Abstract
Acute chest syndrome (ACS) is the leading cause of death in sickle cell disease. Severe ACS often develops in the course of a vaso-occlusive crisis (VOC), but currently there are no predictors for its development. Secretory phospholipase A2(sPLA2), a potent inflammatory mediator, is elevated in ACS, and previous work suggests that sPLA2 predicts impending ACS. We prospectively evaluated sPLA2concentration during 21 admissions for VOC; 6 of these patients went on to develop ACS. Elevation of sPLA2 was detected all 6 patients 24 to 48 hours before ACS was clinically diagnosed. Adding the requirement for fever raised the specificity of sPLA2 to 87% while retaining 100% sensitivity. These data indicate that sPLA2 can be useful in alerting the clinician to patients with impending ACS. In addition, sPLA2 may be useful for instituting early therapies to prevent or reduce the clinical morbidity of ACS.
Introduction
Acute chest syndrome (ACS) is the leading cause of death in sickle cell disease (SCD), and most patients with SCD experience at least one episode of ACS.1-3 Repeated episodes of ACS predict an early death and can result in chronic lung disease.4,5 Half of ACS episodes occur in association with vaso-occlusive crisis (VOC), and these events are generally severe, particularly when secondary to pulmonary fat embolism.6,7Despite the substantial morbidity associated with ACS, there are no predictors for its development. Patients often present initially with pain and a negative chest radiograph and rapidly deteriorate before ACS is diagnosed.8 9
Secretory phospholipase A2 (sPLA2) is a potent inflammatory mediator that has been implicated in the pathology of a variety of inflammatory conditions, including arthritis, sepsis, and multi-organ failure.10,11Up-regulation of sPLA2 results in the hydrolysis of phospholipids to produce free fatty acids and lysophospholipids, both of which result in acute lung injury.12,13 When arachidonic acid is the fatty acid product of sPLA2, the production of leukotrienes, thromboxanes, and prostaglandins can also result. We previously documented that ACS patients have a dramatic increase in sPLA2 and that the degree of sPLA2elevation correlates with the severity of lung injury.14Our preliminary findings of sPLA2 elevation in ACS also indicated that sPLA2 rose 24 to 48 hours before ACS was detected clinically, suggesting that sPLA2 levels might be useful in predicting ACS in patients with sickle cell disease. As these initial studies were retrospective, we undertook a prospective study to determine the accuracy of sPLA2 in predicting ACS in hospitalized patients with VOC.
Patients, materials, and methods
Patients
We prospectively followed 14 SCD patients during 21 hospital admissions for VOC. Only patients admitted with VOC and without ACS at the time of admission were eligible for the study. All patient care was overseen by pediatric house staff and a pediatric hematologist/oncologist, who were unaware of the study. Patients with VOC are treated under a standardized clinical practice guideline that includes incentive spirometry. Chest radiographs were performed when there was a clinical suspicion for ACS but at least twice for each patient. Serum for sPLA2 determination was collected daily during hospitalization. All patients had serum drawn for sPLA2 levels at least 3 times during hospitalization. VOC was defined as a hospitalization requiring parenteral narcotics that was not attributable to other causes. ACS was defined as the presence of a new pulmonary infiltrate in combination with fever, chest pain, or respiratory symptoms. There were 14 patients in the study; 13 had hemoglobin SS and 1 had hemoglobin S-β0 thalassemia. The average age of the patients was 12.6 ± 4.9 years (range, 1.5-20 years; median, 13.5 years). Vital signs, patient symptoms, physical examination findings, and complete blood count were performed daily. Age-appropriate respiratory and auscultatory findings were defined as outlined in the National Acute Chest Syndrome Study.15 The study was approved by the local institutional review board.
Secretory phospholipase A2 measurements
Serum samples collected for sPLA2 determination were run after patients were discharged. An elevated sPLA2concentration was defined as 100 ng/mL on the basis of findings from previous studies.14 Personnel performing the sPLA2 assay were blinded to the clinical status of the patients. Secretory phospholipase A2 concentration was determined with a sandwich enzyme-linked immunosorbent assay (ELISA) modified from Smith et al16 using 2 different monoclonal antibodies against human type IIa sPLA2.17Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of sPLA2 were calculated only on levels obtained in the 3 days before ACS was clinically diagnosed. A χ2 analysis with Yates correction was used for comparison of frequency of elevated sPLA2 in the VOC and ACS groups.
