Abstract
The host defense roles of neutrophil elastase in a porcine skin wound chamber model were explored. Analysis of wound fluid by acid-urea polyacrylamide gel electrophoresis, Western blot, and bacterial overlay confirmed that the neutrophil-derived protegrins constituted the major stable antimicrobial polypeptide in the wound fluid. The application to the wound of 0.10 and 0.25 mM N-methoxysuccinyl-alanine-alanine-proline-valine (AAPV) chloromethyl ketone, a specific neutrophil elastase inhibitor (NEI), blocked the proteolytic activation of protegrins and diminished the associated antimicrobial activity as detected by radial diffusion assay againstStaphylococcus epidermidis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Candida albicans or by bacterial gel overlay against S epidermidis and E coli. The application of the related cathepsin G inhibitor (CGI), benzyloxycarbonyl-glycine-leucine-phenylalanine (ZGLF) chloromethyl ketone, had no effect. In wound chambers that received 106 colony-forming unit (CFU)/mL of S epidermidis, the presence of NEI significantly decreased the 24-hour clearance of bacteria from the wound compared to wounds treated with CGI or solvent only. Neither inhibitor, at 0.10 or 0.25 mM concentration, affected leukocyte accumulation or degranulation in the wound chambers. The in vitro microbicidal decrement due to NEI was restored by an amount of the specific protegrin (PG-1), which was equivalent to the measured difference of protegrin between control and inhibited chambers. Administration of 1 μg/mL exogenous PG-1 4 hours after chamber preparation was sufficient to normalize in vivo antimicrobial activity. Although pharmacologic NEIs are promising candidates as anti-inflammatory drugs, they may impair host defense in part by inhibiting the activation of cathelicidins by neutrophil elastase.
Introduction
Neutrophil elastase is an abundant serine protease of mammalian granulocytes,1 where it is concentrated in the granules that are mainly destined to fuse to phagosomes.2 There is abundant evidence that elastase can contribute to inflammatory tissue injury,3-5 and various natural and synthetic inhibitors of elastase are under development as pharmaceuticals to diminish tissue damage in inflammatory diseases.6 In contrast, knowledge about functions of neutrophil elastase in host defense is very limited. Patients with Chediak-Higashi syndrome lack neutrophil elastase in mature neutrophils and suffer from recurrent bacterial infections, but other abnormalities coexisting in this disease obscure a possible causal relationship.7 Mice made homozygous for a disrupted neutrophil elastase gene have diminished resistance to Klebsiella pneumoniae and Escherichia coli sepsis.8Although one possible target for elastase is a bacterial outer-membrane protein OmpA,9 it is not clear how the lack of neutrophil elastase causes the host defense defect. In contrast, mice that were made deficient in the neutrophil protease cathepsin G were phenotypically normal when challenged with Staphylococcus aureus, K pneumoniae and E coli.10
Neutrophil elastase has modest direct antimicrobial effects11 that are most apparent in low-ionic–strength media, and these could synergize with other antimicrobial proteins of phagocytic granules. Alternatively, elastase may act indirectly by proteolytic activation of other antimicrobial systems. Importantly, the precursors of cathelicidins, abundant antimicrobial peptides of mammalian neutrophils, are activated by catalytic amounts of neutrophil elastase but not by other related proteases.12-16 Although the neutrophils of many if not all mammalian species contain cathelicidins, porcine neutrophils are unusually well endowed with these peptides, including protegrins, PR-39, prophenins, and porcine myeloid antimicrobial peptides (PMAPs)17-24; however, porcine neutrophils have not been reported to contain any peptides of the defensin family, peptides that are abundant in human neutrophils.
Porcine neutrophils are the sole known sources of protegrins, potently and broadly microbicidal peptides of 16-18 residues.18 We previously showed that porcine neutrophils, when stimulated with phorbol myristate acetate (PMA) or latex beads, generated extracellular microbicidal activity against Listeria monocytogenes orE coli by elastase-mediated activation of secreted proprotegrins,16 and we noted that the activity of protegrins was a major contributor to the antibacterial activity of neutrophil secretions. We hypothesized that inhibition of neutrophil elastase would block the activation of antimicrobial cathelicidins and impair local antimicrobial defenses. In the present study, using a porcine skin wound model and the local application of a specific inhibitor for neutrophil elastase, we explored the effects of the inhibitor on antimicrobial properties of wound fluid in vivo.
Materials and methods
Reagents
Protegrin-1 (PG-1) and PG-3 (gifts from Dr Robert I. Lehrer, University of California at Los Angeles (UCLA) Department of Medicine, Los Angeles, CA) are synthetic peptides based on their natural sequence.18 Because PG-1 and PG-3 have similar antimicrobial activities against a broad range of microbes18 and because PG-3 was in limited supply, PG-1 was used for the majority of experiments. Proprotegrin-3 (pPG-3) was prepared in a recombinant baculovirus/insect cell system.15 N-methoxysuccinyl-alanine-alanine-proline-valine (AAPV) chloromethyl ketone (Sigma Chemical Co, St Louis, MO) is a specific neutrophil elastase inhibitor (NEI). Benzyloxycarbonyl-glycine-leucine-phenylalanine (ZGLF) chloromethyl ketone (Enzyme Systems Products, Livermore, CA) is a specific cathepsin G inhibitor (CGI). Both inhibitors were dissolved in 100% ethanol at a stock concentration of 25 mM. Porcine neutrophil elastase was not available commercially. We instead used human neutrophil elastase (HNE) (Elastin Products Company, Owensville, MO) and the structurally distinct porcine pancreatic elastase (Sigma), both shown previously to process proprotegrin to protegrin in vitro.15
Microbes and culture conditions
E coli ML-35p, L monocytogenes strain EGD, S aureus (human clinical isolate, UCLA Clinical Microbiology Facility), Candida albicans (human clinical isolate), and Staphylococcus epidermidis (human clinical isolate, used for in vitro assays) were cultured 18 hours at 37°C in 50 mL 3% trypticase soy broth (TSB) (all strains described in Lee et al25). S epidermidis, isolated from healthy porcine skin and used for in vivo assays, was also typed at the UCLA Clinical Microbiology Facility and frozen (−80°C) as a 3% TSB/20% glycerol stock. A wild type parent strain of Pseudomonas aeruginosa, H10326 (gift from Dr Robert E. W. Hancock (University of British Columbia, Vancouver, British Columbia, Canada), was cultured 18 hours at 30°C. Immediately prior to use each strain was subcultured in 50 mL 3% TSB at either 1:100 dilution (L monocytogenes, S aureus, S epidermidis, C albicans and P aeruginosa) or 1:1000 dilution (E coli) for 2.5 hours at 37°C in an environmental shaking incubator (250 rpm) to obtain microbes in mid-logarithmic growth phase. Subcultures were then centrifuged at 1400g for 10 minutes, washed once in Hank's balanced salt solution (HBSS) without phenol red (Life Technologies, Gaithersburg, MD), resuspended in 5 mL HBSS, and diluted to their desired concentrations in HBSS. For bacteria, an OD625 = 1.0 was equivalent to 2.5 × 108 CFU/mL. For fungi, an OD450 = 1.0 was equivalent to 2.5 × 108 CFU/mL.
