA for 120 hours to indicate the levels of endogenous p18 and p27 present in fully differentiated MEL cells. For further details see “Materials and methods.” (B) The effect of exogenous p18 and p27 on CDK activities in undifferentiated and differentiated MEL cell transfectants. Extracts prepared from the indicated transfectant clones cultured in the presence of 5 mmol/L HMBA and either in the absence (−) or presence (+) of Dox were prepared at the indicated times and assayed for the indicated CDK activity by IP-kinase assays as described in “Materials and methods.”
The biological activity of the transfected gene products was ascertained by measuring their effect on endogenous kinase activities and proliferation. Induction of p18 transfectants with Dox caused a complete inhibition of the CDK4 activity (Figure 3B), but it was not accompanied by any effect on the doubling time or accumulation of cells in G1 (Table 1). On the other hand, induction of p27 transfectants with Dox led to a nearly complete inhibition of the CDK2 and CDK4 activities (Figure 3B), an almost 4-fold increase in cell generation time, and a dramatic accumulation of cells in G1 (Table 1). Unexpectedly, induction of p18 or p27 did not inhibit CDK6 activity (Figure 3B). Induction of either p18 or p27 did not alter the rate of HMBA-induced differentiation, as measured by the rate of accumulation of benzidine-stained hemoglobinized cells, nor did it cause spontaneous differentiation (data not shown). We believe that induction of p18 and p27 failed to inhibit CDK6, whereas it did lead to inhibition of CDK4 because CDK6 is located in the nucleus of undifferentiated MEL cells, and CDK4 is found in the cytoplasm (I.M., F.R., A.I.S., unpublished data, 2000). Perhaps the levels of synthesis of the exogenous p18 and p27 are not high enough to complex with both CDKs, resulting in preferential association of the inhibitor with CDK4 located in the cytoplasm.
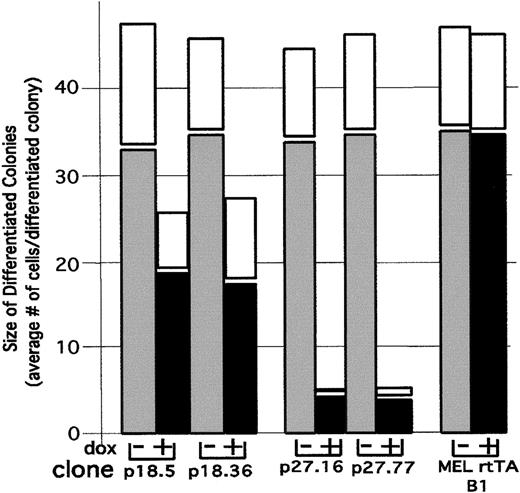
The proliferation capacity of differentiating cells can be measured by plasma clot assays in which the size of colonies arising from individual cells is determined after several days of growth in the clot in the absence of the differentiation inducer. Cells that have not committed to differentiate produce very large colonies consisting of hundreds to thousands of undifferentiated cells. On the other hand, cells that have committed to differentiate by prior exposure to a differentiation inducer, in this case HMBA, give rise to small colonies consisting of fewer than 64 cells, all of which stain positively for hemoglobin with benzidine. If cells are treated with HMBA for 12 hours and then plated in plasma clots, the average number of cells in a differentiated colony is 45 after 7 days of incubation (Figure4). Thus, once differentiation is triggered, the differentiating cells usually undergo an average of 5-6 cell divisions before withdrawing permanently from the cell cycle.
Effect of p18 and p27 on proliferation of differentiated cells.
We cultured p18 and p27 MEL cell transfectants in the presence of 5 mmol/L HMBA for 12 hours. HMBA was washed out, and the transfectants were cultured in the presence of 1 μg/mL Dox for 12 hours. The cells were plated in plasma clots and incubated for 4 days (solid bars) and 7 days (open bars) at 37°C in the absence of both HMBA and Dox. The clots were then stained with benzidine and hematoxylin as described in “Materials and methods.” The proliferative capacity of cells that were committed to differentiation was determined by counting the number of cells in at least 100 colonies that stained positive with benzidine.
