Abstract
In studies aimed at further characterizing the cellular immunodeficiency of the Wiskott-Aldrich syndrome (WAS), we found that T lymphocytes from WAS patients display abnormal chemotaxis in response to the T-cell chemoattractant stromal cell–derived factor (SDF)-1. The Wiskott- Aldrich syndrome protein (WASP), together with the Rho family GTPase Cdc42, control stimulus-induced actin cytoskeleton rearrangements that are involved in cell motility. Because WASP is an effector of Cdc42, we further studied how Cdc42 and WASP are involved in SDF-1–induced chemotaxis of T lymphocytes. We provide here direct evidence that SDF-1 activates Cdc42. We then specifically investigated the role of the interaction between Cdc42 and WASP in SDF-1–responsive cells. This was achieved by abrogating this interaction with a recombinant polypeptide (TAT-CRIB), comprising the Cdc42/Rac interactive binding (CRIB) domain of WASP and a human immunodeficiency virus–TAT peptide that renders the fusion protein cell-permeant. This TAT-CRIB protein was shown to bind specifically to Cdc42-GTP and to inhibit the chemotactic response of a T-cell line to SDF-1. Altogether, these data demonstrate that Cdc42-WASP interaction is critical for SDF-1–induced chemotaxis of T cells.
Introduction
The Wiskott-Aldrich syndrome (WAS) is an X-linked hereditary disease associated with mutations in theWASP gene and characterized by thrombocytopenia, with small platelet size, eczema, and increased susceptibility to infections.1 Recent reports have helped to understand some of the function of the WAS protein (WASP), and many findings are consistent with the hypothesis that WASP has an important role in controlling the actin cytoskeleton. Indeed, WASP contains, at its C-terminal region, verprolin and cofilin homology domains2found in other cytoskeletal-associated proteins and, also, a Ccd42/Rac interactive binding (CRIB) domain that binds to the small GTPase Cdc42.3-5 It was also recently demonstrated that WASP could interact with the actin-related protein (Arp2/3) complex to induce actin polymerization.6-11 Actin cytoskeleton rearrangements are essential for cell motility, and WASP-deficient cells display impaired migration. Indeed, chemotaxis was shown to be deficient in WAS; in several cell lineages including macrophages and monocytes in response to MCP-1, MIP-1α, or CSF-1 12,13; and dendritic cells in response to RANTES (regulated on activation normal T cells expressed and secreted).14 In contrast, we previously reported that megakaryocytes derived by in vitro culture of WAS patient CD34+ cells migrated normally in response to stromal cell–derived factor (SDF)-1 despite abnormal filopodia formation.15 Little is known, however, with regard to T-cell chemotaxis in WAS because, to our knowledge, a single report has addressed this question.16 We therefore studied the ability of peripheral blood CD3+ T lymphocytes from WAS patients to respond to SDF-1, a very efficient chemoattractant for peripheral T cells.
In recent years, it has become clear that the dynamics of actin cytoskeleton is controlled in part by the Rho family GTPases. Cdc42, Rac, and Rho have been shown to regulate cortical actin polymerization events leading respectively to filopodia, lamellipodia, or stress fibers formation.17 These functions are mediated through effector proteins that, upon binding to the small GTPases, either become activated or undergo conformational changes that allow for new protein interactions to occur. Well-characterized effectors of Cdc42 that contain a CRIB domain include the PAK family serine kinases18-20 and WASP.5 We therefore investigated whether the interaction between WASP and Cdc42 could be involved in the SDF-1–induced chemotaxis of T lymphocytes.
Patients, materials, and methods
Cell preparation and migration assays
Fresh blood samples were harvested from WAS patients and normal donors during medical evaluations. Informed consent was obtained in all cases (patients and controls) in accordance with the institutional guidelines of the Committee on Human Investigation. Clinical phenotype and gene mutation for all patients are given in Table1. Peripheral blood mononuclear cells (PBMCs) were purified over a Ficoll-metrizoate gradient. The human T-cell line CEM was grown in MEM α medium (Life Technologies, Cergy Pontoise, France) supplemented with 10% fetal calf serum, antibiotics, and l-glutamin.
Migration assays were performed using 5 μm (mononuclear cells) or 8 μm (CEM cells) pore filters (Transwell, 24-well cell clusters; Costar, Cambridge, MA). Cell suspensions (2.5 × 105cells in a 100 μL volume) were placed into the upper chamber, whereas 600 μL of medium with or without recombinant human SDF-1 (300 ng/mL) (R & D Systems; Minneapolis, MN) was introduced in the lower chamber. The chambers were incubated for 1 hour at 37°C in 5% CO2and 95% air. The cells in the upper and in the bottom chamber were recovered separately in the same volume for counting. For human mononuclear cells, the different cell fractions were then labeled with a phycoerythrin–anti-CD3 monoclonal antibody and analyzed by flow cytometry. Percentage of migrating cells was determined as follows: [number of cells migrating (lower chamber)/ total number of cells (cells in the lower chamber + remaining cells in the upper chamber)]. All assays were done in triplicate.
