Abstract
Quiescence has been thought to be required for the retention of the full biological potential of pluripotent hematopoietic stem cells (PHSCs). This hypothesis has been challenged recently by the observation that all murine PHSCs cycle continuously and constantly contribute to steady-state blood cell production. It was asked whether these observations could be extrapolated to describe hematopoiesis in higher mammals. In this series of experiments, the replicative history of PHSCs was examined in baboons by continuously administering bromodeoxyuridine (BrdU) for more than 85 weeks. The results indicate that under steady-state conditions, PHSCs remain largely quiescent but do cycle, albeit at a far lower rate than previously reported for rodent PHSCs. BrdU-labeled cycling PHSCs and progenitor cells were shown to have an extensive proliferative capacity and to contribute to blood cell production for prolonged periods of time. The proportion of PHSCs entering cell cycle could, however, be rapidly increased by the in vivo administration of granulocyte-colony stimulating factor. These data indicate that during steady-state hematopoiesis, baboon PHSCs require prolonged periods of time to cycle and that the proportion of PHSCs in cycle is not fixed but can be altered by external stimuli. The relative quiescence of PHSCs observed in this nonhuman primate model, in contrast to murine PHSCs, might explain the current barriers to genetic modification and ex vivo expansion of human PHSCs.
Introduction
Mammalian blood cell production is a tightly regulated process in which a hierarchy of cycling hematopoietic stem cell populations contributes to active hematopoiesis.1-5The formed elements of blood ultimately originate from rare pluripotent hematopoietic stem cells (PHSCs), which are capable of self-renewal and differentiation into cells belonging to each hematopoietic lineage.6,7 PHSCs contribute to steady-state hematopoiesis and respond to a variety of stressful conditions without actually depleting their number. It is commonly believed that PHSCs remain quiescent during steady-state hematopoiesis and that stem cell quiescence plays a pivotal role in determining the self-renewal and proliferative potential of PHSCs.8 9
In 1965, Kay10 postulated that only a few PHSC clones actively contribute to blood cell production at any given time and that only after exhaustion of these active clones would additional PHSCs self-replicate and actively contribute to blood cell production. Experimental evidence supporting this clonal succession hypothesis was first gained by studies of the contribution of murine blood cell production by PHSCs that had been genetically modified by retroviral vectors.11,12 The contribution of individual PHSCs to blood cell production was monitored by tracking retroviral integration sites in mature blood cells. Abkowitz and coworkers13 also addressed this hypothesis by performing stem cell transplants in female cats who were heterozygotes for the X-linked gene glucose 6-phosphate dehydrogenase. They observed wide fluctuations in clonal contributions to hematopoiesis following initial engraftment but found that long-term reconstitution was due to a limited number of stem cell clones.13 The validity of such reconstitution assays as an appropriate test of the clonal succession hypothesis has been questioned since the kinetics of hematopoietic reconstitution following stem cell transplantation bears little resemblance to the events occurring during steady-state hematopoiesis.14
The clonal succession hypothesis has recently been challenged by studies in which the proliferation history of PHSCs has been directly analyzed by monitoring the incorporation of a nucleotide analogue into the DNA of cycling cells in mice.15-17 The incorporation of bromodeoxyuridine (BrdU) into primitive hematopoietic progenitors was assessed by exposing mice to BrdU in order to label the nuclei of dividing cells. These studies were further aided by the phenotypic characterization and identification of PHSCs. These studies indicate that murine PHSCs enter the cell cycle in an asynchronous manner and that virtually all PHSCs slowly divide over a period of 1.5 to 3.0 months.16,17 Many murine PHSCs were found to contribute simultaneously to steady-state blood cell production. Moreover, a large fraction of murine PHSCs could be driven to self-replicate prior to their mobilization into the peripheral blood (PB) if stressed by the in vivo administration of cyclophosphamide and granulocyte-colony stimulating factor (G-CSF).18
Since important differences are known to exist between the PHSCs of large animals and rodents,19 we attempted to study PHSC quiescence during steady-state hematopoiesis and following stress induced by the administration of G-CSF. The studies were pursued owing to the uncertainty of whether the conclusions drawn from rodent experimentation could be used to further expand our understanding of human hematopoiesis. As the subject of these studies, we chose nonhuman primates in which steady-state blood cell production as well as engraftment kinetics following stem cell transplantation closely resembles that of humans.7,20,21 We also used a noninvasive method to track cycling PHSCs in vivo by monitoring the incorporation of BrdU into cycling PHSCs.16,17 Since BrdU is incorporated into the genomic DNA of dividing cells, bound BrdU can be traced in successive generations of dividing cells.22The present data indicate that a large fraction of PHSCs in baboons remains quiescent during steady-state conditions but that during an 85-week period of observation, most PHSCs do indeed cycle at an apparently much slower rate than is observed in murine PHSCs. The fraction of PHSCs cycling at any one time does not, however, appear to be fixed since PHSC cycling in vivo can be rapidly increased by the administration of G-CSF.
Methods
Nonhuman primate model
Healthy young adult female baboons (Papio anubis)weighing 9 to 10 kg were used for this study. The animals were housed under conditions approved by the Association for the Assessment and Accreditation of Laboratory Animal Care. The studies were performed under protocols approved by the Animal Care Committee of the University of Illinois at Chicago. Animals were provided with water, biscuits, and fruit throughout the study. Three baboons (PA6588, PA6589, and PA6644) were continuously fed BrdU for 52, 84, and 18 weeks, respectively. Two additional baboons (PA6243 and PA5957) were fed BrdU while undergoing 5 days of G-CSF treatment.
Continuous administration of BrdU to baboons
A stock solution of BrdU (Sigma Chemical, St Louis, MO) was prepared in sterile water at a concentration of 20 mg/mL and stored at −20°C in the dark. BrdU was administered continuously to baboons via their drinking water and food in divided doses. The intake of water and biscuits containing BrdU was monitored daily. The animals uniformly ingested the biscuits and water containing BrdU since their alternative food supply was limited. During the period of study, each animal ingested between 280 and 330 mg BrdU per day.