Effect of p18 and p27 on proliferation of differentiated cells.
We cultured p18 and p27 MEL cell transfectants in the presence of 5 mmol/L HMBA for 12 hours. HMBA was washed out, and the transfectants were cultured in the presence of 1 μg/mL Dox for 12 hours. The cells were plated in plasma clots and incubated for 4 days (solid bars) and 7 days (open bars) at 37°C in the absence of both HMBA and Dox. The clots were then stained with benzidine and hematoxylin as described in “Materials and methods.” The proliferative capacity of cells that were committed to differentiation was determined by counting the number of cells in at least 100 colonies that stained positive with benzidine.
To ascertain whether premature induction of p18 or p27 would cause early cell cycle withdrawal in differentiating cells, we treated transfectants for 12 hours with HMBA, which is sufficient time to cause 20% of the cells to become committed to differentiation. We then removed the HMBA and cultured the cells for 12 hours in the presence or absence of Dox. Finally, Dox was removed, and the cells were plated in plasma clots in the absence of both HMBA and Dox and incubated for 4 days. Despite the fact that induction of exogenous p18 by Dox in these transfectants caused a complete inhibition of CDK4 activity in the cells (Figure 3B), it caused only a moderate decrease in the size of differentiated (benzidine-positive) colonies (Figure 4). On the other hand, treatment of p27 transfectants with Dox caused a dramatic decrease in the size of differentiated colonies, to an average size of 4 cells per colony (Figure 4). Even though the colonies were reduced in size, all of the cells in the colonies stained positive with benzidine and appeared to have normal levels of hemoglobin.
To determine whether the smaller colonies produced by induction of exogenous p27 had indeed undergone permanent cell cycle withdrawal or were merely retarded in their growth, we incubated the plasma clots for an additional 3 days. We observed virtually no increase in average colony size with further incubation (Figure 4, white bars). The observed permanent cell cycle withdrawal in Dox-treated p27 transfectants is in contrast to the behavior of the parental line and p18 transfectants, in which increased incubation time in the clots resulted in an increase in colony size (Figure 4, white bars). These results indicate that induction of p27 in differentiating cells causes them to withdraw prematurely and permanently from the cell cycle. This is in contrast to the effect of exogenous p27 in undifferentiated MEL cells, in which it causes an accumulation of cells in G1 (Table 1) that is fully reversible upon removing Dox (data not shown). Because p27 inhibits both CDK2 and CDK4 activities in differentiating cells, whereas p18 inhibits only CDK4, the results imply that inhibition of both kinases is required for cells to undergo terminal cell division. Finally, because 16% of the Dox-treated differentiated colonies in p27 transfectants consisted of single cells which stained positive for hemoglobin, we believe that once differentiation is triggered, the cells need not proliferate in order to differentiate.