Plasmid constructs and TAT-CRIB protein purification
Complementary DNA encoding for a 120–amino acid peptide (residues 201-321) of WASP that contains the CRIB motif was amplified by polymerase chain reaction using appropriate oligonucleotides and cloned in PGEX-4T1 (Amersham Pharmacia Biotech, Saclay, France) for glutathione-S-transferase (GST)-CRIB production or C-terminal of the TAT peptide in vector pTAT-HA23 for pTAT-CRIB production. Mutations in the CRIB motif were introduced by site-directed mutagenesis (QuickChange, Stratagene, Amsterdam, The Netherlands) with oligonucleotide primers bearing the desired sequence changes. All constructs and mutations were verified by DNA sequencing. The pTAT-CRIB vectors were transformed into Escherichia coli (BL21) and proteins expressed by isopropyl thiogalactoside induction. Recombinant TAT-CRIB proteins were purified by sequential chromatography on Nickel-Agarose (Amersham Pharmacia) and anion exchange chromatography.23
Affinity precipitation of Cdc42-GTP
Immobilized GST-CRIB was mixed with CEM T-cell lysates at 4°C for 1 hour. After washing, Cdc42-GTP was extracted in 2 × sample buffer and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by transfer to polyvinylidene fluoride (PVDF) membranes. Bound Cdc42 was detected using a polyclonal antibody against Cdc42,24 and immunoreactive bands were visualized using an enhanced chemiluminescence (ECL) detection system (Amersham Pharmacia).
Immunoprecipitation and kinase assay of PAK
CEM cells were lysed in 40 mM HEPES (pH 7.4), 1% Nonidet P-40, 100 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 25 mM NaF, 1 mM sodium orthovanadate, 10 μg/mL aprotinin, and 100 μg/mL leupeptin. PAKs were immunoprecipitated at 4°C for 2 hours with anti–PAK-2 (Santa Cruz, CA) or anti–PAK-4 antibodies (a gift from A. Abo, Onyx Pharmaceuticals, Richmond, CA). The immune complexes were recovered using protein G–coupled agarose. PAK activity was determined in an in vitro kinase assay by mixing 30 μL of immune complexes with 30 μL of a phosphorylation mixture containing (final concentration) 40 mM HEPES (pH 7.4), 100 mM NaCl, 10 mM MgCl2, 1 mM MnCl2, 20 μM adenosine triphosphate, and 185 kBq (5 μCi) [γ-32P] adenosine triphosphate together with 5 μg myelin basic protein as a substrate, at 30°C for 20 minutes as described.25 The reaction was stopped by boiling in 2 × sample buffer and analyzed by SDS-PAGE followed by autoradiography.
Western blot anti-WASP
Cells were lysed in 50 mM Tris, 0.5% Nonidet P-40, 250 mM NaCl, 5 mM ethylenediaminetetraacetic acid, and a protease inhibitor cocktail (Boehringer Mannheim, Germany). Lysates were extracted in 2 × sample buffer. Forty-microgram proteins were separated by SDS-PAGE followed by transfer to PVDF membranes. Actin and WASP were detected using a monoclonal anti-WASP antibody (a gift from D. Nelson, National Institutes of Health, Bethesda, MD) and a monoclonal anti-β actin antibody (Sigma, St Louis, MO), respectively, and immunoreactive bands were visualized using an ECL detection system.