Porcine skin wound fluid generation
All animal procedures were approved by the UCLA Chancellor's Animal Research Committee. Porcine skin wound model was created as described earlier with slight modifications.27 Female Yorkshire pigs (40-60 pounds) used in the experiments were fed a Minipig porcine diet (LabDiet 5P94, ProLab, Brentwood, MO). Wounds were generated under general anesthesia with 10 mg/kg tiletamine (Telazol; Fort Dodge, Cherry Hill, NJ) and 2 mg/kg xylazine (AnaSed; Lloyd Laboratories, Shenandoah, IA). The skin was surgically sanitized with 7.5% povidone iodine scrub followed by 70% isopropanol. Medium partial-thickness excisional wounds (2-cm wide,2 1- to 1.5-mm deep) were created with a scalpel in duplicate or quadruplicate in the regions immediately caudal to the inferior angle of the scapula. Polyvinyl chambers (P.A. Medical Inc, Columbia, TN) were attached and secured by tape over each wound. Each chamber received 2 mL sterile HBSS without phenol red and with or without protease inhibitors (0.1, 0.25, 0.5, 1, or 2 mM) or their solvent. Bacteria or fungi at 1 × 106 CFU/mL final concentration was also included in some chambers. Wound fluids in the chambers were removed by aspiration at 4, 24, or 48 hours.
Analysis of porcine wound fluid
Total and differential leukocyte counts were performed for each chamber (Diff-Quik; Dade Behring, Newark, DE). To quantify microbes, the fluid was separated into cell-free supernatant and cell pellet by centrifugation at 210g for 3 minutes. In some experiments, the cell pellet was lysed by 3 10-second bursts of sonication in HBSS. For either cell-free supernatant or cell lysate, microbial CFUs were determined by spreading on antibiotic-free TSA plates. In a preliminary set of experiments, microbial CFUs were determined in wound fluid before and after the cells and debris were removed by centrifugation at 210g for 3 minutes. The CFUs did not differ significantly (data not shown). Therefore, subsequent antibacterial activity assays and biochemical analyses of wound fluids were performed on the cell-free supernatant.
Neutrophil elastase and cathepsin G inhibition assays
In a modification of a previously described procedure,28 2 times the concentrations of NEI (312 nM to 80 μM) and HNE (625 nM to 5 μM) or CGI (63 nM to 8 μM) and human cathepsin G (125 nM to 1 μM) were reconstituted in 1 × phosphate-buffered saline (PBS). NEI with elastase or CGI with cathepsin G (5 μL each) were admixed in a checkerboard fashion in a 96-well microtiter plate (Falcon flexible U-bottom; Becton-Dickinson Labware, Franklin Lakes, NJ) and incubated in a humidified chamber for 1 hour at 37°C. We added 75 μL of a neutrophil elastase-specific chromogenic substrate, which comprises 0.8 mM methoxysuccinyl-AAPV-P-nitroanalide in 0.1 M Tris-HCl (tris[hydroxymethyl] aminomethane–hydrochloride)/0.15 M sodium chloride (NaCl) (pH 7.5), or cathepsin G-specific chromogenic substrate, which comprises 3 mM succinyl-AAPF-P-nitroanalide in 0.1 M Tris-HCl (pH 7.5). After 20 minutes of elastase or 1.5 hours of cathepsin G incubation at 37°C, the reaction was stopped with 40 μL of 50 μM soybean trypsin inhibitor in 0.1 M Tris-HCl/0.15 M NaCl (pH 7.5). Absorbance was read at 405 nm in a variable wavelength spectrophotometer (SpectraMAX 250 Kinetic Microplate Spectrophotometer; Molecular Devices, Sunnyvale, CA).
Colony-forming unit assay
A CFU assay was performed to determine the viable bacterial or fungal concentration in porcine skin wound fluids. Briefly, 25-100 μL wound fluid was diluted in HBSS, and aliquots were plated on TSA plates. After incubation for 18 hours at 37°C, the colonies were counted, and the CFU/mL concentrations were calculated.
Radial diffusion assays
Radial diffusion assays (RDAs) were performed as described previously.29 Briefly, the underlay consisted of 1% agarose and 1:100 dilution TSB in 10 mM sodium phosphate (NaP), a low-ionic buffer (pH 7.4), or in 10 mM NaP with 100 mM NaCl, a higher-ionic buffer (pH 7.4). Overlay consisted of 6% TSB and 1% agarose in PBS for all assays. We mixed 4 × 106bacteria or fungi with 10 mL gel-underlay solutions kept molten at 48°C and poured into 100 cm2 square petri dishes. A series of 3.2-mm diameter wells were punched after the agarose solidified, and 5 μL wound fluids or standard PG-1 or PG-3 solution was added into designated wells. Plates were incubated at 37°C for 3 hours to allow for peptide diffusion. The microbe-laden underlay was then covered with 10 mL molten overlay, and the plates were allowed to harden. Antimicrobial activity was identified as a clear zone around the well, which was absent of microbial growth after 18 hours incubation at 37°C. The activity was represented in radial diffusion units (RDUs) defined as: [diameter of clear zone in mm − 3.2 mm] × 10. Assays were performed in duplicate and repeated at least once.
Gel-overlay assay
The gel-overlay assay was performed as described previously.29 Briefly, proteins and peptides from 100 μL wound fluid were concentrated using 10 μL Strataclean resin (Stratagene) and separated by acid-urea polyacrylamide gel electrophoresis (AU-PAGE). The gel was washed 20 minutes in 10 mM NaP, then it was placed on a premade 1% agarose plate containing 10 mM NaP with 100 mM NaCl (pH 7.4), 1:100 dilution TSB, 1% porcine serum, and 4 × 106L monocytogenes or E coli. The plate was then incubated at 37°C for 3 hours to allow the proteins and peptides in the polyacrylamide gel to diffuse into the underlying bacterial layer. The polyacrylamide gel was then removed, and the bacterial layer was overlaid with a nutrient layer that contained 6% TSB in 1% agarose. Clear zones without bacterial growth represented antibacterial activity. Duplicate AU-gels were electrophoresed and Coomassie-stained to correlate antibacterial activity with protein bands.