Synergistic action of CDK2 and CDK4 delays terminal cell division and causes extensive proliferation of hemoglobinized cells
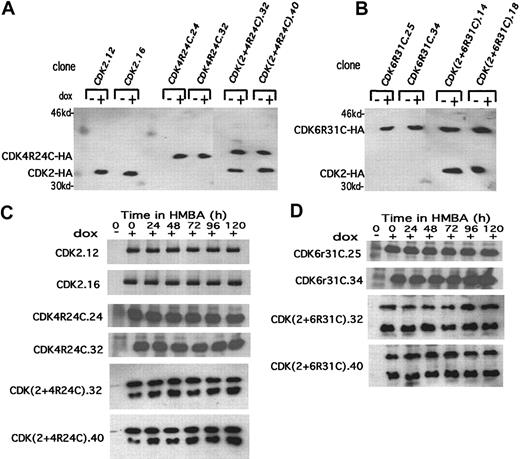
An alternative approach for examining the requirement for inhibition of CDK2 and CDK4 activities in terminal cell division is (1) to introduce expression vectors encoding these CDKs into MEL cells and (2) to determine whether inducing their synthesis during the period when the activities of the endogenous kinases decline promotes extended proliferation of differentiated cells. We initially generated MEL cell transfectants expressing a tetracycline-controlled human CDK4 fusion protein containing a C-terminal HA tag. However, while these transfectants express readily detectable exogenous CDK4 kinase activity in undifferentiated cells, the activity of the exogenous CDK4, like that of endogenous CDK4, was inhibited during differentiation (data not shown), presumably due to the rise of the endogenous CDKIs during differentiation. To avoid inhibition of the exogenous CDK4 by the endogenous CDK inhibitors, we used a mutant form of CDK4 (R24C)22 that is resistant to inhibition by the INK4 family of CDKIs. We generated MEL cell lines expressing a tetracycline-controlled human CDK4R24C fusion protein containing a C-terminal HA tag. We also generated lines expressing a tetracycline-controlled HA-tagged human CDK2 and lines expressing both proteins by supertransfecting a CDK4R24C transfectant (clone 24) with the CDK2 expression construct. Two clones of each type that showed highly inducible expression were chosen for further analysis (Figure5A). These clones exhibited comparable protein levels of the 2 exogenous HA-tagged CDKs, and expression of these proteins could be maintained throughout differentiation (Figure 5C).
Inducible expression of exogenous CDK2, CDK4R24C, and CDK6R31C in MEL cell transfectants.
(A, B) The extracts were prepared from the indicated CDK-HA MEL cell transfectants cultured in the absence (−) or presence (+) of Dox for 36 hours. The levels of exogenous HA-tagged CDKs were determined by immunoblotting with anti-HA–specific antibody. (C, D) Extracts were prepared from the indicated CDK-HA MEL cell transfectants that had been cultured in the presence of Dox for 24 hours (0 time in the figure) followed by treatment with 5 mmol/L HMBA for the indicated times. The levels of exogenous HA-tagged CDKs were determined by immunoblotting as in panels A and B. The upper band in the 2 lower sections of panel C is CDK4R24C-HA, and the lower band is CDK2-HA. The upper band in the 2 lower sections of panel D is CDK6R31C-HA, and the lower band is CDK2-HA.
Inducible expression of exogenous CDK2, CDK4R24C, and CDK6R31C in MEL cell transfectants.
(A, B) The extracts were prepared from the indicated CDK-HA MEL cell transfectants cultured in the absence (−) or presence (+) of Dox for 36 hours. The levels of exogenous HA-tagged CDKs were determined by immunoblotting with anti-HA–specific antibody. (C, D) Extracts were prepared from the indicated CDK-HA MEL cell transfectants that had been cultured in the presence of Dox for 24 hours (0 time in the figure) followed by treatment with 5 mmol/L HMBA for the indicated times. The levels of exogenous HA-tagged CDKs were determined by immunoblotting as in panels A and B. The upper band in the 2 lower sections of panel C is CDK4R24C-HA, and the lower band is CDK2-HA. The upper band in the 2 lower sections of panel D is CDK6R31C-HA, and the lower band is CDK2-HA.