Stem cell mobilization and collection
G-CSF (a gift of Amgen, Thousand Oaks, CA) was administered at a dose of 100 μg/kg as daily subcutaneous injections for 5 days in the lateral aspect of the thighs of 2 additional animals (PA6243 and PA5957). This dose of human recombinant G-CSF has previously been shown to be optimal for the mobilization of baboon hematopoietic stem cells that are capable of reconstituting hematopoiesis in lethally irradiated nonhuman primates.20,21 Starting 2 days prior to the first day of G-CSF administration, animals were fed water and biscuits containing BrdU as described above. Animals were leukapheresed on the fifth day of G-CSF administration by means of a standard protocol.20 21 The leukaphereses were performed with animals under ketamine sedation (10 mg/kg body weight intramuscular) followed by thiopental (25 μg/kg body weight) induction and general oral endotracheal anesthesia (isoflurane, 1% to 2%). A Cobe spectra leukapheresis unit (Cobe, Lakewood, CO) was primed with approximately 180 mL irradiated whole blood obtained from another ABO-compatible baboon blood donor. Venous access for both the draw and return lines was created by a surgical cut down of the right femoral vein where a double-lumen 7 French pediatric hemodialysis catheter (Medcomp, Harleysville, PA) was inserted. Before leukapheresis, each animal was anticoagulated with heparin (50 U/kg administered as an intravenous bolus following cannulation, followed by a continuous intravenous infusion at a rate of 10 U/kg/h). Acid citrate dextrose (ACD-A) (Baxter/Fenwal, Deerfield, IL) was administered as a continuous intravenous infusion at the rate of 1 mL/25 mL whole blood entering the machine. Flow rates of approximately 20 to 25 mL/min were achieved in order to process approximately 3 blood volumes during the period of leukapheresis. The leukapheresis product (LP) was collected into a single plastic blood collection bag (Baxter) containing 20 mL ACD-A. The CD34+ cells in the LP were analyzed for their incorporation of BrdU.
PHSC isolation
Every 2 to 5 weeks, bone marrow (BM) samples were obtained from the iliac crests or humeri of chronically BrdU-fed animals (PA6588, PA6589, PA6644) following ketamine (10 mg/kg) and xylazine (1 mg/kg) sedation. The collection and separation of BM cells, PB cells, and cells within the LP were performed as previously described.7 The heparinized BM, PB, or LPs were diluted 1:8 in phosphate-buffered saline (PBS), and the mononuclear cell fraction was obtained by centrifugation over 60% Percoll (Pharmacia, Uppsala, Sweden) at 500g for 30 minutes at room temperature. CD34+ cells were selected from low-density bone marrow cells (LDBMCs) by magnetic activated cell sorting (Miltenyi Biotec, Auburn, CA) by means of a mouse immunoglobulin (Ig)–M monoclonal antibody (mAb) 12-8 (CD34)23 (a gift of Dr Robert G. Andrews, Fred Hutchinson Cancer Research Center, Seattle, WA) and anti–mouse IgM microbeads (Miltenyi Biotec).
Hoechst/Rhodamine staining and flow-cytometric isolation of the CD34+ cell subpopulations
The fluorescent dyes Hoechst 33342 (Ho) and Rhodamine 123 (Rho) (Molecular Probes, Eugene, OR) were used to obtain subpopulations of CD34+ cells enriched for primitive hematopoietic progenitors.16 24-30 CD34+ cells were suspended at a concentration of 106 cells per milliliter in 0.1 μg/mL Rho and incubated in the dark for 30 minutes at 37°C. The cells were then centrifuged and resuspended in 10 μM Ho and incubated at 37°C for 1 hour in the dark. The cells were washed twice in ice-cold PBS containing 0.2% bovine serum albumin (BSA) (Sigma Chemical) and kept on ice until sorting. Cell sorting was performed by means of a FACSVantage (Becton Dickinson, San Jose, CA). Green fluorescence pulses (Rho) were collected through a fluorescein isothiocyanate (FITC) 530-nm filter, with a bandwidth of 15 nm. UV emissions (Ho) were reflected by a 440 dichroic long pass mirror and collected by a 424DF44 filter. Cells were sorted at a rate of 2000 cells per second and collected in polypropylene tubes containing media with 20% fetal bovine serum (FBS).
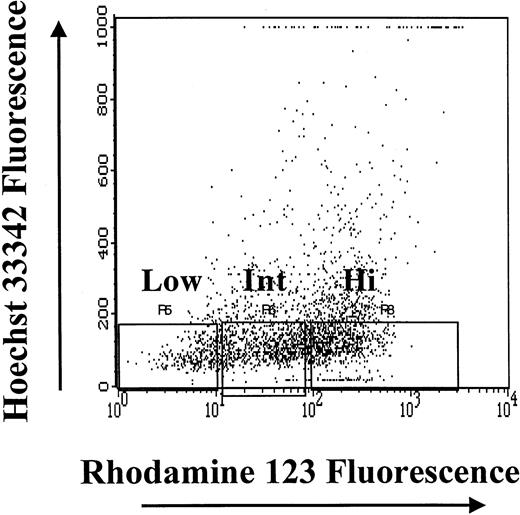
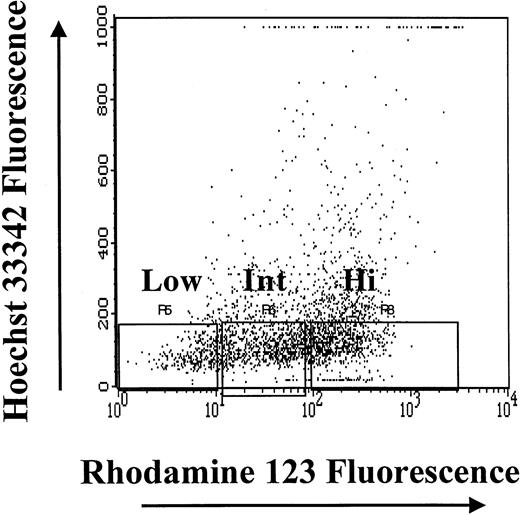
CD34+ cells that had the least amount of DNA (HoLow) were arbitrarily subdivided into Rho low (RhoLow), Rho intermediate (RhoInt), and Rho high (RhoHi) populations depending on Rho fluorescence, as previously described.16,24 25 The purity of magnetic cell–sorted CD34+ cells ranged from 90% to 93%; the CD34+HoLow/RhoLow cells sorted flow-cytometrically were virtually 100% pure. The high degree of purity of the CD34+HoLow/RhoLowcells was achieved by gating out contaminating mature cells on the basis of light-scatter properties and excluding HoInt and HoHi cells (Figure 1). Sufficient numbers of BM cells were not always available to analyze the BrdU content of both CD34+ cells and the CD34+HoLow/RhoLow subpopulation. At such times, analysis of the BrdU content of CD34+HoLow/RhoLow cells was considered a priority, and the available CD34+ cells were devoted to the analysis of the CD34+HoLow/RhoLow cell subpopulation.