Western blot analysis
For Western blot analysis, after AU-PAGE, proteins were electroblotted to Immobilon-P PVDF membranes (Millipore, Bedford, MA) in 0.7% acetic acid. The blots were probed with 1:1000 dilution of monoclonal antibody (mAb) anti-PG (recognizes PG-1 and PG-3) and a 1:1000 dilution of rabbit antimouse immunoglobulin G (IgG) alkaline-phosphatase conjugate, and then they were developed in 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium solution. Synthetic PG-1 and PG-3 were used as protegrin standards.
Lysoplate assay for muramidase activity of lysozyme
Briefly, a mixture of 0.5 mg/mL lyophilizedMicrococcus lysodeiktikus (M luteus) and 1% low EEO agarose in 66 mM NaP buffer [pH 7.0] was kept at 45°C and poured in a level sterile dish.30 Evenly spaced 3-mm wells were punched in the solidified agar, and a 5-μL test sample was introduced into each well. Lysozyme enzymatic activity was determined by measuring the zone-of-clearance diameters after an 18-hour incubation at room temperature.
The effect of NEI on superoxide anion production by porcine neutrophils
Porcine neutrophil isolation and superoxide anion (· O2−) production assay were performed as described earlier.31 Briefly, to isolate porcine neutrophils, 30 mL ethylenediamine tetraacetic acid (EDTA)–treated blood (final concentration, 5 mM EDTA) was mixed with 30 mL 3% dextran in PBS and was incubated for 30 minutes at room temperature. The supernatant was carefully removed from the dextran-sedimented blood, overlaid on 10 mL Histopaque 1.077 (Sigma), and centrifuged at 300g for 25 minutes at room temperature. Neutrophils were collected from the bottom of the tube, and contaminating red blood cells were lysed with 10 mL 0.2% NaCl for 30 seconds. Isotonicity was restored with 10 mL 1.6% NaCl. Neutrophils (more than 95% of the cells) were washed once in ice-cold PBS solution without divalent cations and kept on ice.
The superoxide anion (· O2−) production of neutrophils in response to opsonized bacteria was measured by the superoxide dismutase (SOD)–inhibitable reduction of ferricytochrome c. Each measurement required four samples: (1) 1 × 106 cells in 500 μL HBSS mixed with 100 μL (80 μM) ferricytochrome c (Sigma), 250 μL (1 × 107) E coli in 10% fresh porcine serum, and 100 μL (300 U) SOD (Sigma) in 12 × 75-mm tubes; (2) cells treated with ferricytochrome c only; (3) cells treated with bacteria and ferricytochrome c; and (4) cells treated with ferricytochrome c and SOD. In parallel experiments, the neutrophils were treated with 1 mM NEI or CGI for 30 minutes before they were mixed with ferricytochrome c. The mixtures were incubated at 37°C for 20 minutes and then centrifuged at 4°C (350g) for 5 minutes. We transferred 200-μL cell-free supernatant to flat-bottom 96-well plates, and OD was read at 550 nm using HBSS as the blank. Production of · O2− was calculated according to the following formula: nM · O2− per 1 × 106 cells per 20 minutes equals [(OD3 + OD4) − (OD1 + OD2)] × 190.5.
Statistical analysis
Unless otherwise stated, assays were performed in at least triplicate for each independent experiment. Where applicable, results from duplicate chambers were averaged for each pig tested. Sets of independent experiments were compared with a paired t test when analyzing individual experiments of inhibitor-treated wound fluids against solvent-treated fluids (SigmaStat; SPSS Inc, Chicago, IL). Error bars represent the SEM.
Results
Protegrins are the principal antibacterial substances in porcine wound fluid
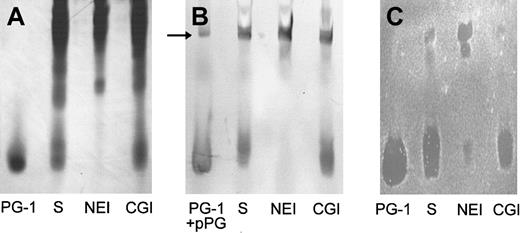
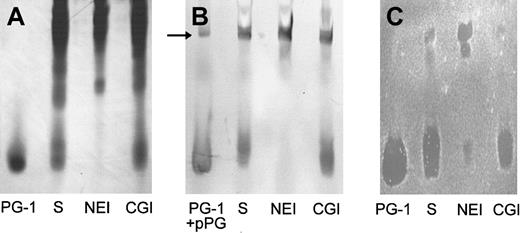
Because in previous studies we identified protegrins as the principal stable antimicrobial substances secreted by porcine neutrophils,16 the antibacterial potency of wound fluid was determined relative to the activity of synthetic protegrins. To identify the antimicrobial components, the composition and activity of wound fluid generated from chambers injected with HBSS and solvent only (n = 4) was evaluated using AU-PAGE, gel-overlay antibacterial assay, and Western blot with a mAb to protegrins. Wound fluid was extracted and subjected to AU-PAGE, in which proteins and peptides were separated based on their positive-charge density. Distinct bands near the dye front of the AU-gel were identified as protegrins: They migrated similarly to the synthetic PG-1 (Figure1A, lane S), reacted in a Western blot with mAb anti-PG (Figure 1B, lane S), and were shown to have antibacterial activity in a gel-overlay assay against E coli(Figure 1C, lane S) and S epidermidis (not shown). As judged by the overlay assay, protegrins were the predominant antibacterial components in porcine skin wound fluid. Moreover, under these conditions (10 mM NaP/100 mM NaCl) there was no evidence of direct antimicrobial activity of neutrophil elastase.
The effect of protease inhibitors on the production of antibacterial protegrins in wound fluid.
Wound fluids (100 μL) induced by HBSS with solvent only (S), with 0.25 mM N-methoxysuccinyl-AAPV chloromethyl ketone (NEI), or with 0.25 mM ZGLF chloromethyl ketone (CGI) were analyzed by (A) Coomassie-stained AU-PAGE, (B) Western blot with anti-PG mAb, and (C)E coli gel overlay. We used 1 μg PG-1 as a standard. We also used 1 μg proprotegrin (pPG) as a control in the Western blot (panel B, arrow).
The effect of protease inhibitors on the production of antibacterial protegrins in wound fluid.
Wound fluids (100 μL) induced by HBSS with solvent only (S), with 0.25 mM N-methoxysuccinyl-AAPV chloromethyl ketone (NEI), or with 0.25 mM ZGLF chloromethyl ketone (CGI) were analyzed by (A) Coomassie-stained AU-PAGE, (B) Western blot with anti-PG mAb, and (C)E coli gel overlay. We used 1 μg PG-1 as a standard. We also used 1 μg proprotegrin (pPG) as a control in the Western blot (panel B, arrow).