Results
T lymphocytes from WAS patients display abnormal chemotaxis in response to SDF-1
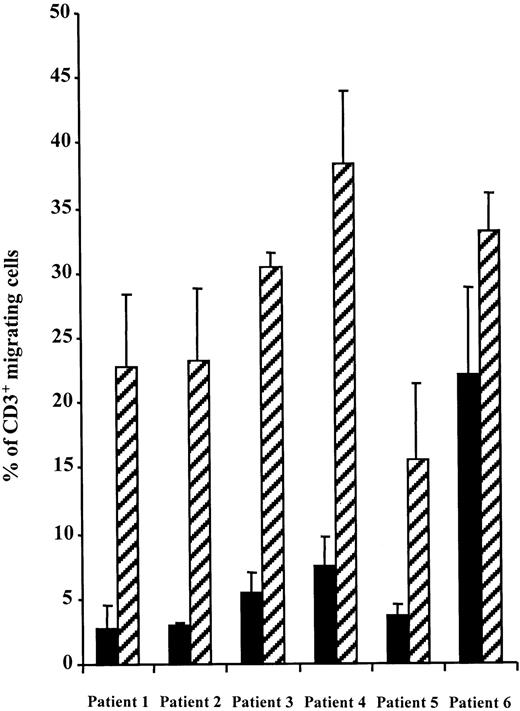
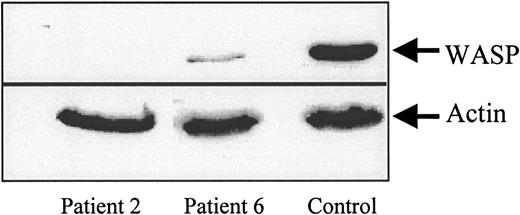
Freshly isolated PBMCs from WAS patients were tested for their chemotactic response to SDF-1 in a Transwell migration assay in parallel with PBMCs from normal donors. CD3+ T cells from 5 of 6 patients showed a dramatic reduction in chemotaxis (Figure1). However, migration was not completely abolished because 2.7% to 7.5% of CD3+ cells were recovered in the lower chamber as opposed to 0.4% in the absence of SDF-1. In patient no. 6, T-lymphocyte migration was partly reduced to about 70% of the control. Interestingly, the WASP gene mutation in this patient leads to a stop codon at position 494, likely resulting in expression of a partly functional protein. This was confirmed by a Western blot analysis of T-cell lysates from patients no. 6 and 2 as compared with T cells of control (Figure2). Indeed, in patient no. 2, whose gene mutation leads to a stop codon at position 83, no WASP protein could be detected in lymphocytes while, in patient no. 6, WASP protein appeared to be expressed although at a much lower level than in control lymphocytes.
SDF-1–induced chemotaxis is impaired in WAS CD3+ lymphocytes.
The migration of PBMCs from normal donors or WAS patients in response to SDF-1 (300 ng/mL) was studied in a Transwell assay. Data representing the percentage of migrating CD3+ cells are expressed as the mean of triplicate ± SE. No differences were observed between patients' and controls' CD3+ cells in the absence of SDF-1 (range 0.3%-0.5% migrating cells) (not shown). (▪), patients; (▨), normal donors.
SDF-1–induced chemotaxis is impaired in WAS CD3+ lymphocytes.
The migration of PBMCs from normal donors or WAS patients in response to SDF-1 (300 ng/mL) was studied in a Transwell assay. Data representing the percentage of migrating CD3+ cells are expressed as the mean of triplicate ± SE. No differences were observed between patients' and controls' CD3+ cells in the absence of SDF-1 (range 0.3%-0.5% migrating cells) (not shown). (▪), patients; (▨), normal donors.
Western blot analysis of WASP in blood lymphocytes of patients no. 2 and 6 and normal donor (control).
Protein extracts (40 μg) from patients no. 2 and 6 or from a normal donor T lymphocytes were separated by SDS-PAGE followed by transfer to PVDF membranes. Actin and WASP were detected using a monoclonal anti-WASP antibody (a gift from D. Nelson) and a monoclonal anti-β actin antibody (Sigma), respectively, and immunoreactive bands were visualized using an ECL detection system.
Western blot analysis of WASP in blood lymphocytes of patients no. 2 and 6 and normal donor (control).
Protein extracts (40 μg) from patients no. 2 and 6 or from a normal donor T lymphocytes were separated by SDS-PAGE followed by transfer to PVDF membranes. Actin and WASP were detected using a monoclonal anti-WASP antibody (a gift from D. Nelson) and a monoclonal anti-β actin antibody (Sigma), respectively, and immunoreactive bands were visualized using an ECL detection system.
These results thus suggest that WASP is required for T-cell chemotaxis in response to SDF-1.