Isolation of subpopulations of CD34+ BM cells on the basis of the relative intensity of Hoechst 33342 and Rhodamine 123 fluorescence.
After gating on live cells, the CD34+HoLow/RhoLow, CD34+HoLow/RhoInt, and CD34+HoLow/RhoHi subpopulations were isolated flow-cytometrically.
Isolation of subpopulations of CD34+ BM cells on the basis of the relative intensity of Hoechst 33342 and Rhodamine 123 fluorescence.
After gating on live cells, the CD34+HoLow/RhoLow, CD34+HoLow/RhoInt, and CD34+HoLow/RhoHi subpopulations were isolated flow-cytometrically.
Colony-forming cell assays
Colony-forming cells (CFCs) were assayed under standard conditions in semisolid media as previously described.7Briefly, 1 × 103 to 2 × 103 cells were plated in duplicate cultures containing 1 mL Iscove's modified Dulbecco's medium (IMDM) with 1.1% methylcellulose, 30% FBS, 5 × 10−5 M 2-mercaptoethanol (Methocult, Stem Cell Technologies, Vancouver, BC, Canada), to which was added a cocktail of growth factors, including 100 ng/mL recombinant human stem cell factor (SCF), 100 ng/mL Flt3 ligand, 10 ng/mL interleukin (IL)–3, 10 ng/mL IL-6, 10 ng/mL granulocyte/macrophage colony stimulating factor (GM-CSF) (all purchased from R&D Systems, Minneapolis, MN) and 5 U/mL erythropoietin (a gift of Amgen). The cells were plated into 35-mm tissue-culture dishes (Costar, Corning, NY), and after 14 days' incubation at 37°C in a 100%-humidified atmosphere containing 5% CO2, the colonies were scored with an inverted microscope with the use of standard criteria.7
Cobblestone area-forming cell assays
The ability of primitive hematopoietic cells to form cobblestone areas (CAs) in long-term marrow cultures has been used as an in vitro surrogate PHSC assay.7,31,32 Baboon cobblestone area-forming cells (CAFCs) give rise to colonies composed of undifferentiated, uniformly sized, round, refractile cells arranged in a compact manner when cocultured with murine stromal fibroblasts in the presence of human cytokines for 5 weeks.7 BM CD34+ cells or CD34+HoLow/RhoHi, CD34+HoLow/RhoInt, and CD34+HoLow/RhoLow cells were plated in limiting dilution in flat-bottomed 96-well plates (Costar) onto confluent, irradiated (7000 cGy) monolayers of the murine stromal fibroblast line M2-10B4 (a gift of C. Eaves, Vancouver, BC, Canada). Each well contained 200 μL of a 50:50 mixture of IMDM and RPMI with 10% FBS (Hyclone Laboratories, Logan, UT). A cocktail of growth factors, including 100 ng/mL SCF, 100 ng/mL leukemia inhibitory factor (a gift of Amgen), 50 ng/mL IL-3, 50 ng/mL IL-6, and 50 ng/mL GM-CSF, was added to these assays. The cytokine cocktail has been previously shown in our laboratory to be optimal for the development of baboon CAs (data not shown). The cultures were fed weekly by replacement of one half of the culture volume with fresh medium containing the above cytokines at 2 times the previously defined concentration. After 5 weeks of culture in a humidified incubator at 37°C containing 5% CO2, the number of CAs was scored with an inverted microscope by means of standard criteria.32 The CAFC frequency was computed by means of minimization of chi by regression to the cell number at which 37% of wells were negative for CA formation, with 95% statistical precision.33
Analysis of BrdU incorporation and propidium iodide staining
Analysis of the BrdU content of different subsets of BM cells was performed every 2 to 5 weeks in animals (PA6588, PA6589, and PA6644) continuously fed BrdU. In addition, BM samples from one animal (PA6588) were examined 20 days and then 159 days after the cessation of BrdU administration. Granulocytes and lymphocytes were flow-cytometrically isolated from BM, and their BrdU content was analyzed on the basis of their light-scatter properties. The percentage of BrdU+ cells present in any particular cell subpopulation was flow-cytometrically determined. BrdU incorporation was detected by staining with anti-BrdU mAb conjugated with FITC (Becton Dickinson). The percentage of BrdU+ granulocytes was obtained by subtracting (1) the percentage of granulocytes that nonspecifically bound to the anti-BrdU antibody isolated from a control animal that had not received BrdU from (2) the percentage of BrdU+ BM granulocytes isolated from an animal that had been fed BrdU chronically. The relative mean fluorescence intensity (MFI) of granulocytes and BrdU-pulsed KG1a cells was determined by dividing the geometric mean fluorescence of BrdU-labeled cells by the geometric mean fluorescence of control (BrdU-nonexposed) cells.
The number of CD34+ cells and CD34+HoLow/RhoLow cells that were BrdU+ at any one time provided a history of PHSC replication for that particular animal. BrdU staining was performed as described previously with minor modifications.16 34-36Briefly, cells were fixed in 70% ethanol; to avoid the formation of aggregates, the cell suspension was added dropwise onto 70% ethanol while vortexing and kept on ice for 20 minutes and stored at −20°C in the dark until analysis. During analysis, cells were washed with PBS and treated with 0.1% ribonuclease type 1A (Sigma Chemical) at 37°C for 20 minutes. The DNA was denatured by means of 2N hydrochloric acid containing 0.8% fresh Triton X. The acid was then neutralized with 0.1 M Na2B4O7. The cells were subsequently incubated with 20 μL FITC-conjugated anti-BrdU mAb in a staining solution containing freshly prepared 1% Tween-20 and 0.2% BSA.