Elastase inhibition blocks the activation of proprotegrin in vivo
We showed previously that protegrins are stored in porcine neutrophils as inactive proprotegrins and that neutrophil elastase is required for in vitro activation of protegrins.15,16 To investigate if elastase was the converting enzyme that was responsible for the activation of protegrins in vivo, we employed NEI or CGI, specific inhibitors for either neutrophil elastase or cathepsin G, respectively, both of which are abundant serine proteases from neutrophil granules. The CGI also served as a control for the potential effect of the chloromethyl ketone moiety on the antibacterial and biochemical properties of porcine skin wound and as a control for the reported antichemotactic effects of inhibitors of neutrophil elastase and cathepsin G.32 As shown in Figure 1A (AU-PAGE) and Figure 1B (Western blot), protegrins were generated in saline-induced wound fluid (HBSS with solvent, lane S). The wound fluid was induced in the presence of CGI, but the characteristic protegrin bands were not detected in the wound fluid that was induced in the presence of NEI. In the gel-overlay assay (Figure 1C), protegrins were the predominant antibacterial components in the normal wound fluid and the CGI-treated wound fluid. However, if NEI was present during wound fluid production, the antibacterial activity corresponding to the protegrin bands was severely reduced. Two bactericidal bands not usually seen in wound fluid and migrating much more slowly were present in the upper part of the AU-gel. To examine these additional bands further we used 1 μg pPG-3 in the Western analysis and identified one band in the wound fluid (Figure 1B, arrow) that comigrated with pPG-3 and was reactive with mAb anti-PG.
In vitro inhibition of neutrophil elastase by NEI and cathepsin G by CGI
To determine the inhibitory capacity of the specific NEI, 0.312-2.5 μM ≈ 8.7-70 μg/mL HNE was coincubated with 0.16-40 μM NEI and subjected to a chromogenic assay of residual elastase activity (data not shown). NEI inhibited the activity of HNE in a stoichiometric ratio of 1. In a similar set of experiments, 0.063-0.5 μM ≈ 1.7-13.5 μg/mL human cathepsin G was inhibited by an equimolar ratio of its specific inhibitor, CGI (data not shown).
At moderate concentrations, NEI or CGI have no effect on superoxide production, neutrophil influx, and degranulation
In experiments with neutrophils from 3 pigs, superoxide was measured by the cytochrome c/SOD assay during a 20-minute incubation of 1 × 106 neutrophils with 1 × 107 opsonized E coli with or without the addition of 1 mM NEI or CGI. There were no differences in superoxide production in a pair-wise comparison between solvent and NEI- or CGI-treated wound fluid (P > .39; data not shown) (n = 3). This confirmed the previously reported lack of effect of the cell-impermeable protease inhibitors, NEI and CGI, on the production of superoxide.33
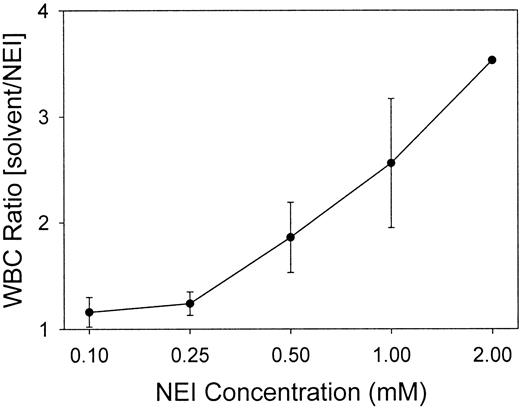
White blood cells (WBCs) were quantitated for each chamber to determine if the inhibitors influenced the influx of WBCs into the wounds. Figure2 indicates that at NEI concentrations of 0.1 and 0.25 mM, the ratio of WBCs in the solvent-treated to NEI-treated chambers was 1, demonstrating no difference in the cell numbers between the control and inhibited chambers (P > .5). The WBC counts in 0.25 mM CGI-treated chambers were also unchanged from the solvent-only control fluid (data not shown). However, as compared with the solvent control, increasing the NEI concentration to 0.5-2.0 mM reduced the number of WBCs recovered from the NEI-inhibited chambers by 2- to 4-fold. Regardless of NEI concentration, the control chambers contained 94.6% ± 0.6% WBCs as neutrophils (n = 29), and inhibitor chambers (both NEI and CGI) contained an equivalent percentage (94.0% ± 0.6%) (n = 29). Subsequent experiments were performed using either 0.10 or 0.25 mM NEI to obviate antichemotactic effects.
Low concentrations of NEI do not affect the recruitment of WBCs into the wound chambers.
WBCs in the chambers were counted at 24 hours and expressed as ratios of inhibitor-treated to solvent-treated chambers. A ratio of 1 indicates an equal number of leukocytes in the solvent and NEI chambers. Low NEI concentrations (0.10 and 0.25 mM), but not higher concentrations (0.5, 1.0, and 2.0 mM), maintained an equal WBC count in a pair-wise comparison between the solvent and NEI chambers (n = 2-7, except for the 2.0 mM data-point, which represents only one experiment). Error bars represent SEM.
Low concentrations of NEI do not affect the recruitment of WBCs into the wound chambers.
WBCs in the chambers were counted at 24 hours and expressed as ratios of inhibitor-treated to solvent-treated chambers. A ratio of 1 indicates an equal number of leukocytes in the solvent and NEI chambers. Low NEI concentrations (0.10 and 0.25 mM), but not higher concentrations (0.5, 1.0, and 2.0 mM), maintained an equal WBC count in a pair-wise comparison between the solvent and NEI chambers (n = 2-7, except for the 2.0 mM data-point, which represents only one experiment). Error bars represent SEM.
To measure the effect of the protease inhibitors on degranulation, the enzymatic activity of lysozyme, which is stored in both the azurophil and specific granules of the neutrophil,34 was measured in solvent-only control and NEI- and CGI-inhibited wound fluids using a lysoplate assay: solvent only, 1.05 ± 0.07 μg/mL (n = 13); NEI, 1.01 ± 0.08 μg/mL (n = 10); CGI, 0.94 ± 0.09 μg/mL (n = 3). No significant difference in lysozyme activity was measured in a pair-wise comparison between the wound fluids (P > .5).