SDF-1 activates Cdc42 in a T-cell line
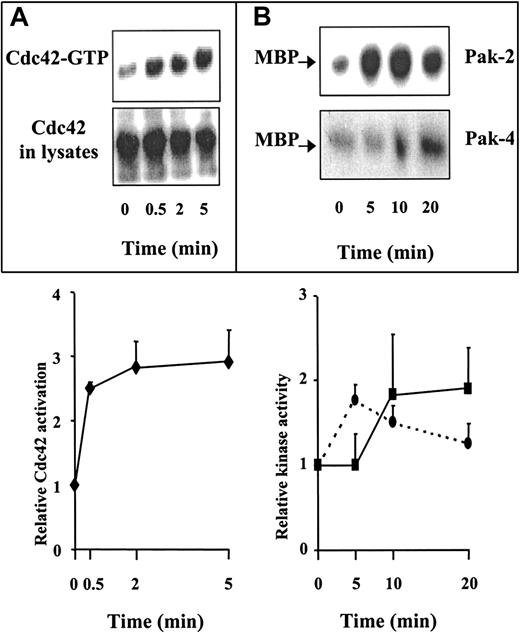
Because WASP is a known effector of Cdc42,3-5 we hypothesized that SDF-1 signaling involves a Cdc42-WASP pathway and that WASP functions as a Cdc42 effector in SDF-1–induced T-cell chemotaxis. This hypothesis implies that SDF-1 activates Cdc42. This was demonstrated using a GST fusion protein containing the CRIB motif of WASP that specifically binds the GTP-bound form of Cdc42. This protein was used to pull down activated Cdc42 from extracts of resting or SDF-1–stimulated CEM cells, a human T-cell line that migrates in response to SDF-1. As shown in Figure3A, cell stimulation with SDF-1 induced a marked increase in activated Cdc42 after 30 seconds that reached a maximum after 2 minutes. In addition, we showed that SDF-1 also activates the Cdc42 downstream effector kinases PAK-2 and PAK-4 (Figure3B). Of note, the kinetics of activation was slightly different for PAK-2 and PAK-4, peaking at 5 minutes for PAK-2 and at 10 to 20 minutes for PAK-4. It is unclear why these kinetics do not closely parallel that of Cdc42 activation.
SDF-1 activates Cdc42 and PAK in CEM cells.
(A) SDF-1 activates Cdc42. Cells deprived of serum were treated with 500 ng/mL SDF-1 for the indicated times, and Cdc42-GTP was pulled down using GST-CRIB. The top panel shows the kinetics of accumulated Cdc42 in the GTP-bound form that associates with GST-CRIB; the lower panel shows the total amount of Cdc42 in 4% of total cell extract. The bands corresponding to Cdc42-GTP were quantified by scanning densitometry, and the results shown are the mean ± SE of 3 independent experiments. (B) SDF-1 increases PAK-2 and PAK-4 kinase activity. Cells were stimulated as above and lysed. The lysates were immunoprecipitated with specific antibodies to PAK-2 or PAK-4. PAK activity was determined by in vitro kinase assay followed by SDS-PAGE and autoradiography. Quantitative analyses were performed by scanning densitometry, and the results shown are the mean ± SE of 5 (PAK-2) and 3 (PAK-4) independent experiments. (– –), Pak-2; (–
–), Pak-2; (– –), Pak-4.
–), Pak-4.
SDF-1 activates Cdc42 and PAK in CEM cells.
(A) SDF-1 activates Cdc42. Cells deprived of serum were treated with 500 ng/mL SDF-1 for the indicated times, and Cdc42-GTP was pulled down using GST-CRIB. The top panel shows the kinetics of accumulated Cdc42 in the GTP-bound form that associates with GST-CRIB; the lower panel shows the total amount of Cdc42 in 4% of total cell extract. The bands corresponding to Cdc42-GTP were quantified by scanning densitometry, and the results shown are the mean ± SE of 3 independent experiments. (B) SDF-1 increases PAK-2 and PAK-4 kinase activity. Cells were stimulated as above and lysed. The lysates were immunoprecipitated with specific antibodies to PAK-2 or PAK-4. PAK activity was determined by in vitro kinase assay followed by SDS-PAGE and autoradiography. Quantitative analyses were performed by scanning densitometry, and the results shown are the mean ± SE of 5 (PAK-2) and 3 (PAK-4) independent experiments. (– –), Pak-2; (–
–), Pak-2; (– –), Pak-4.
–), Pak-4.