KG1a cells (purchased from American Type Culture Collection, Rockville, MD), a human myeloid leukemia cell line in an exponential growth phase that had or had not been pulsed with BrdU (10 μM for 40 minutes at 37°C) in vitro served as positive and negative controls, respectively, during BrdU analysis. In addition, LDBMCs that had not been exposed in vitro to BrdU were similarly stained with anti-BrdU/propidium iodide (PI) to serve as an additional negative control.
The cell cycle status of the BrdU+ cells was determined by PI (Sigma Chemical) staining. To analyze the DNA content of BrdU+ cells, the cells were stained with 5 μg/mL PI. The BrdU quantitation and DNA content of the cells were analyzed with a FACSCalibur (Becton Dickinson) by means of Cell Quest software (Becton Dickinson). To analyze only the cell cycle status without BrdU labeling, CD34+ cells isolated either from PB, LP, or the BM of baboons were fixed and stained with PI to determine the percentage of cells at different phases of the cell cycle. Data files containing forward- and side-scatter peak signals as well as width and areas of the PI signal were collected. Doublet events were excluded by gating on the PI signal-width channel. The cell cycle analysis was performed with ModFit LT software (Verity Software House, Topham, ME), and at least 20 000 events were collected for analysis of each sample.
Results
Isolation of subpopulations of baboon BM CD34+HoLow cells by intensity of Rho 123 fluorescence
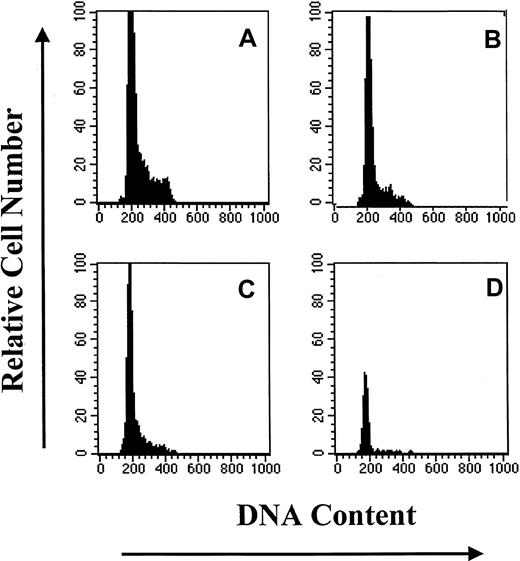
Subpopulations of primitive hematopoietic progenitor cells were isolated flow-cytometrically from CD34+HoLow BM cells on the basis of Rho fluorescence (Figure 1). The CD34+HoLow/RhoLow cell population was rare in steady-state BM, representing only 10.4% ± 0.3% (mean ± SE) of the CD34+ selected cells from 12 different samples from 3 animals. The CD34+HoLow/RhoInt and CD34+HoLow/RhoHi cells composed 19.9% ± 2.4% (mean ± SE) and 28.2% ± 3.6% (mean ± SE), respectively, of the total CD34+ cells. The remaining 41.5% ± 4.9% of CD34+ cells were HoHi-HoInt and were more mature cells with a greater quantity of DNA. An analysis of the cell cycle status of CD34+ cells and their subpopulations was performed. In Figure 2, a representative analysis is shown. The CD34+ cell population was largely quiescent with more than 85% of the cells in G0/G1. Increasing numbers of cells resided in G0/G1 as Rho fluorescence diminished, with approximately 96% of CD34+HoLow/RhoLow cells in G0/G1. On the basis of light-scatter properties and Ho/Rho fluorescence, the CD34+HoLowRhoLow cells were judged to be devoid of mature cells and 100% pure. These data directly demonstrate that the CD34+ cell compartment is hierarchically ordered on the basis of quiescence during steady-state conditions.
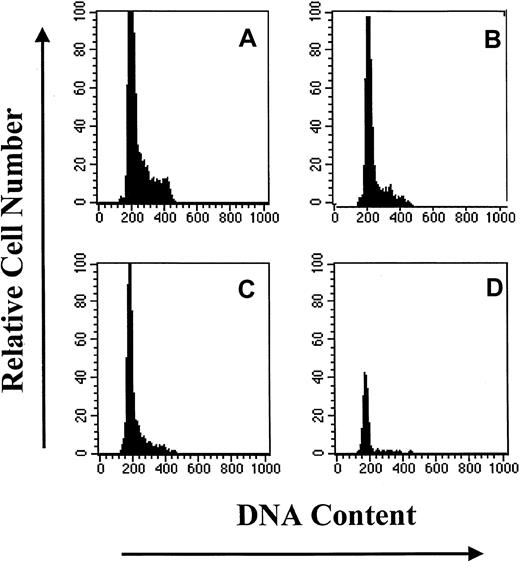
DNA histograms of subpopulations of CD34+ BM cells based upon PI staining.
Similar results were obtained in 2 additional experiments. (A) CD34+. (B) CD34+HoLow/RhoHi. (C) CD34+HoLow/RhoInt. (D) CD34HoLow/RhoLow.
DNA histograms of subpopulations of CD34+ BM cells based upon PI staining.
Similar results were obtained in 2 additional experiments. (A) CD34+. (B) CD34+HoLow/RhoHi. (C) CD34+HoLow/RhoInt. (D) CD34HoLow/RhoLow.
Cloning efficiency of CD34+ stem cell subpopulations
Progenitor cell and CAFC assays were used to determine the proliferative capacity of each of the sorted cell populations. Although each fraction contained significant numbers of progenitor cells and CAFCs (Table 1), the progenitor cell cloning efficiency and CAFC numbers decreased with increasing Rho fluorescence.
In vivo turnover of CD34+HoLow/RhoLow BM cells
To determine whether CD34+HoLow/RhoLow cells were dormant or cycled rather slowly during steady-state conditions, BrdU incorporation was monitored over a 21-month period. Hematopoietic cell production was assessed during the period of BrdU administration to detect any deleterious effects of BrdU on hematopoiesis. White blood cell and platelet counts and hematocrit levels of the animals receiving BrdU remained within a normal range throughout the period of study, indicating a lack of documentable hematopoietic toxicity (data not shown).