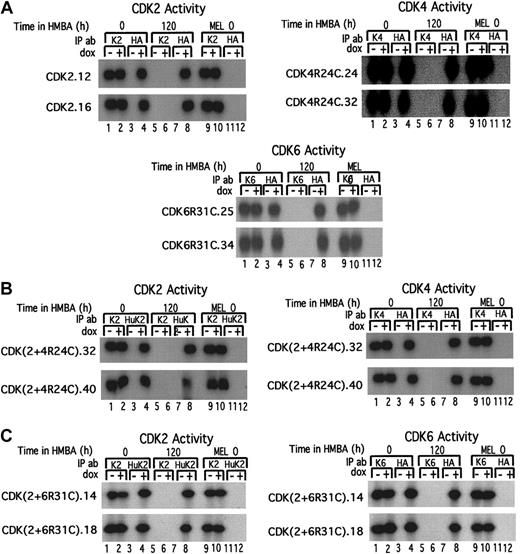
To directly assess the activities of exogenous CDKs and to compare their activities in the transfectants to that of the endogenous CDKs, we performed in vitro kinase assays for each specific CDK using extracts from the transfected cells. The specificity of the immunoprecipitation procedures used for the respective exogenous and endogenous CDKs was demonstrated by several results. First, CDK activity was not recovered in anti-HA immunoprecipitates of extracts from untransfected MEL cells (Figure 6, lanes 11 and 12 in each panel). Nor was CDK activity recovered in each anti-CDK immunoprecipitate of transfectants that were treated with HMBA plus Dox. This occurred because HMBA treatment leads to the loss of all endogenous CDK activities (Figure 6, lanes 5 and 6 in each panel). However, abundant Dox-induced CDK activities were recovered from the same cell extracts immunoprecipitated with anti-HA antibody (Figure 6, lane 8 in each panel). Furthermore, the activity of each exogenous kinase in the transfectants was strongly induced by Dox (Figure 6, compare lanes 3 and 4 in each panel) to levels that are comparable to the endogenous CDK levels (Figure 6, compare lanes 4 and 1 or 2 in each panel). Moreover, whereas all endogenous CDK activities are lost during HMBA-induced differentiation (Figure 6, lanes 5 and 6 in each panel), when HMBA-treated transfected cells are also treated with Dox, which induces the transfected CDK genes, the respectiveCDK activities are maintained in the treated cells at levels comparable to the activities present in undifferentiated cells (Figure6, compare lanes 1 or 2 and lane 8 in each panel). The generation time of the CDK transfectants was unaffected by induction of the exogenous CDKs with Dox, suggesting that CDK activity is present in excess in the undifferentiated cells.
Comparison of exogenous and endogenous kinase activities in undifferentiated and differentiated MEL cell CDK transfectants.
The indicated MEL cell transfectants were cultured in the absence (0) or presence (120) of HMBA for 120 hours and then further cultured in the absence (−) or presence (+) of Dox for 36 hours. Cell extracts were prepared and immunoprecipitated with antibodies specific for the endogenous murine CDKs (K2, K4, and K6) or the exogenous human HA-tagged CDKs (HA or HuK2). In doubly transfected cells (panels B and C) assays of exogenous kinase activities were performed by first immunoprecipitating with an antibody specific for the exogenous human CDK2 (HuK2), and after checking that the exogenous CDK2 was completely removed, the supernatant was immunoprecipitated with an anti-HA antibody specific for the exogenous HA-tagged (B) CDK4R24C or (C) CDK6R31C. The immunoprecipitates were incubated with γ-32P–ATP (γ-phosphorous 32–adenosine 5′-triphosphate) in the presence of either H1 or the GST-Rb C-terminal fragment, and the reactions were resolved by SDS-PAGE. For further details see “Materials and methods.”
Comparison of exogenous and endogenous kinase activities in undifferentiated and differentiated MEL cell CDK transfectants.
The indicated MEL cell transfectants were cultured in the absence (0) or presence (120) of HMBA for 120 hours and then further cultured in the absence (−) or presence (+) of Dox for 36 hours. Cell extracts were prepared and immunoprecipitated with antibodies specific for the endogenous murine CDKs (K2, K4, and K6) or the exogenous human HA-tagged CDKs (HA or HuK2). In doubly transfected cells (panels B and C) assays of exogenous kinase activities were performed by first immunoprecipitating with an antibody specific for the exogenous human CDK2 (HuK2), and after checking that the exogenous CDK2 was completely removed, the supernatant was immunoprecipitated with an anti-HA antibody specific for the exogenous HA-tagged (B) CDK4R24C or (C) CDK6R31C. The immunoprecipitates were incubated with γ-32P–ATP (γ-phosphorous 32–adenosine 5′-triphosphate) in the presence of either H1 or the GST-Rb C-terminal fragment, and the reactions were resolved by SDS-PAGE. For further details see “Materials and methods.”