The interaction between Cdc42 and CRIB-containing protein(s) is needed for SDF-1–induced chemotaxis in a T-cell line
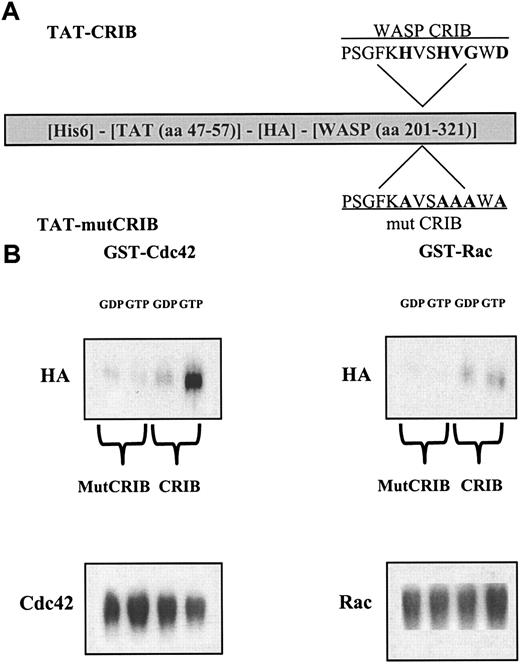
To specifically investigate the interaction between Cdc42 and CRIB-containing protein(s), we designed a peptide that can be introduced into unmodified cells and directly antagonize this interaction. A fusion protein was constructed containing an extended CRIB domain of WASP, with high-affinity binding to activated Cdc42,26 fused to the human immunodeficiency virus (HIV)-TAT protein transduction domain.23 As a control, the same protein bearing mutations at 5 conserved residues that are critical for the interaction of CRIB with Cdc4227 28 was produced (Figure 4a). In vitro interaction experiments confirmed that TAT-CRIB associates specifically with GTP-loaded Cdc42, as opposed to GDP-Cdc42 or Rac, and that this association is completely abolished by the amino acid substitutions introduced in TAT-mutCRIB (Figure 4B).
Protein interactions.
(A) Characterization of TAT-CRIB and TAT-mutCRIB proteins. Features and functional domains of the fusion proteins: The fusion proteins are about 29 kd in size and contain a 6-histidine tag (His6), amino acids 47 to 57 of the HIV-TAT protein, a hemagglutinin tag (HA), and the extended CRIB domain of WASP. Critical residues in the CRIB domain and their substitution to alanine are shown in bold. (B) TAT-CRIB associates specifically with Cdc42-GTP. GST-Cdc42 or GST-Rac (2.5 μg) were coupled on GSH-agarose beads and loaded with GDP or GTP in 0.5 μM MgCl2 buffer. Purified TAT fusion proteins (100 ng) were added for 1 hour in a phosphate-buffered saline buffer containing 5 mM NH42SO4, 10 mM MgCl2, 300 mM NaCl, and 0.1 mg/mL bovine serum albumin. Following 3 washes, proteins bound to the beads were recovered, separated by 10% SDS-PAGE, and immunoblotted with the following antibodies: 12CA5 anti-HA–tag to visualize TAT-CRIB, rabbit anti-Cdc42 antiserum, or monoclonal anti-Rac for loading controls.
Protein interactions.
(A) Characterization of TAT-CRIB and TAT-mutCRIB proteins. Features and functional domains of the fusion proteins: The fusion proteins are about 29 kd in size and contain a 6-histidine tag (His6), amino acids 47 to 57 of the HIV-TAT protein, a hemagglutinin tag (HA), and the extended CRIB domain of WASP. Critical residues in the CRIB domain and their substitution to alanine are shown in bold. (B) TAT-CRIB associates specifically with Cdc42-GTP. GST-Cdc42 or GST-Rac (2.5 μg) were coupled on GSH-agarose beads and loaded with GDP or GTP in 0.5 μM MgCl2 buffer. Purified TAT fusion proteins (100 ng) were added for 1 hour in a phosphate-buffered saline buffer containing 5 mM NH42SO4, 10 mM MgCl2, 300 mM NaCl, and 0.1 mg/mL bovine serum albumin. Following 3 washes, proteins bound to the beads were recovered, separated by 10% SDS-PAGE, and immunoblotted with the following antibodies: 12CA5 anti-HA–tag to visualize TAT-CRIB, rabbit anti-Cdc42 antiserum, or monoclonal anti-Rac for loading controls.
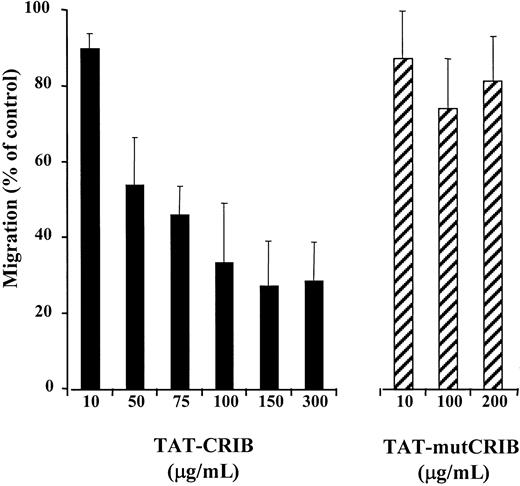
To set the conditions for entry of the fusion protein into cells, preliminary experiments using TAT-CRIB fused to a green fluorescent protein were performed. The intracellular concentration of the protein, as detected by fluorescence-activated cell sorter analysis, peaked at 2 hours of incubation (not shown). Therefore, CEM cells were incubated with either TAT-CRIB or TAT-mutCRIB for 2 hours before being tested for SDF-1–induced migration. As shown in Figure5, TAT-CRIB did inhibit SDF-1–induced migration in a dose-dependent manner, whereas TAT-mutCRIB had no significant effect. A 50% migration inhibition was obtained when cells were incubated with about 75 μg/mL TAT-CRIB. At higher TAT-CRIB concentrations, a 25% residual migration was observed, indicating that migration was not completely inhibited.