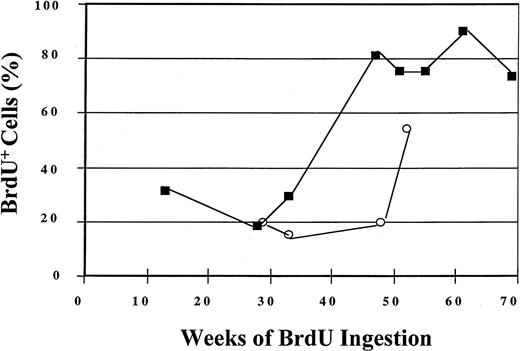
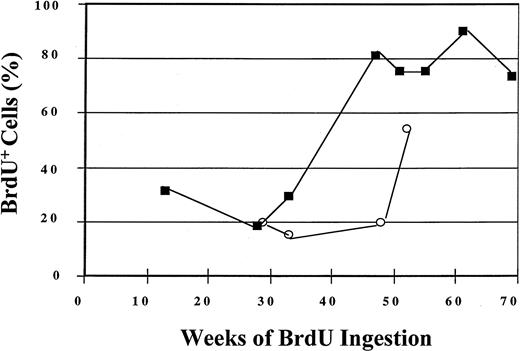
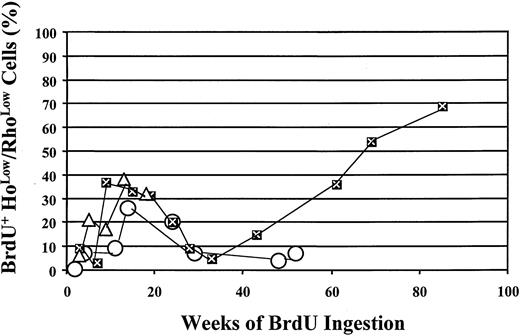
During the year-long period of BrdU labeling, 56% of CD34+cells in one animal (PA6588) and 84% of CD34+ cells in another animal (PA6589) underwent self-replication as assessed by BrdU labeling (Figure 3). The replicative history of CD34+ BM cells is shown for 52 and 69 weeks for the 2 animals, respectively, in Figure 3. The labeling of the most primitive subpopulation of CD34+ cells is shown in Figure4. Within 11 weeks of BrdU feeding, only 9% of the CD34+HoLow/RhoLow cells in one of the animals (PA6588) had become BrdU+ while 37% of the CD34+HoLow/RhoLow cells in the other animal (PA6589) were BrdU labeled. Although continued BrdU feeding of one animal (PA6588) never resulted in an increase in the number of BrdU+CD34+HoLow/RhoLow cells beyond 26%, continued feeding of BrdU to the second animal (PA6589) led to increasing numbers of BrdU-labeled CD34+HoLow/RhoLow cells so that by week 85, about 70% of this rare cell population had become BrdU+, indicative of PHSC cycling at an extremely slow rate (Figure 4). A third baboon (PA6644) received BrdU for a shorter period of time, and 32% of the CD34+HoLow/RhoLow cells were labeled BrdU by 18 weeks (Figure 4). The pattern of BrdU labeling was similar but distinct for each of the animals studied. Such differences probably represent normal biological variations.
Labeling of BM CD34+ cells by BrdU after 52 and 69 weeks of BrdU ingestion by 2 baboons.
BM CD34+ cells were analyzed for BrdU labeling by means of anti-BrdU/PI staining and flow-cytometric detection. The open circles represent data obtained from baboon PA6588, and the closed squares represent data obtained from baboon PA6589.
Labeling of BM CD34+ cells by BrdU after 52 and 69 weeks of BrdU ingestion by 2 baboons.
BM CD34+ cells were analyzed for BrdU labeling by means of anti-BrdU/PI staining and flow-cytometric detection. The open circles represent data obtained from baboon PA6588, and the closed squares represent data obtained from baboon PA6589.
Incorporation of BrdU by the CD34+Holow/RhoLow cells after prolonged BrdU administration.
Open circles represent data obtained from baboon PA6588, while closed squares with the “X” indicate data obtained from baboon PA6589, and open triangles represent the data from PA6644. CD34+HoLow/RhoLow cells were isolated flow-cytometrically and analyzed for BrdU incorporation by means of anti-BrdU/PI staining. The percentage of BrdU+cells is plotted against the number of weeks of BrdU ingestion, at the end of which time BM aspirates were obtained.
Incorporation of BrdU by the CD34+Holow/RhoLow cells after prolonged BrdU administration.
Open circles represent data obtained from baboon PA6588, while closed squares with the “X” indicate data obtained from baboon PA6589, and open triangles represent the data from PA6644. CD34+HoLow/RhoLow cells were isolated flow-cytometrically and analyzed for BrdU incorporation by means of anti-BrdU/PI staining. The percentage of BrdU+cells is plotted against the number of weeks of BrdU ingestion, at the end of which time BM aspirates were obtained.
To determine whether these cycling progenitor cells were responsible for active blood cell production, the BrdU content of BM granulocytes was repeatedly analyzed. Since granulocytes are derived from relatively rapidly cycling granulocytic precursors and survive only a few hours, we believed that BrdU labeling would also serve as an indicator of in vivo BrdU saturation.37 Within 1 to 2 weeks of BrdU ingestion, more than 85% to 90% of the granulocytes in the BM had become BrdU+. Similar degrees of labeling of PB granulocytes with BrdU were observed (at week 15, in PA6644, 93% of BM granulocytes were BrdU+ and 98% of PB granulocytes were BrdU+). This similarity of effect of BM and PB granulocytes is not surprising since the transit time of BM granulocytes to the PB has been calculated to be 2 to 3 days).37 38 On an average, 85% to 90% of granulocytes were labeled with BrdU throughout the period of study (Figure5A).
Incorporation of BrdU by BM granuloctyes and its persistence after ingestion has stopped.