Elastase inhibition impairs the in vivo clearance of bacteria from wound fluid
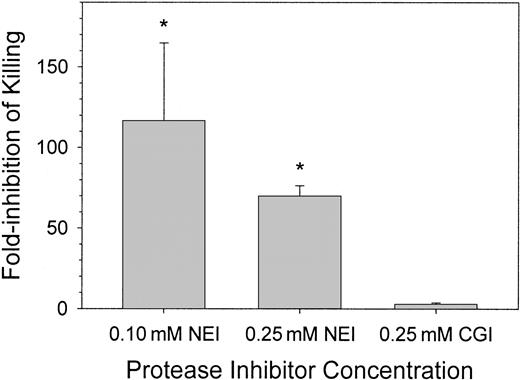
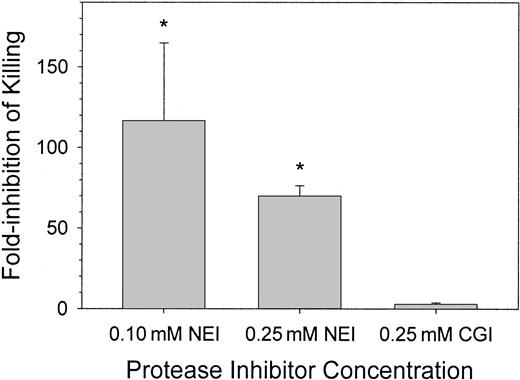
We observed that the common spontaneous infections of the wound fluid with porcine skin-derived coagulase-negative S epidermidis resulted in 100-times more bacteria in NEI-treated wound fluid than in CGI-treated wound fluid (data not shown). To examine the effects of protease inhibitors on the resistance of the skin wound to a defined bacterial inoculum, we introduced 1-2 × 106 CFU/mL porcine-derived S epidermidis into the wound with or without NEI or CGI and examined the fate of the bacteria 4 and 24 hours later. In Figure3, the effect of inhibitors on antimicrobial activity was expressed as “fold-inhibition of killing,” defined as the ratio of CFU counts in the control and inhibitor-treated chambers. At 4 hours, there were no significant differences in CFU counts between saline (solvent only) control and inhibitor-treated chambers (not shown). At 24 hours, there was an approximate 115-fold and 70-fold reduction in microbicidal activity in wound fluids treated with 0.10 mM and 0.25 mM NEI, respectively, compared with solvent-treated control wound fluids (P < .001). NEI-treated wound chambers exposed to E coli, C albicans, or P aeruginosa behaved similarly (data not shown). Wound fluids treated with 0.25 mM CGI showed no difference in bacterial CFUs compared with control (Figure 3, right panel; P > .5). Although the chambers treated with 0.10 mM NEI showed more variation in antimicrobial activity than those treated with 0.25 mM NEI, the in vivo antimicrobial activities of these 2 concentrations of NEI were not significantly different from each other (P > .2). NEI at a concentration of 0.25 mM was thus used in the remaining experiments.
Clearance of bacteria from wound fluid treated with NEI, CGI, or solvent.
S epidermidis (porcine skin isolate) CFUs were counted 24 hours after wound chamber preparation. Graphs indicate fold-inhibition of killing by the protease inhibitor NEI (n = 3-8) or CGI (n = 3) compared pair-wise to solvent chambers (n = 3-8). Asterisks indicate a significant difference in killing compared to solvent-treated chambers (P < .001). Error bars represent SEM.
Clearance of bacteria from wound fluid treated with NEI, CGI, or solvent.
S epidermidis (porcine skin isolate) CFUs were counted 24 hours after wound chamber preparation. Graphs indicate fold-inhibition of killing by the protease inhibitor NEI (n = 3-8) or CGI (n = 3) compared pair-wise to solvent chambers (n = 3-8). Asterisks indicate a significant difference in killing compared to solvent-treated chambers (P < .001). Error bars represent SEM.
Inhibiting neutrophil elastase decreases the ex vivo antimicrobial activity of wound fluid
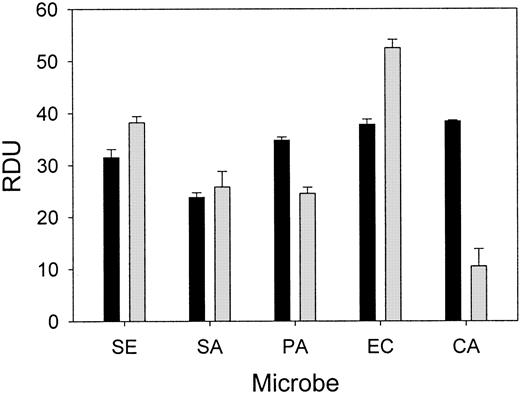
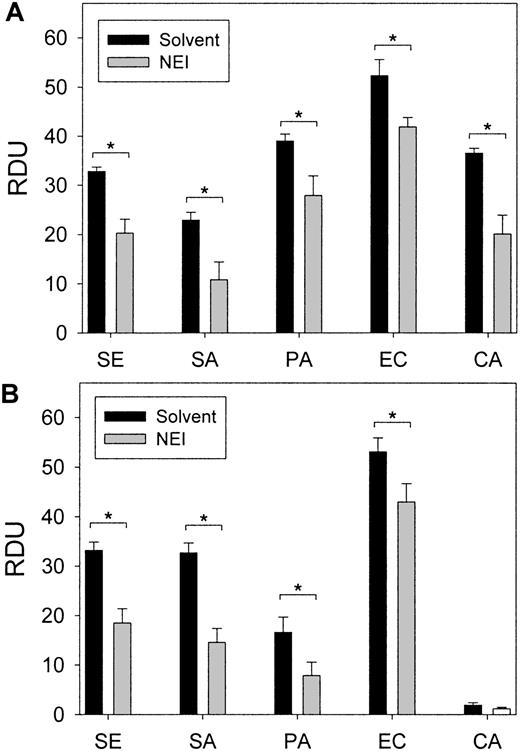
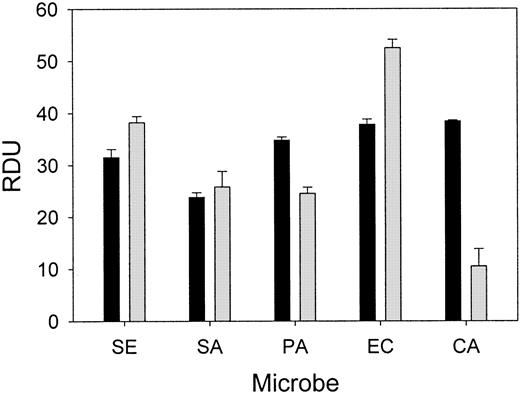
Wound fluid collected from each chamber at 24 hours was subjected to a radial diffusion assay to measure ex vivo antimicrobial activity. The antibacterial activity of the wound fluid was determined by an RDA with our laboratory test strains of S epidermidis,S aureus, P aeruginosa, E coli, andC albicans in either low-ionic (10 mM NaP) or higher-ionic (10 mM NaP/100 mM NaCl) buffer conditions (Figure4). The antimicrobial activity of fluid formed in chambers without added microbes or inhibitor was similar to fluid from chambers containing bacteria and solvent (control). However, the in vitro antimicrobial activity of the wound fluid formed in the presence of NEI was diminished both under low-ionic conditions (P < .05; Figure 5A) and higher-ionic conditions (P < .05, except for C albicans; Figure 5B), but CGI had no effect (not shown). Semiquantitative Western analysis revealed that the concentration of protegrin was markedly reduced in the inhibitor chambers (35.3 ± 6.0 μg/mL) compared with control chambers (86.6 ± 16.8 μg/mL;P < .001) (n = 13-17). These data taken together would indicate that protegrin concentration and total antimicrobial activity of porcine wound fluid are directly correlated.