TAT-CRIB inhibits SDF-1–induced chemotaxis.
CEM cells were tested for SDF-1–induced chemotaxis after a 2-hour incubation in medium containing increasing concentrations of TAT-CRIB or TAT-mutCRIB (and in normal medium to assess control migration). Data are presented as the percentage of control migration and are expressed as mean + SE of 4 independent experiments.
TAT-CRIB inhibits SDF-1–induced chemotaxis.
CEM cells were tested for SDF-1–induced chemotaxis after a 2-hour incubation in medium containing increasing concentrations of TAT-CRIB or TAT-mutCRIB (and in normal medium to assess control migration). Data are presented as the percentage of control migration and are expressed as mean + SE of 4 independent experiments.
TAT-CRIB does not inhibit actin polymerization but inhibits PAK-2 kinase activity
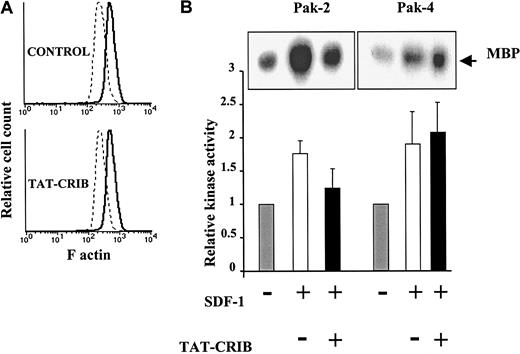
To test whether disrupting Cdc42/CRIB interaction also affected SDF1-induced actin polymerization,29 CEM cells that had been preincubated with TAT-CRIB or not were stained for F-actin and analyzed by cytofluorimetry. As shown in Figure6A, TAT-CRIB, when used at a concentration that blocked cell migration, did not modify the F-actin content in resting cells or its increase in SDF-1–stimulated cells.
Effects of cell treatment with TAT-CRIB.
(A) TAT-CRIB does not inhibit SDF-1–induced actin polymerization. After a 2-hour incubation in medium containing 300 μg/mL TAT-CRIB or not (control), CEM cells were stimulated with SDF-1 (300 ng/mL) for 30 seconds at 37°C. Cells were then fixed in paraformaldehyde, permeabilized, and incubated with fluorescein isothiocynate–labeled phalloidin. Analysis of fluorescein isothiocynate intensity on a FACSort (Becton Dickinson) showed that the baseline content of F-actin (dotted line) and its increase following SDF-1 stimulation (plain line) are not altered by TAT-CRIB. (B) Effects of TAT-CRIB on PAK-2 and -4 activities. Cells were preincubated with 300 μg/mL TAT-CRIB (+) or not (−) for 2 hours. Cells were then left unstimulated or were stimulated with 500 ng/mL SDF-1 for 5 (PAK-2) or 20 minutes (PAK-4) and lysed. The lysates were immunoprecipitated with anti–PAK-2 or anti–PAK-4, and PAK activity was determined by in vitro kinase assay. Data shown represent mean + SE of 4 (PAK-2) or 3 (PAK-4) independent experiments. Representative autoradiograms are shown in insets.
Effects of cell treatment with TAT-CRIB.
(A) TAT-CRIB does not inhibit SDF-1–induced actin polymerization. After a 2-hour incubation in medium containing 300 μg/mL TAT-CRIB or not (control), CEM cells were stimulated with SDF-1 (300 ng/mL) for 30 seconds at 37°C. Cells were then fixed in paraformaldehyde, permeabilized, and incubated with fluorescein isothiocynate–labeled phalloidin. Analysis of fluorescein isothiocynate intensity on a FACSort (Becton Dickinson) showed that the baseline content of F-actin (dotted line) and its increase following SDF-1 stimulation (plain line) are not altered by TAT-CRIB. (B) Effects of TAT-CRIB on PAK-2 and -4 activities. Cells were preincubated with 300 μg/mL TAT-CRIB (+) or not (−) for 2 hours. Cells were then left unstimulated or were stimulated with 500 ng/mL SDF-1 for 5 (PAK-2) or 20 minutes (PAK-4) and lysed. The lysates were immunoprecipitated with anti–PAK-2 or anti–PAK-4, and PAK activity was determined by in vitro kinase assay. Data shown represent mean + SE of 4 (PAK-2) or 3 (PAK-4) independent experiments. Representative autoradiograms are shown in insets.