(A) BrdU incorporation by BM granulocytes following prolonged administration of BrdU in vivo. BM granulocytes were isolated from chronically BrdU-fed animal at different time points and analyzed for their BrdU incorporation flow-cytometrically by means of an anti-BrdU mAb conjugated with FITC and PI. The open circles indicate data obtained from one unperturbed animal (PA6588); the closed squares indicate data from a second baboon (PA6589). (B) Persistence of BrdU+ granulocytes following the cessation of BrdU ingestion. Granulocytes were isolated from one of the animals who had been continuously fed BrdU for 52 weeks, and the percentage of BrdU+ granulocytes on the day of cessation of BrdU feeding is indicated as Day-0. Samples were obtained 20 days and 159 days after withdrawal of BrdU. Granulocytes from both of these samples were isolated and analyzed for their BrdU content. The bars represent the percentage of granulocytes that were BrdU+ on the day BrdU feeding was halted, as well as 20 days and 159 days after withdrawal of BrdU.
Incorporation of BrdU by BM granuloctyes and its persistence after ingestion has stopped.
(A) BrdU incorporation by BM granulocytes following prolonged administration of BrdU in vivo. BM granulocytes were isolated from chronically BrdU-fed animal at different time points and analyzed for their BrdU incorporation flow-cytometrically by means of an anti-BrdU mAb conjugated with FITC and PI. The open circles indicate data obtained from one unperturbed animal (PA6588); the closed squares indicate data from a second baboon (PA6589). (B) Persistence of BrdU+ granulocytes following the cessation of BrdU ingestion. Granulocytes were isolated from one of the animals who had been continuously fed BrdU for 52 weeks, and the percentage of BrdU+ granulocytes on the day of cessation of BrdU feeding is indicated as Day-0. Samples were obtained 20 days and 159 days after withdrawal of BrdU. Granulocytes from both of these samples were isolated and analyzed for their BrdU content. The bars represent the percentage of granulocytes that were BrdU+ on the day BrdU feeding was halted, as well as 20 days and 159 days after withdrawal of BrdU.
To further test the contribution of cycling progenitor cells and/or PHSCs to blood cell production, BrdU administration was halted after 52 weeks in one of the animals (PA6588). At the time of BrdU withdrawal, 96% of granulocytes had become BrdU+. BM samples were obtained at 20 days and 159 days after the withdrawal of BrdU. It was anticipated that after this period of time, free BrdU capable of labeling newly divided progenitor cells would no longer be present.22 After 20 days of BrdU withdrawal, 89% of the granulocytes were BrdU+, while 71% of the granulocytes remained BrdU+ 159 days after the cessation of BrdU ingestion (Figure 5B). These findings suggest that the previously labeled fraction of PHSCs had an extensive proliferative capacity and was capable of contributing to active blood cell production for sustained periods of time. At 159 days after the withdrawal of BrdU, a significant number (29%) of BrdU− granulocytes were detected (Figure 6C). These BrdU− granulocytes were probably the product of the proliferation and differentiation of PHSCs and progenitor cells that cycled following the interuption of the prolonged period of BrdU feeding.
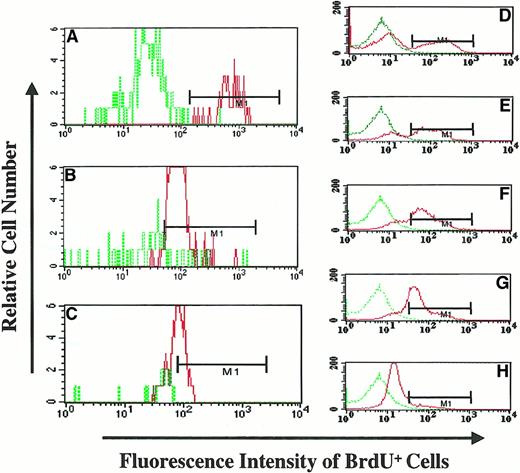
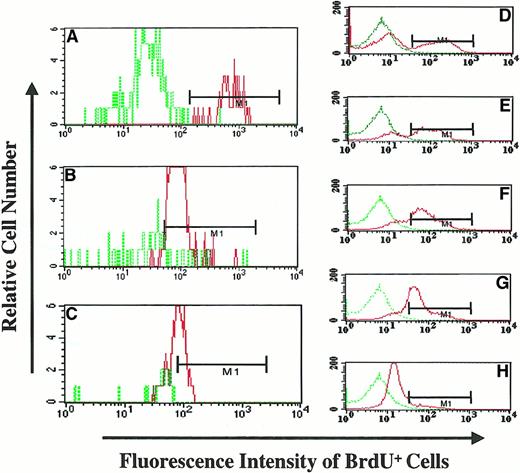
BrdU fluorescence intensity of granulocytes following withdrawal of BrdU feeding (PA6588).
(A) (B) (C) The BrdU fluorescence of BM granulocytes from the animal at different time points following withdrawal of BrdU (Day-0, panel A; Day-20 panel B; and Day 159, panel C) was analyzed flow-cytometrically. The MFI was determined by dividing the geometric mean fluorescence of BrdU-labeled granulocytes by the geometric mean fluorescence of control (BrdU nonexposed) granulocytes. Dotted green line histograms represent the MFI of granulocytes from BM of an animal not exposed to BrdU; continuous red line histograms indicate the MFI of granulocytes from the BrdU-fed animal following the withdrawal of BrdU. (D) (E) (F) (G) (H) The MFI of KG1a cells exposed to BrdU for 9 hours and cultured for an additional 4 days in the absence of BrdU. Dotted green line histograms represent the MFI of KG1a cells not pulsed with BrdU (BrdU−), and continuous red line histograms represents the MFI of BrdU-pulsed KG1a cells at different time points. The relative decrease of MFI of KG1a cells was used to determine the number of cell divisions that KG1a cells underwent during this time period; this was done by analyzing the decrease in MFI.
BrdU fluorescence intensity of granulocytes following withdrawal of BrdU feeding (PA6588).
(A) (B) (C) The BrdU fluorescence of BM granulocytes from the animal at different time points following withdrawal of BrdU (Day-0, panel A; Day-20 panel B; and Day 159, panel C) was analyzed flow-cytometrically. The MFI was determined by dividing the geometric mean fluorescence of BrdU-labeled granulocytes by the geometric mean fluorescence of control (BrdU nonexposed) granulocytes. Dotted green line histograms represent the MFI of granulocytes from BM of an animal not exposed to BrdU; continuous red line histograms indicate the MFI of granulocytes from the BrdU-fed animal following the withdrawal of BrdU. (D) (E) (F) (G) (H) The MFI of KG1a cells exposed to BrdU for 9 hours and cultured for an additional 4 days in the absence of BrdU. Dotted green line histograms represent the MFI of KG1a cells not pulsed with BrdU (BrdU−), and continuous red line histograms represents the MFI of BrdU-pulsed KG1a cells at different time points. The relative decrease of MFI of KG1a cells was used to determine the number of cell divisions that KG1a cells underwent during this time period; this was done by analyzing the decrease in MFI.