Porcine wound fluid has natural antimicrobial activity ex vivo.
Wound fluids were subjected to RDAs against S epidermidis(SE), S aureus (SA), P aeruginosa (PA), E coli (EC), and C albicans (CA) in low-ionic conditions (▪, 10 mM NaP) and higher-ionic conditions (░, 10 mM NaP/100 mM NaCl) (n = 4). Antimicrobial activity is expressed as RDUs. Error bars represent SEM.
Porcine wound fluid has natural antimicrobial activity ex vivo.
Wound fluids were subjected to RDAs against S epidermidis(SE), S aureus (SA), P aeruginosa (PA), E coli (EC), and C albicans (CA) in low-ionic conditions (▪, 10 mM NaP) and higher-ionic conditions (░, 10 mM NaP/100 mM NaCl) (n = 4). Antimicrobial activity is expressed as RDUs. Error bars represent SEM.
The effect of NEI treatment in vivo on the antimicrobial activity of wound fluids ex vivo.
Wound fluids were subjected to RDAs against S epidermidis,S aureus, P aeruginosa, E coli, and C albicans in (A) low-ionic conditions (10 mM NaP) and (B) higher-ionic conditions (10 mM NaP/100 mM NaCl). Antimicrobial activity, expressed as RDUs, was significantly decreased in NEI-treated fluids in a pair-wise comparison to solvent-only controls (P < .05, indicated by asterisks) (n = 10-16). Error bars represent SEM.
The effect of NEI treatment in vivo on the antimicrobial activity of wound fluids ex vivo.
Wound fluids were subjected to RDAs against S epidermidis,S aureus, P aeruginosa, E coli, and C albicans in (A) low-ionic conditions (10 mM NaP) and (B) higher-ionic conditions (10 mM NaP/100 mM NaCl). Antimicrobial activity, expressed as RDUs, was significantly decreased in NEI-treated fluids in a pair-wise comparison to solvent-only controls (P < .05, indicated by asterisks) (n = 10-16). Error bars represent SEM.
Because there was still an appreciable amount of ex vivo antimicrobial activity in the NEI-treated fluids, it is likely that unprocessed proprotegrin (Figure 1) or peptides processed from other unidentified pathways play a role in the antimicrobial activity of wound fluid. Other antimicrobial peptides identified in porcine wound fluid, including the prophenins,35 PR-39,36 and PMAPs,37 may also be processed to an active peptide by other enzymes or in their proform may contribute to the antimicrobial activity of wound fluid.
The antimicrobial activity of NEI-inhibited wound fluids can be restored in vitro by the addition of neutrophil elastase or PG-1
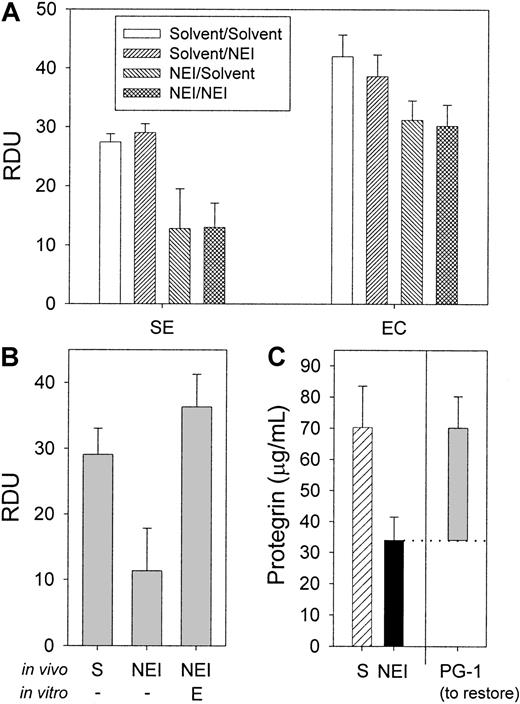
Additional amounts of NEI added to collected wound fluids in vitro at 0.25 mM final concentration did not alter the antimicrobial activity of the fluids (Figure 6A;P > .5) (n = 6), indicating that NEI modified the antimicrobial activity of the fluid during its formation in vivo. If NEI acts predominantly by inhibiting the conversion of cathelicidin precursors into active protegrins, then adding excess elastase should restore this function. Figure 6B demonstrates that adding a final concentration of 100 μg/mL HNE to inhibited wound fluid in an RDA (10 mM NaP) fully restored the activity of inhibited fluid. It required 200 μg/mL of the less-specific porcine pancreatic elastase to only partially restore the antimicrobial activity (data not shown). Of note, neutrophil elastase alone had little antimicrobial activity (1.5 ± 0.5 RDU) (n = 3) in these permissive buffer conditions (data not shown).
The effect of NEI, neutrophil elastase, and PG-1 added in vitro on the antimicrobial activity of porcine wound fluid.
(A) To determine the effect of NEI on the in vitro antimicrobial activity, wound fluids were either treated with solvent in vivo (24-hour treatment) and in vitro (2-hour treatment) (“solvent/solvent”); solvent in vivo and 0.25 mM NEI in vitro (“solvent/NEI”); 0.25 mM NEI in vivo and solvent in vitro (“NEI/solvent”); or 0.25 mM NEI in vivo and in vitro (“NEI/NEI”). Antimicrobial activity was measured by RDA. NEI added in vitro to the wound fluids had no effect on antimicrobial activity against S epidermidis and E coli under the assay's most sensitive conditions (10 mM NaP) (n = 3). (B) Neutrophil elastase (E) added in vitro at 100 μg/mL final concentration to wound fluid that had been treated with NEI in vivo fully restored the antimicrobial activity under RDA against E coli compared with solvent-only control (S) and NEI-treated wound fluids (n = 3). (C) The average concentrations of protegrin in wound fluid treated in vivo with solvent (hatched bar) NEI (solid bar) were determined by semiquantitative Western analysis (n = 3). The in vitro antimicrobial activity of the same wound fluid that had been treated in vivo with NEI could be restored to that of the solvent-only control with the addition of an average of 36 μg/mL PG-1 (RDA against E coli, shaded bar) (n = 3). Note that the difference in measured protegrin concentration between the NEI- and solvent-treated fluids is equivalent to the restorative concentration of protegrin. Error bars represent SEM.
The effect of NEI, neutrophil elastase, and PG-1 added in vitro on the antimicrobial activity of porcine wound fluid.