Results and discussion
Of the 21 patients who had VOC admissions in the study, 6 went on to develop ACS. With the use of a threshold value of 100 ng/mL, sPLA2 concentration was elevated in all patients who developed ACS in the 24 to 48 hours before this diagnosis was made clinically. The maximal sPLA2 concentration for the ACS group (mean, 849 ng/mL; median, 399 ng/mL) was significantly higher than for the uncomplicated VOC group (mean, 132 ng/mL; median, 88 ng/mL) (P = .009). Comparing the frequency of an elevated sPLA2 in the 2 groups in the first 3 days revealed that ACS patients were more than 20 times more likely to have an elevated sPLA2 than VOC patients (odds ratio = 24.8; 95% confidence interval, 1.168-527.5; P = .023). The specificity of sPLA2 elevation for predicting ACS was 67% (Table 1), while the sensitivity for sPLA2was excellent (100%). We then examined whether adding additional clinical parameters occurring at the time of sPLA2elevation would increase the specificity and overall accuracy of sPLA2. Sensitivity and specificity were calculated for sPLA2 elevation in combination with fever, chest pain, respiratory symptoms, or auscultatory findings (Table 1). The addition of the requirement for fever in combination with an elevated sPLA2 raised the specificity of sPLA2 to 87% while retaining 100% sensitivity. Overall accuracy improved to 90%. The addition of chest pain, respiratory symptoms, or auscultatory findings increased the specificity of sPLA2; however, it significantly decreased the overall sensitivity of sPLA2. Complete blood count results did not improve the predictive value of sPLA2 as hemoglobin, platelet count, and white blood cell count changes did not occur before ACS was diagnosed clinically.
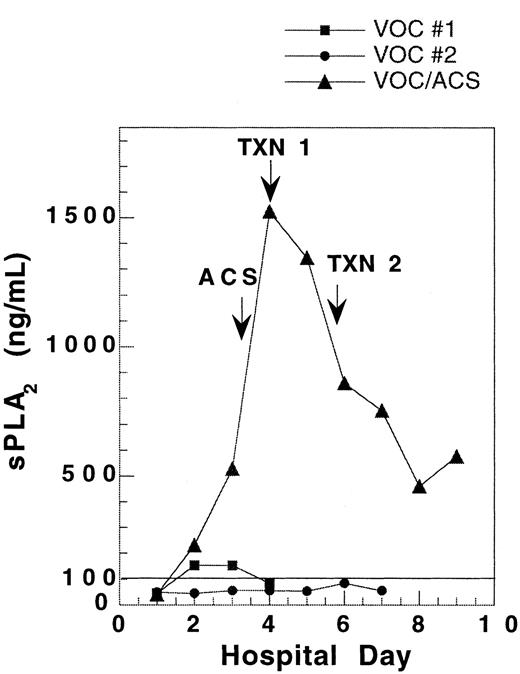
One patient was followed during 3 admissions for VOC, including one instance in which ACS developed 2 days after admission. The sPLA2 concentration during the ACS admission rose to 10-fold higher than in the VOC admissions. Comparison of sPLA2 concentration during these 3 admissions reveals a substantially different pattern of sPLA2 for the 2 uncomplicated VOC admissions as compared with the admission where the patient developed ACS with severe hypoxia (Figure1).
Pattern of sPLA2 concentration in a single SCD patient hospitalized for vaso-occlusive crisis on 3 occasions.
During 2 hospitalizations for uncomplicated vaso-occlusive crisis(VOC), sPLA2 concentration remained low. When the patient developed ACS 2 days into an admission for VOC, the sPLA2 rose to levels 10-fold higher than in uncomplicated VOC. VOC no. 1, ▪; VOC no. 2, ●; VOC/ACS, ▴.
Pattern of sPLA2 concentration in a single SCD patient hospitalized for vaso-occlusive crisis on 3 occasions.
During 2 hospitalizations for uncomplicated vaso-occlusive crisis(VOC), sPLA2 concentration remained low. When the patient developed ACS 2 days into an admission for VOC, the sPLA2 rose to levels 10-fold higher than in uncomplicated VOC. VOC no. 1, ▪; VOC no. 2, ●; VOC/ACS, ▴.
ACS often develops rapidly in patients with SCD and results in substantial morbidity for these patients. Patients often present with few findings and then rapidly become critically ill. Previously, no predictors as to which patients will develop ACS have been identified, but our data indicate that sPLA2 elevation predicts impending ACS 24 to 48 hours before it is diagnosed clinically. Elevated sPLA2 in combination with fever is particularly helpful in identifying patients who will develop ACS. Other, more traditional indicators of pulmonary disease, including chest pain, respiratory symptoms, and auscultatory findings, either occur too late or do not occur in many patients who will develop ACS. Therefore, sPLA2 appears to be a useful parameter for the clinician following SCD patients with VOC. The time required to perform the sPLA2 ELISA outlined in the present study makes it impractical for clinical use, but a rapid, fluorescence-based assay is now available.18 With a rapid assay, sPLA2levels could be monitored daily, and as sPLA2 levels rise, the clinician would be alerted to the impending development of ACS. Increased clinical vigilance and therapeutic measures should be focused on those patients at highest risk of ACS. Pilot data indicate early transfusion may ameliorate the course of ACS.15 19 A clinical trial that uses sPLA2's predictive capabilities to determine if early transfusion will affect the clinical course of ACS is now underway.
Acknowledgment
The authors thank Sandra Larkin for technical assistance.
Supported in part by National Institutes of Health grants RR01271 and HL-20985.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Lori A. Styles, Dept of Hematology/Oncology, Children's Hospital Oakland, 747 52nd St, Oakland, CA 94609; e-mail:lstyles@landminds.com.