In an attempt to quantitate the number of cell divisions required to result in the reduction of MFI, we used, as a model system, KG1a cells, which divide in a predictable fashion. KG1a cells were pulsed for 9 hours, washed, and then cultured in the absence of BrdU for 4 days. Aliquots of cells were analyzed to determine the MFI and the percentage reduction of BrdU+ cells from day 0 to day 4. The relative MFI of BrdU-pulsed cells was determined by dividing the MFI of individual samples by the MFI of a negative control (KG1a cells not pulsed with BrdU). The doubling time of the KG1a cells was measured during their exponential growth phase and was determined to be approximately 22.5 hours. A gradual reduction of the relative MFI (2.5-fold) was observed in BrdU-pulsed KG1a cells over 4 days (Figure 6D-H). Thus, the reduction in relative MFI was estimated to be the result of KG1a cells undergoing approximately 4 cell divisions. We then applied the data generated with KG1a cells to our in vivo baboon model in order to estimate the number of cell divisions required for the reduction of MFI in granulocytes following the withdrawal of BrdU. Following the withdrawal of BrdU (20 days and 159 days), the relative MFI of granulocytes was reduced approximately 9.9-fold and 14.5-fold, respectively, as compared with granulocytes on the day BrdU was halted (Figure 6A-C). This fold reduction of BrdU fluorescence corresponded to a minimum of 15.8 cell divisions (20 days after withdrawal) and 23 cell divisions (159 days after withdrawal) required to achieve the observed reduction in BrdU fluorescence. These findings are a reflection of the extensive proliferative capacity of the PHSCs and progenitor cells previously labeled by long-term BrdU administration.
Perturbation of PHSC quiescence by in vivo growth factor administration
G-CSF administration results in mobilization of PHSCs into the PB.21,39-43 Stem cell mobilization has been reported to be preceded by PHSC cycling within the BM.18 To test whether the quiescence of PHSCs observed in steady-state BM could be altered by perturbations in the BM microenvironment, G-CSF was administered daily to 2 additional adult baboons (PA6243 and PA5957). During the 5-day period of G-CSF administration, baboons were continuously fed BrdU. Both the LP and BM were analyzed for the presence of BrdU+cells. Surprisingly, after 5 days of BrdU ingestion, in animals receiving G-CSF (PA5957, PA6243), 66% and 52% of the CD34+ cells, respectively, and 23% and 11% of the CD34+HoLow/RhoLow cells in the LP, respectively, had become BrdU+ (Figure7). Similarly, when the cytokine-primed BM from one of the animals (PA6243) was analyzed after 5 days of G-CSF administration, 32% of BM CD34+ cells were found to be BrdU+ (data not shown). This contrasts with the longer time required to achieve a similar degree of BrdU labeling of CD34+ or CD34+HoLow/RhoLow cells during steady-state conditions (Figures 3 and 4). To exclude the possibility that any alteration of the phenotype of PHSCs occurred as a consequence of G-CSF administration, we determined the CAFC content of G-CSF mobilized PB CD34+HoLow/RhoHi, CD34+HoLow/RhoInt, and CD34+HoLow/RhoLow cells from both of these animals (PA5957 and PA6243). The number of CAFCs assayed from similar phenotype subpopulations of mobilized PB and BM were compared. The mobilized PB CD34+HoLow/RhoLowcells contained 46 ± 7 CAFCs per 104 cells plated; CD34+HoLow/RhoInt contained 28 ± 23 CAFCs per 104 cells plated, whereas CD34+HoLow/RhoHi cells contained 5 ± 0.5 CAFCs per 104 cells plated. These numbers of mobilized PB CAFCs present were comparable to the number assayed from the same phenotype subpopulations isolated from BM (Table 1). Prior to and following G-CSF administration, the fraction of BM and PB CD34+ cells engaged in active DNA synthesis (S/G2/M) phase was determined.
Incorporation of BrdU by various subsets of primitive hematopoietic cells following G-CSF mobilization.
Low-density cells from the leukapheresis products were enriched for CD34+ cells, and the CD34+Ho/Rho subpopulations were isolated flow-cytometrically. Cells from these various subpopulations were analyzed for their BrdU content flow-cytometrically by means of anti-BrdU/PI staining. Each bar represents data derived from the indicated population of cells in each of 2 animals. ░ indicates PA 5957, ▨ indicates PA6243.
Incorporation of BrdU by various subsets of primitive hematopoietic cells following G-CSF mobilization.
Low-density cells from the leukapheresis products were enriched for CD34+ cells, and the CD34+Ho/Rho subpopulations were isolated flow-cytometrically. Cells from these various subpopulations were analyzed for their BrdU content flow-cytometrically by means of anti-BrdU/PI staining. Each bar represents data derived from the indicated population of cells in each of 2 animals. ░ indicates PA 5957, ▨ indicates PA6243.
Prior to the administration of G-CSF, 19% of CD34+ cells in BM were in the S/G2/M phase of the cell cycle, while after 5 days of G-CSF administration, a similar proportion of BM CD34+ cells remained in S/G2/M (15%) (Table2). By contrast, the CD34+cells in the LP following G-CSF mobilization were largely quiescent, with more than 98% of the LP CD34+ cells remaining in G0/G1 (Table 2). The absolute number of CD34+ cells in the BM at day 0 was 87 CD34+cells per microliter, and following G-CSF mobilization, it was increased to 449 CD34+ cells per microliter. At day 0, on the other hand, 1.2 CD34+ cells per microliter were present in the PB; these increased to 148 CD34+ cells per microliter following G-CSF administration. Thus, a 5-fold increase in the absolute number of CD34+ cells in the BM and a 123-fold increase in the absolute number of CD34+ cells in the mobilized PB were observed. These findings indicate that G-CSF treatment induces a fraction of primitive hematopoietic progenitor cells to enter cell cycle prior to the mobilization. Following egress into the PB, the vast majority of these progenitor cells re-enter a quiescent phase.