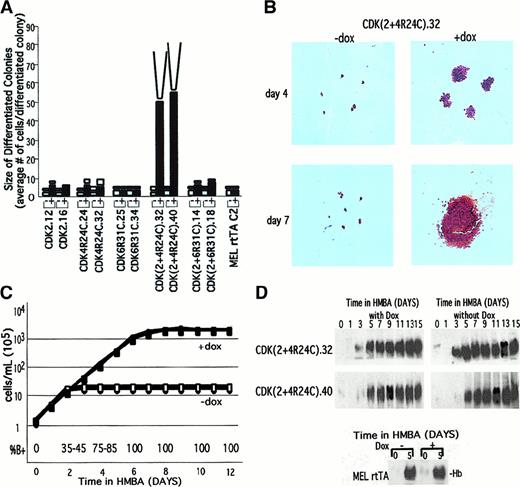
To determine whether restoration of CDK2 or CDK4 activity in differentiated cells prevents their withdrawal from the cell cycle, we measured the size of the colonies arising from differentiated transfected cells by plasma clot assays. Transfectants expressing either CDK2 or CDK4R24C or both proteins, were treated for 48 hours in liquid culture with HMBA and then plated in plasma clots in the presence or absence of Dox. After 48 hours of HMBA treatment, the average size of benzidine-positive hemoglobinized colonies produced from the parental MEL rtTA line (as well as MEL cells) is approximately 4 cells (Figure 7A,B). The small size of these colonies is due to the differentiated cells undergoing most of their terminal cell divisions in liquid culture, prior to plating in plasma clots. The differentiated colonies produced from each type of transfectant were similarly small in size when the cells were plated in plasma clots lacking Dox. However, in clots containing Dox, transfectants CDK2 and CDK4R24C produced differentiated colonies that were about twice as large, indicating that either CDK has the capacity to cause a modest increase in the number of cell divisions that can be undertaken by differentiating cells.
Synergistic activities of CDK2 and CDK4 in causing extensive proliferation of fully differentiated cells.
(A) The indicated MEL cell CDK transfectants were cultured in the presence of 5 mmol/L HMBA for 48 hours and then plated in plasma clots either in the absence (−) or presence (+) of Dox. The plasma clots were analyzed as described in the legend of Figure 4 after 3 days (■) and 7 days (□) of incubation at 37°C. (B) Plasma clots of a doubly transfected cell line were photographed (original magnification × 10) after staining with benzidine and hematoxylin as described in “Materials and methods.” (C) Logarithmically growing CDK(2 + 4R24C).32 and CDK(2 + 4R24C).40 were treated at 1 × 105 cells per mL with 5 mmol/L HMBA in the presence (shaded symbols) or absence (open symbols) of Dox. The cell densities were maintained at less than 1 × 106 cells per mL by subculturing to 2 × 105 cells per mL with the indicated growth medium every 24 hours as required. The cell densities were measured daily with a Coulter counter (Coulter Electronics, Miami, FL), and the cumulative cell densities were calculated. (D) Total cellular extracts were prepared at the indicated times from cells treated as described in panel C. The extracts were analyzed by nondenaturing PAGE and immunoblotting with an antibody specific for mouse hemoglobin. The lower section shows hemoglobin levels in the rtTA MEL cell parental line after 5 days of treatment with HMBA, with and without Dox.
Synergistic activities of CDK2 and CDK4 in causing extensive proliferation of fully differentiated cells.