(A) To determine the effect of NEI on the in vitro antimicrobial activity, wound fluids were either treated with solvent in vivo (24-hour treatment) and in vitro (2-hour treatment) (“solvent/solvent”); solvent in vivo and 0.25 mM NEI in vitro (“solvent/NEI”); 0.25 mM NEI in vivo and solvent in vitro (“NEI/solvent”); or 0.25 mM NEI in vivo and in vitro (“NEI/NEI”). Antimicrobial activity was measured by RDA. NEI added in vitro to the wound fluids had no effect on antimicrobial activity against S epidermidis and E coli under the assay's most sensitive conditions (10 mM NaP) (n = 3). (B) Neutrophil elastase (E) added in vitro at 100 μg/mL final concentration to wound fluid that had been treated with NEI in vivo fully restored the antimicrobial activity under RDA against E coli compared with solvent-only control (S) and NEI-treated wound fluids (n = 3). (C) The average concentrations of protegrin in wound fluid treated in vivo with solvent (hatched bar) NEI (solid bar) were determined by semiquantitative Western analysis (n = 3). The in vitro antimicrobial activity of the same wound fluid that had been treated in vivo with NEI could be restored to that of the solvent-only control with the addition of an average of 36 μg/mL PG-1 (RDA against E coli, shaded bar) (n = 3). Note that the difference in measured protegrin concentration between the NEI- and solvent-treated fluids is equivalent to the restorative concentration of protegrin. Error bars represent SEM.
The ability of only 100 μg/mL elastase (3.6 μM) to restore antimicrobial activity of wound fluid generated in the presence of 0.25 mM NEI indicated that the elastase-inhibitory activity of the fluid dissipated by the time of collection. To confirm the transient effect of NEI, we measured the residual inhibitory activity of NEI-treated wound fluid. Wound fluids from 3 separate NEI-treated chambers did not appreciably inhibit the elastolytic activity of 100, 250, or 500 μg/mL HNE (Alphasin Elastase Diffusion Plate, Elastin Products)(data not shown). Therefore, by the end of the 24-hour in vivo experiment, the NEI either diffused into the circulation, was bound, or was otherwise inactivated.
The concentration of PG-1 required to restore the inhibited antimicrobial activity of NEI-treated wound fluid was determined by PG-1 titration in RDAs. Figure 6C (shaded bar) indicates that an average of 36 μg/mL PG-1 was necessary to fully restore the antimicrobial activity of NEI-treated fluids against E coli(n = 3). Similar results were obtained with S epidermidis(data not shown). The added PG-1 also brought the total concentration of protegrin to that in control wound fluids, as measured by Western analysis (Figure 6C, hatched bar; P > .5). The ability of PG-1 to fully restore the in vitro activity of NEI-inhibited wound fluid is further evidence that NEI acts predominantly by inhibiting protegrin activation.
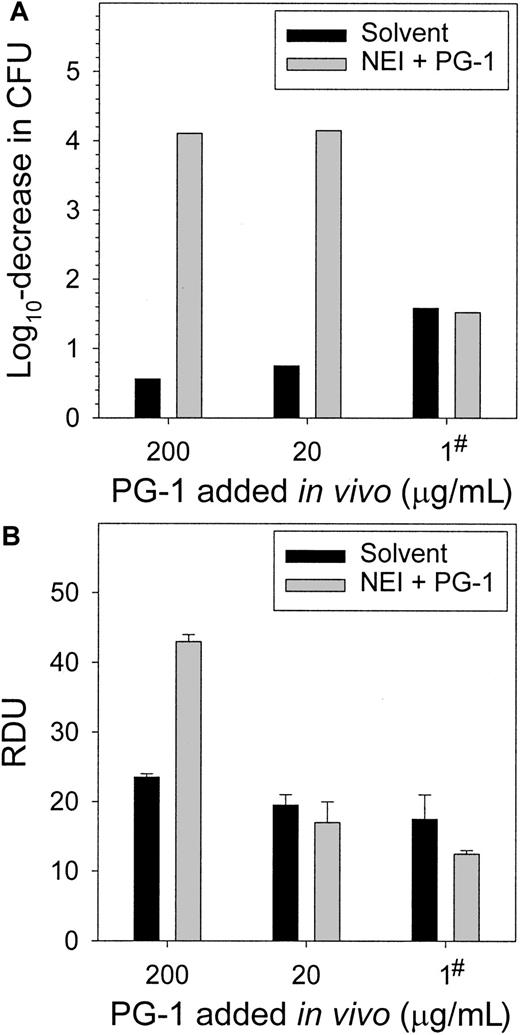
Supplementing wound chambers with PG-1 in vivo restores the microbicidal function of NEI-treated fluid
To correct the protegrin reduction caused by the NEI, PG-1 was added to wound chambers concurrently with NEI and test bacteria. Two concentrations of PG-1 (20 and 200 μg/mL) completely cleared the chambers of S epidermidis CFUs (Figure7A). The addition of PG-1 at the onset of the experiment likely killed the bacteria before colonization, proliferation, or both could occur. To approximate the more gradual time course of protegrin release, in the third experiment we added 1 μg/mL PG-1 4 hours after wound chamber preparation. This was sufficient to restore the in vivo antimicrobial activity to that of the solvent-only controls. The fluids were subjected to RDAs againstS epidermidis (Figure 7B) and E coli, S aureus, P aeruginosa, and C albicans (not shown). The fluids from NEI-inhibited chambers to which 20 μg/mL or 1 μg/mL PG-1 was added in vivo did not differ in antimicrobial activity from the noninhibited control (P > .25), while the addition of 200 μg/mL PG-1 overcompensated for the NEI-inhibition (P < .003).
Supplementing wound chambers in vivo with exogenous PG-1 restores the microbicidal activity of NEI-treated wound fluid.
(A) The complete clearance of S epidermidis from wound fluid, as measured by a CFU assay at 24 hours, was achieved by adding either 20 or 200 μg/mL PG-1 during wound chamber preparation. PG-1 (1 μg/mL) added 4 hours after chamber preparation normalized the activity of the NEI-treated fluid to that of the solvent-treated control (denoted by #), as determined by a CFU assay at 24 hours. (B) Wound fluids from panel A were subjected to RDAs against S epidermidis. The addition of 20 μg/mL PG-1 in vivo concurrently with NEI and bacteria (t = 0) or 1 μg/mL PG-1 at 4 hours restored the antimicrobial activity of NEI-treated wound fluid (n = 3).
Supplementing wound chambers in vivo with exogenous PG-1 restores the microbicidal activity of NEI-treated wound fluid.
(A) The complete clearance of S epidermidis from wound fluid, as measured by a CFU assay at 24 hours, was achieved by adding either 20 or 200 μg/mL PG-1 during wound chamber preparation. PG-1 (1 μg/mL) added 4 hours after chamber preparation normalized the activity of the NEI-treated fluid to that of the solvent-treated control (denoted by #), as determined by a CFU assay at 24 hours. (B) Wound fluids from panel A were subjected to RDAs against S epidermidis. The addition of 20 μg/mL PG-1 in vivo concurrently with NEI and bacteria (t = 0) or 1 μg/mL PG-1 at 4 hours restored the antimicrobial activity of NEI-treated wound fluid (n = 3).