Discussion
Our data clearly demonstrate in nonhuman primates that the primitive hematopoietic stem cell compartment remains largely dormant at any one time during steady-state conditions but that these cells do indeed cycle over a relatively long period of time. During the period of study, the fraction of CD34+ cells that were BrdU+ was consistently greater than the CD34+HoLow/RhoLow population. These findings are consistent with the presence of greater numbers of less quiescent, more differentiated progenitor cells in the CD34+ cell population and the enrichment for more quiescent stem cells in CD34+HoLow/RhoLowsubpopulation. Although no hematological toxicity was observed in the animals receiving BrdU for several months, it remains possible that the increased incorporation of BrdU over time is a response to BrdU toxicity. Although this possibility is unlikely, it cannot be excluded.
Since more than 90% of the granulocytes from these animals were BrdU+ during the observation period, we conclude that the cycling PHSCs or their progenitor cells contribute to active steady-state blood cell production. The continued production of BrdU+ granulocytes 159 days after the cessation of BrdU feeding further suggests that the previously labeled primitive hematopoietic cells continue to contribute to hematopoiesis for several months. The persistent production of BrdU+ granulocytes is the result of the extensive number of cell divisions that the previously labeled PHSCs and progenitor cells were estimated to have undergone following BrdU withdrawal.
We have determined that progressively greater numbers of PHSCs do indeed cycle over time. Since we were limited by the number of animals that could be studied, we were unable to calculate the actual doubling time of CD34+HoLow/RhoLow cells. However, the period required for the majority of PHSCs to cycle does appear to be considerably longer than that previously reported in rodents.15-17 The differences in the behavior of rodent and primate PHSCs appear to be primarily a function of the time required for the stem cell compartment to cycle. This slow cycling behavior of baboon PHSCs is in agreement with the data generated by other researchers in other large animal models, including cats and rhesus macaques, and in humans.13,19,44-48 In particular, on the basis of measurements of telomere shortening in humans, Rufer et al44 have previously reported that a rapid expansion of stem cells occurs early in life, followed by a marked decrease in the rate of stem cell divisions in the years that follow. They concluded that 15 to 30 stem cell divisions occur during the first half-year of life followed by less than 1 stem cell division per year in following years. Our finding of slow-cycling PHSCs in adult baboons is consistent with the time frame observed by Rufer and coworkers.44
In this study, the dormant state of the majority of PHSCs in baboons could be dramatically altered by the administration of an external stimulus, G-CSF. As expected, G-CSF resulted in a significant increase in the absolute numbers of CD34+ cells in both BM and PB. Almost 60% of the CD34+ cells and 17% of the CD34+HoLow/RhoLow cells in the LP were BrdU+, suggesting that they had divided prior to their mobilization into the PB. More than 10 weeks were required to achieve a similar degree of BrdU labeling of BM CD34+HoLow/RhoLow cells in animals that had not received G-CSF. The percentage of cycling cells (S/G2/M) in BM at any given time was not increased following G-CSF administration, suggesting that cycling CD34+ cells exit from the BM. Since virtually all of the LP CD34+ cells were in G0/G1, we conclude that the CD34+ cells, which had cycled prior to their exit from the BM, had returned to a quiescent state following their mobilization. These findings are in agreement with previous reports.16,49-51 Since not all LP CD34+ cells and CD34+HoLow/RhoLow cells were BrdU+, BM stem cell cycling appears, in the baboon at least, not to be an absolute requirement for G-CSF mobilization of PHSCs.52 The increased stem cell cycling observed following G-CSF administration suggests that the dormancy status of PHSCs can be modified by extrinsic stimuli. However, our data do indicate that only a small fraction of PHSCs responds to this short course of G-CSF. Whether this proportion would increase with a longer course of G-CSF administration requires further investigation.
The intrinsic behavior patterns of baboon and rodent PHSCs are probably genetically determined and might underlie the barriers that have been encountered during attempts to expand PHSCs ex vivo and/or genetically modify large animal PHSCs as compared with rodents.53-58 Although studies of murine hematopoiesis have produced important insights into our fundamental understanding of mammalian blood cell production, the present studies indicate that many of the conclusions derived from rodent studies may not be directly extrapolated to descriptions of the behavior of PHSCs in larger species. Important species-dependent differences in the patterns of PHSC cycling require one to limit conclusions about PHSC behavior to the strain or species in which the data are generated. Whether the differences in stem cell kinetics in large animals as compared with mice represents an evolutionary adaptation to increased size and longevity remains the subject of speculation. The increased numbers of dividing stem cells observed following G-CSF administration, however, may be representative of a variety of potential extrinsic stimuli that might alter PHSC behavior.
The behavior of PHSCs during steady-state hematopoiesis and their response to microenvironmental stimuli reported here are best portrayed by a stem cell model in which most PHSCs normally cycle very slowly but remain capable of responding to a variety of environmental stimuli by accelerating this process. Since a cellular hierarchy of PHSCs has been previously reported and also documented here,59-63 it remains possible that the fraction of PHSCs that do not cycle during steady-state conditions or following G-CSF administration might represent the most primitive subpopulation of quiescent PHSCs. Such a model of hematopoiesis, in which there are quiescent and activated PHSCs within the stem cell compartments, has previously been described by Killmann64 (the sleeper-to-feeder stem cell hypothesis). The relevance of this model to large animal steady-state hematopoiesis will require further study.
We thank Manuel B. Borce and Matthew Heckler for their excellent assistance in the administration and daily monitoring of BrdU feeding of the animals; Drs Yi-Hsiang Chen, Amittha Wickrema, and John E. Brandt for their critical reading of the manuscript; Paul A. Weiss for his technical support in FACSVantage sort; and Elen Rosler for her assistance in long-term stem cell culture. N.M. was awarded a post-doctoral fellowship from W. M. Keck Foundation.
C.S. was supported partially by the Pillsburg Foundation and the Living Institute for Surgical Studies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ronald Hoffman, Hematology/Oncology Section, University of Illinois College of Medicine, 900 South Ashland Ave, Chicago, IL 60607-7173; e-mail: ronhoff@uic.edu.