(A) The indicated MEL cell CDK transfectants were cultured in the presence of 5 mmol/L HMBA for 48 hours and then plated in plasma clots either in the absence (−) or presence (+) of Dox. The plasma clots were analyzed as described in the legend of Figure 4 after 3 days (■) and 7 days (□) of incubation at 37°C. (B) Plasma clots of a doubly transfected cell line were photographed (original magnification × 10) after staining with benzidine and hematoxylin as described in “Materials and methods.” (C) Logarithmically growing CDK(2 + 4R24C).32 and CDK(2 + 4R24C).40 were treated at 1 × 105 cells per mL with 5 mmol/L HMBA in the presence (shaded symbols) or absence (open symbols) of Dox. The cell densities were maintained at less than 1 × 106 cells per mL by subculturing to 2 × 105 cells per mL with the indicated growth medium every 24 hours as required. The cell densities were measured daily with a Coulter counter (Coulter Electronics, Miami, FL), and the cumulative cell densities were calculated. (D) Total cellular extracts were prepared at the indicated times from cells treated as described in panel C. The extracts were analyzed by nondenaturing PAGE and immunoblotting with an antibody specific for mouse hemoglobin. The lower section shows hemoglobin levels in the rtTA MEL cell parental line after 5 days of treatment with HMBA, with and without Dox.
Much more potent, however, are the combined activities of CDK2 and CDK4R24C. Remarkably, the double transfectants showed a very large increase in the size of differentiated colonies, which grew to contain on average about 50 cells (Figure 7A,B). This effect was strictly dependent on the presence of Dox in the plasma clots. To determine whether the observed Dox-dependent expansion of differentiated cells produced from the double transfectants represents a limit, we incubated the clots for an additional 4 days. The differentiated colonies became very large, too large to effectively count the number of cells in each colony (Figure 7A,B). Note that within these large colonies produced at 3 days and 7 days in the clot, most cells in the colony contain hemoglobin, as judged by benzidine staining (Figure 7B).
Because it was not possible to accurately estimate the extent of proliferation in plasma clots of Dox-treated differentiated double transfectants, we measured their growth and differentiation in liquid cultures containing HMBA and Dox. Figure 7C shows the cumulative cell densities of the cultures. In the absence of Dox these cells, similar to HMBA-treated MEL cells, grew exponentially, with a doubling time of 11.8 hours for about 2-3 days and then gradually ceased dividing as cells became terminally differentiated. The final cumulative cell density of these cultures was about 1.2 × 106 cells per mL. However, when the double transfectants were treated with both HMBA and Dox, the cells grew logarithmically for up to 7-8 days. The doubling time of these cultures was 14.3 hours, which was very similar to the time in the early phase of growth in HMBA-treated parental MEL cells, prior to their undergoing differentiation. The final cumulative cell density of these cultures was about 1.8 × 108 cells per mL, at least 100 times higher than that observed in the absence of Dox. These results indicate that the maintenance of both CDK activities in differentiating cells allows the cells to undergo at least 6-7 additional cell divisions while still maintaining their differentiated phenotype, as judged by the fact that 100% of the cells stained positively with benzidine (Figure 7C, bottom). Moreover, cell extracts prepared from these cells contained as much hemoglobin as fully differentiated MEL cells (Figure 7D). We conclude that restoration of CDK2 and CDK4 activities in differentiated cells prevents their withdrawal from the cell cycle and allows them to proliferate extensively. Nevertheless, ultimately even these cells cease dividing (Figure 7C and “Discussion”).
CDK6 cannot substitute for CDK4 in differentiating cells
To date functional differences have not been established for the 2 cyclin D–dependent kinases,23 and it was of interest to determine whether or not CDK6 could, like CDK4, synergize with CDK2 to promote extended proliferation in differentiating cells. To test this possibility we prepared MEL cell transfectants expressing Dox-inducible HA-tagged CDK6. Using a similar rationale to that used for the CDK4R24C transfections, we used a newly constructed mutant form of CDK6, designed and characterized by D. Franklin and Y. Xiong (University of North Carolina, Chapel Hill), which does not bind and is resistant to inhibition by the INK4 inhibitors.24 Both single and double (with CDK2) transfectants of CDK6R31C were prepared and analyzed as described above (Figure 5B,D). Both types of transfectants exhibited levels of exogenous CDK6 activity comparable to that of endogenous CDK6 activity (Figure 6A,C, compare lanes 4 and 1 or 2 in right panels) as well as to the exogenous CDK4 activity in undifferentiated and differentiated transfectants described in the previous section (Figure 6A,C, lane 8, right panels).