Because the PAK family kinases are implicated in actin polymerization,20,30 31 we investigated the effect of TAT-CRIB on PAK kinase activation in an in vitro kinase assay. Cell treatment with TAT-CRIB resulted in significant inhibition of PAK-2 kinase activity (Figure 6B). In contrast, the kinase activity of PAK-4 was not modified by cell treatment with TAT-CRIB (Figure 6B).
Discussion
Although the structure of WASP and its implication in cytoskeleton regulation are well described, the exact mechanism by which abnormal or absent WASP in lymphocytes leads to immunodeficiency is not fully understood. It has been proposed that WAS may be related in part to cell trafficking alterations.32 Although impaired chemotaxis has already been described in other lineages, it is the first time that abnormal chemotaxis is reported in WAS T cells, a relevant finding in the context of an immunodeficiency. Indeed, we found that SDF-1–induced chemotaxis of T cells from 5 of 6 patients was dramatically reduced. We found also that migration was slightly impaired in one patient whose gene mutation leads to reduced WASP expression. Moreover, we found that abnormal T-cell chemotaxis in these patients did correlate with the severity of the disease as assessed by clinical scores measuring immunodeficiency, infections, and autoimmune manifestations. Indeed, patients no. 1 to 5 displayed a severe clinical score (score 5 in 4 cases and score 4 in 1 case) while patient no. 6 displayed a score 2 with a minor X-linked thrombocytopenia associated with mild eczema.1 These data, which have to be confirmed in a larger series of patients, suggest that abnormal T-cell locomotion could be critical in the outcome of immunodeficiency in this disease. These results also suggest that WASP is required for T-cell chemotaxis in response to SDF-1.
WASP is a known effector of Cdc42.3-5 A variety of cell stimuli lead to activation (GTP loading) of Cdc42, which then switches to an activated conformation allowing its interaction with CRIB domain–containing proteins such as WASP or the PAK family kinases.18,20,33 These effector proteins then trigger signaling pathways that control cytoskeleton rearrangements leading to filopodia formation.17 We thus hypothesized that SDF-1 signaling involves a Cdc42-WASP pathway and that WASP functions as a Cdc42 effector in SDF-1–induced T-cell chemotaxis. In support of this hypothesis, we first demonstrated, using a direct biochemical approach, that SDF-1 activates Cdc42.
Thus, on one hand, the patient studies reported above indicate that WASP is required for SDF-1–induced chemotaxis and, on the other hand, we have shown that SDF-1 activates Cdc42. We therefore investigated specifically whether the interaction between Cdc42 and CRIB-containing protein(s) was involved in SDF-1 signaling. For this purpose, the HIV-TAT polybasic transduction domain was used to introduce into otherwise unmodified cells a CRIB peptide that directly antagonizes this interaction. As a control, we designed a TAT-mutCRIB peptide that was shown not to bind Cdc42-GTP. That TAT-CRIB interacts with GTP-Cdc42 while TAT-mutCRIB does not indicates that the inhibition of migration observed in the presence of TAT-CRIB is the consequence of TAT-CRIB binding to GTP-Cdc42 and preventing Cdc42/CRIB interaction with endogenous effectors. These data, together with the demonstration that WASP is needed for SDF-1–induced migration, lead to the conclusion that the interaction between Cdc42 and WASP is required for SDF-1–induced chemotaxis in T cells.
That TAT-CRIB peptide did not inhibit SDF-1–induced actin polymerization suggests that actin polymerization can be regulated independently of the Cdc42-WASP pathway. It is of interest that in studying a limited number of WAS patients (not shown), SDF-1–induced actin polymerization was found not to be consistently abnormal. This finding is in complete agreement with those of Rengan et al, who recently reported that actin polymerization was normal in WAS hematopoietic lineages.34 Because the PAK family kinases have also been reported to play a role in actin polymerization20,30,31 and because we demonstrated that kinase activity of both proteins was increased by SDF-1 stimulation, the effect of TAT-CRIB on PAK kinase activation was investigated in an in vitro kinase assay. Cell treatment with TAT-CRIB resulted in significant inhibition of PAK-2 kinase activity. This result is consistent with TAT-CRIB inhibiting the ability of Cdc42-GTP to interact with its effectors, because PAK-2 activation is known to depend on the interaction of Cdc42-GTP with the PAK CRIB domain.18,19 In contrast, the kinase activity of PAK-4 was not modified by cell treatment with TAT-CRIB. This was not unexpected because, while Cdc42 may regulate the subcellular localization of PAK-4, PAK-4 kinase activity has been shown to be independent of its interaction with Cdc42.20 It is thus possible that the normal kinase activity of PAK-4 in TAT-CRIB–treated cells could account for normal SDF-1–induced actin polymerization. This hypothesis does not, however, exclude that other pathways, eg, those regulated by Rac or Rho, may contribute to SDF-1–induced actin polymerization.