Discussion
The roles of neutrophil elastase and protegrins in wound host defense
This study explored the effects of a specific NEI on innate host defense in porcine skin wounds. The preceding experiments demonstrated that wound fluid generated in the presence of NEI is deficient in mature protegrin content, protegrin-associated antibacterial activity, and total antibacterial activity against a wide range of microbes. Moreover, NEI-treated wounds showed diminished clearance of fungi and Gram-positive and Gram-negative bacteria. We discerned no effects of 0.1-0.25 mM concentrations of NEI on neutrophil recruitment, phagocytic superoxide production, or degranulation of lysozyme. However, higher concentrations of NEI decreased neutrophil recruitment and thus could not be used for this study. CGI, an inhibitor of a neutrophil serine protease structurally similar to neutrophil elastase, had no detectable effects on antimicrobial activity in any of these assays. Taken together these experiments suggest that in the pig model, the NEI N-methoxysuccinyl-AAPV chloromethyl ketone impairs the clearance of skin bacteria from wounds, at least in part by blocking the elastase-mediated activation of antimicrobial protegrins. These findings also support the proposed role of antimicrobial peptides as effector molecules of host defense in mammals.
The reduction of antimicrobial activity caused by NEI could be corrected by replacing the mature protegrin, thereby confirming the important contribution of elastase-processed protegrin to the antimicrobial activity of wound fluid. Initial tests showed that the in vitro antimicrobial activity of wound fluid could be restored to normal levels with an amount of protegrin equivalent to the measured difference between the protegrin concentrations in the control and inhibited chambers. In vivo, 1 μg/mL PG-1 added directly to the chambers 4 hours after bacterial inoculation was sufficient to compensate for the inhibition of local protegrin production. However, 1 μg/mL protegrin is less than the measured difference in approximately 50 μg/mL protegrin concentration between the control and inhibited chambers at 24 hours. Because protegrin is not yet detectable in wound fluid at 4 hours (Western analysis, data not shown), adding even minute amounts of protegrin earlier, when antimicrobial substance concentrations are still low, is likely to have a dramatic effect on the fluid's cumulative microbicidal activity. Finally, as expected from the proposed mechanism, the antimicrobial activity of wound fluid that had formed in the presence of NEI could be restored by adding excess neutrophil elastase in vitro.
No elastase activity was detectable in cell-free wound fluid by the chromogenic assay (data not shown) or with an elastin-based radial diffusion assay (Alphasin Elastase Diffusion Plate, data not shown). Our previous studies with the same neutrophil elastase-specific chromogenic substrate revealed that the enzymatic activity of 106 porcine PMNs was equivalent to 1 μg HNE, ie, similar to that of human PMNs.15 Therefore, the porcine neutrophil elastase concentration, which is derived from the average number of neutrophils present in the wound fluid (approximately 1 × 107 PMN/mL), was approximately 1-10 μg/mL (0.036-0.36 μM), assuming 10% to 100% degranulation and no degradation. The in vivo concentration of NEI (0.1-0.25 mM) thus was at least 270- to 700-fold greater than the amount necessary to inhibit the activity of neutrophil elastase. The lack of elastase activity in the cell-free wound fluid is consistent with the studies of other biological fluids that contain plasma-derived elastase inhibitors. In these fluids, elastase is only biologically active when bound to the cell surface of neutrophils, where it is not inhibited by α-1–antiprotease and other plasma-derived inhibitors but remains accessible to low molecular weight inhibitors.38 Inactive elastase complexes and degradation products were detectable in the wound fluid by Western blot using commercially available mAbs and polyclonal antibodies to HNE (ICN, Costa Mesa, CA, and Accurate Chemical and Scientific Corp, Westbury, NY) (data not shown). We therefore surmise that the conversion of proprotegrin to active protegrin takes place in or on activated PMNs, and that this was the site of inhibition by NEI.
In additional experiments, 200 μg PG-1 administered topically 24 hours after infection reduced bacterial CFUs by 10-fold at 48 hours in wounds inoculated with a highly infective strain of P aeruginosa (A.C., A.M.C., and T.G., unpublished data, September 2000). Indeed, Loury and colleagues39 have shown that topical administration of a protegrin derivative, IB-367, can significantly reduce microflora densities in experimentally induced hamster oral mucositis. Augmentation of the biological levels of antimicrobial peptides may become a useful strategy for the prevention and treatment of localized infections.
Although neutrophil-specific protegrins are the most active antimicrobials in porcine wound fluid, the neutrophils also produce additional microbicidal peptides and proteins, some of which may be processed by other proteases. NEI might also affect the activity of additional serine and cysteine proteases that have not yet been identified. Extrapolation of the effects of neutrophil elastase inhibition to other peptides and mammalian species should therefore be made with caution. Although cathelicidins have also been found in human40-43 and murine neutrophils,44,45neutrophils of other mammals,46 and neutrophils in several mammalian epithelia,47,48 mature cathelicidin peptides vary in their sequences as do their potential elastase cleavage sites. Two rabbit cathelicidins, p15A and p15B,49 apparently do not undergo proteolytic maturation, and they manifest microbicidal activity without it. Moreover, the human myeloid defensins are proteolytically processed to mature forms before storage in granules.50 Thus, further studies will be necessary to determine to what extent the findings in the pig model are applicable to other animals and humans. In the meantime, it may be prudent to monitor for clinical signs of impaired tissue microbicidal activity in experimental subjects who are receiving synthetic elastase inhibitors.
Acknowledgments
We thank Dr Edith M. Porter for critically reviewing this manuscript and Thomas Kang, Shawn McGill, Fernando Vinuela Jr, and John Robert for their technical contributions. We also express our gratitude to Dr Timothy Lawson, Director of the Division of Laboratory Animal Medicine, UCLA, Los Angeles, CA.
Supported by National Research Initiative Grant 98-35204-6594 (T.G. and J.S.) from the U.S. Department of Agriculture, Washington, DC; grant HL46809 (T.G.) and postdoctoral fellowship HL10181 (A.M.C.) from the National Institutes of Health, Bethesda, MD; a postdoctoral fellowship (A.M.C.) from the American Lung Association, New York, NY; and a postdoctoral fellowship (J.S.) from the Cystic Fibrosis Foundation, Bethesda, MD. J.S. is currently employed by the Biologicals Development Group, Pfizer Inc., Groton, CT.
A.M.C. and J.S. contributed equally to this manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tomas Ganz, Department of Medicine, UCLA School of Medicine, Los Angeles, CA 90095-1690; e-mail: tganz@ucla.edu.