Despite the evidence indicating that both single and double CDK6R31C transfectants had exogenous kinase activities comparable to that of the corresponding single and double CDK4R24C transfectants, we did not observe any effect of CDK6R31C on proliferation of differentiating cells, as measured by the size of differentiated colonies (Figure 7A). The ability of CDK4R24C to synergize with CDK2 to produce extremely large differentiated colonies in plasma clot represents a very sensitive assay for the ability of CDK4, along with CDK2, to promote cell division in differentiating cells. We found that the size of differentiated colonies produced by CDK2 and CDK6R31C doubles transfectants in the presence of Dox was not increased at all above that seen in the clones transfected with CDK2 alone. We conclude that CDK6 cannot substitute for CDK4 in promoting cell division in differentiating cells.
Discussion
Mechanism of permanent cell cycle withdrawal
The results reported here show that in differentiating MEL cells, terminal cell division is brought about by induction of 4 CDK inhibitors, their association with CDK2 and CDK4, and the resulting inhibition of the activities of the 2 kinases. These studies provide the most direct support for the view that these changes, which have been observed in several other in vitro differentiation systems,25-28 constitute the “clock” that determines the number of cell divisions which can be undertaken by a differentiating cell. We also suggest that induction of the CDK inhibitors is essential for making cell cycle withdrawal irreversible.29 However, we believe that other events preprogrammed to occur after induction of the CDKIs may also be needed to ensure that the cell cycle exit is irreversible. Although maintenance of CDK2 and CDK4 activities could cause greatly extended proliferation of differentiating cells, they did not proliferate indefinitely. We think that differentiating MEL cells may become refractory to induction of exogenous CDKs due to a decline in cyclin levels at much later stages of differentiation30; reduction in cyclin levels has also been seen in other differentiating systems.31 32 The combination of induction of CDK inhibitors and a decline in cyclin levels may serve to ensure that cell cycle withdrawal is permanent.
CDK4 and CDK6 play different roles at different stages of differentiation
Although differences in the regulation of CDK4 and CDK6 synthesis have sometimes been observed,33-35 to date functional differences between the 2 cyclin D kinases have not been reported.36 37 The results reported here show that inhibition of CDK4 in differentiating cells inhibits their proliferation, and conversely, maintenance of CDK4 activity extends their proliferation. On the other hand, we found that while both CDK4 and CDK6 are active in undifferentiated MEL cells, only inhibition of CDK6 slows their proliferation (I.M. et al, unpublished data, 2000). These observations suggested to us that CDK6 in conjunction with CDK2 promotes proliferation in the undifferentiated cells, whereas CDK4 along with CDK2 promotes proliferation in the differentiating cells. The ability of CDK4R24C to synergize with CDK2 to cause the differentiating cells to undergo many more cell divisions than usual provided us with the opportunity to assess possible functional differences between CDK4 and CDK6. We found that CDK6R31C could not substitute for CDK4R24C in synergizing with CDK2 to extend proliferation of the differentiated cells. We are certain that the CDK6R31C expression vector used in the current work produces a functionally active form of CDK6 because we were able to measure its activity in transfectants undergoing differentiation (Figure 6). Furthermore, we have found that when CDK6R31C transfectants are treated with Dox before initiating differentiation, they are blocked from differentiating (I.M. et al, unpublished data, 2000). This effect was not observed in CDK4R24C transfectants. These results indicate that the early decline in CDK6 plays an important role in the cell's decision to differentiate and further strengthens the view that CDK6 and CDK4 play different roles at different stages of differentiation.