Biologic effects of SDF-1 are mediated by the 7 transmembrane domains, G-protein–coupled CXCR4 receptor.35,36 SDF-1 has been shown to activate several signaling pathways, including phosphoinositide 3-kinase, mitogen-activated protein kinase, protein kinase C, and tyrosine phosphorylation of components of focal adhesion complex.37-40 Our results provide direct evidence that, in addition to these pathways, SDF-1 activates the small GTPase Cdc42 and its effectors. Furthermore, our direct approach emphasizes the critical role of the interaction between Cdc42 and WASP in a pathway controlling a physiologic function in normal cells. The role of Cdc42 in chemotaxis has been previously suggested by the use of dominant negative (N17) Cdc42 in CSF-1–induced chemotaxis of monocytic cells.41However, GTPase's dominant negative mutants are thought to sequester exchange factors, thus preventing the activation of GTPases. In addition, because Cdc42 and Rac share several exchange factors, dominant negative mutant of Cdc42 could lead to blocking Rac as well. In contrast, the approach used here, introducing a cell-permeant CRIB motif of WASP, does not prevent Cdc42 or Rac activation nor, presumably, Cdc42 interactions with potential effectors that do not contain a CRIB domain. In addition, the CRIB-containing sequence of WASP used here has no significant interaction with recombinant Rac protein, as shown in Figure 4, nor with endogenous Rac as assessed by GST pull-down experiments (not shown). As a result, our approach focuses on the role of Cdc42 interaction with its effectors in chemotaxis rather than on the role of Cdc42 activation and demonstrates that the interaction between Cdc42 and WASP is required for SDF-1–induced chemotaxis.
Because Cdc42-WASP interaction was found dispensable for SDF-1–induced actin polymerization, it can be hypothesized that this interaction is necessary to give directionality to cell movement but is not essential for cell locomotion itself. Indeed, Cdc42 is known to play a critical role in cell polarization in budding yeast42 during fibroblast migration43 or antigen presentation in lymphocytes.44 Moreover, it has been demonstrated that macrophages injected with the dominant negative N17Cdc42 were able to migrate but did not polarize in the direction of a CSF-1 gradient.41 Similarly, macrophages from WAS patients were shown to display abnormal chemotaxis whereas their speed of random migration was conserved.13 Accordingly, random movement may explain the residual migration observed in WASP-deficient lymphocytes (Figure 1) or in CEM cells where Cdc42-WASP interaction had been disrupted (Figure 6A).
The pathogenesis of the immunodeficiency in WAS is not completely understood. It has been suggested that altered cytoskeleton function may affect antigen presentation and dendritic cell migration.14 Following the observation that small GTPases of the Rho family, particularly Cdc42, regulate cell apoptosis,45 it has also been proposed that increased apoptosis in lymphocytes may play a critical role in the disease.34 46 Finally, it is possible that the defect in T-cell migration reported here may participate in the immunodeficiency observed in WAS by impairing the capacity of T lymphocytes to home properly to lymphoid organs.
Acknowledgments
We thank Drs S. Dowdy, P. Chavrier, T. Azuma, A. Abo, and D. Nelson for valuable reagents; G. Tchernia, N. Wulffraadt, and S. Blanche for patient blood samples; J. P. Hossle and A. Wechsler for mutation analysis of patient no. 3; and A. Vazquez and D. Duménil for helpful discussions.
Supported by INSERM and in part by Association pour la Recherche contre le Cancer (grant no. 5608 to J.B. and no. 9728 to N.D.), Ligue Nationale contre le Cancer, and by E.C. grant QLGICT199901090/RA015F. J.L.Z. received an INSERM fellowship contributed by the Conseil Regional d'Ile-de-France followed by a grant from Fondation pour la Recherche Médicale.
E.H. and J.L.Z. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jacques Bertoglio, INSERM U461, Faculté de Pharmacie-Paris Sud, 5, Rue Jean-Baptiste Clément, 92296 Chatenay-Malabry, France; e-mail: j.bertoglio@cep.u-psud.fr.