How might such a restriction in biological activity of 2 otherwise very similar enzymes arise? Although hypophosphorylated forms of Rb accumulate in differentiating MEL cells,38 most E2F is found associated with p107 in these cells.39,40 Similar observations have been made in other differentiating cell types.41-43 Thus, although Rb may be a key player in controlling proliferation in growing cells (eg, undifferentiated MEL cells), p107 or possibly p130 may assume this role during terminal differentiation.44-46 Preferential substrate specificity of CDK4 and CDK6 among the Rb-related proteins could explain their different biological activities in differentiating vs undifferentiated MEL cells, respectively. Alternatively, currently unrecognized substrates of the 2 kinases may be involved. The system and transfected cell lines described here should help further our understanding in this area.
Phenotypic differentiation and terminal cell division: 2 independent aspects of cell differentiation
The prevailing view of the relationship of cell division to other aspects of cell differentiation is that there is an intimate connection.47,48 In principle, the control of cell division and control of cell differentiation could be coupled at either the time cells make the decision to differentiate (commitment) or as cells actually execute their phenotypic differentiation program, or both. Studies in mice lacking 1 or more specific CDK inhibitors and in cells derived from such mice have indicated that loss of these inhibitors that normally promote cell cycle exit does not affect the capacity of cells to differentiate.49-51 Furthermore, continued proliferation of certain highly differentiated cell types was observed in such mice indicating that phenotypic differentiation and terminal cell division can be uncoupled.52-54 However, such studies in live animals do not address whether the observed additional cell divisions are due to cell intrinsic vs. cell extrinsic controls, the precise stage of differentiation at which the additional cell divisions occur, the exact number of such extra cell divisions, and whether or not the cellular concentration of gene products characteristic of the differentiated cells is affected. Furthermore, loss of inhibitors may have effects within the cell beyond liberation of CDK activity.
The temporally well-defined in vitro differentiation process provided by the MEL cell system permitted us to overcome most of these limitations. The results obtained with the CDK and CDKI transfectants imply that differentiating erythroid cells do not need to proliferate in order to differentiate and that differentiating cells can proliferate further without affecting the synthesis of gene products that are specific to the mature cells. Thus, our results imply that there need not be a tight coupling between phenotypic differentiation and terminal cell division in differentiating erythroid cells. Recent studies in p27-deficient mice suggested that loss of cell cycle exit controls within the hematopoietic compartment leads to additional cell divisions in progenitors but not in more highly differentiated cells.51 Differences between these results and those reported here may be due to homeostatic mechanisms present in vivo or to compensation by other CDK inhibitors still present in the p27 deficient mice. Alternatively, it may be that differentiating erythroleukemia cells, although similar to normal differentiating erythroblasts, are especially sensitive to abrogation of terminal cell division by exogenous CDK2 plus CDK4. Therefore, it will be important to determine whether proliferation of normal erythroblasts can be extended by maintenance of CDK2 and CDK4 activities in these cells. Successful manipulation of the terminal cell divisions in normal, differentiating cells could have important implications for cell transplantation therapies.
Acknowledgments
We are grateful to David Franklin and Yue Xiong for providing us with constructs, antibodies, technical support, and advice that were essential to the completion of these experiments. We also thank Richard Pestell and Liang Zhu for providing us with critical reagents and advice. We also thank Matthew Scharff, Liang Zhu, Richard Pestell, Stuart Murray, and Natasha Rekhtman for critical reading of the manuscript.
Supported by grant 2P30CA13330 (A.I.S.) from the National Cancer Institute Cancer Center, grants 5T32AG00194 (F.R.) and 5R37CA16368 from the National Institutes of Health (NIH), and grant 5T32GM07288-25 (I.M.) from the NIH/Medical Scientist Training Program, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Arthur I. Skoultchi, Department of Cell Biology, Albert Einstein College of Medicine, 1300 Morris Park Ave, Bronx, NY 10461; e-mail: skoultch@aecom.yu.edu.