The rapid recovery of hematopoiesis after allogeneic blood stem cell transplantation has been attributed to the quality and quantity of hematopoietic progenitors in the blood stem cell grafts from filgrastim-stimulated donors. To determine whether further stimulation with filgrastim after transplantation would affect hematopoietic recovery, a prospective, randomized, controlled study was performed. Forty-two adult recipients of allogeneic blood stem cells from human leukocyte antigen-matched related donors were randomized to receive 10 μg/kg per day filgrastim subcutaneously from day 1 through neutrophil recovery or no growth factor support after transplantation. There was no significant difference between the 2 groups in the number of CD34+ cells infused (median, 4.8 vs 4.3 × 106/kg). Graft-versus-host (GVHD) disease prophylaxis consisted of tacrolimus and steroids for 9 patients and tacrolimus and minimethotrexate for 33 patients. The group receiving filgrastim had a shorter time to neutrophil levels greater than 0.5 × 109/L (day 12 vs day 15, P = .002) and to neutrophil levels greater than 1.0 × 109/L (day 12 vs day 16, P = .01). The filgrastim group also had a trend for earlier discharge (day 16 vs 20, P = .05). There was no significant difference between the groups in time to platelet recovery, number of transfusions, regimen-related toxicity, infection, incidence of GVHD, relapse, survival, or hospital charges. It can be concluded that the administration of filgrastim after allogeneic blood stem cell transplantation shortens the time to neutrophil recovery.
Introduction
Serious and life-threatening infectious complications after marrow or blood stem cell transplantation have been attributed to prolonged neutropenia induced by the myeloablative regimen and to neutrophil dysfunction in the early phase after transplantation.1,2 Hematopoietic growth factors have been evaluated to ameliorate these complications. In preclinical studies, recombinant human granulocyte–colony-stimulating factor (rhG-CSF), such as filgrastim or lenograstim, stimulated hematopoietic proliferation and neutrophil differentiation, up-regulated adhesion molecule expression by neutrophils, and increased neutrophil chemotaxis, phagocytosis, and intracellular killing.3 In clinical studies of filgrastim or lenograstim after autologous marrow or blood stem cell transplantation, the time to neutrophil recovery was shortened significantly, and there was a reduction in days of fever or antibiotic use and in length of hospital stay in the group receiving the growth factor.4-10
The safety and activity of filgrastim have also been evaluated in recipients of allogeneic marrow transplants. In phase 1 and 2 studies, filgrastim has been used at doses of 2 to 20 μg/kg per day after allogeneic marrow transplantation, with mild bone pain at the highest dose level the only significant toxicity noted.11-13 Phase 2 studies with comparison to historical controls have suggested a beneficial effect of filgrastim or lenograstim on neutrophil recovery after allogeneic marrow transplantation, and no adverse effects on acute graft-versus-host disease (GVHD) or relapse were reported.14-19 These results were confirmed on subset analysis of phase 3 studies of lenograstim after marrow transplantation4,5 and in a preliminary report of a controlled trial of filgrastim for recipients of allogeneic marrow transplants.20
Use of filgrastim-mobilized blood stem cells rather than marrow from human leukocyte antigen (HLA)-identical donors has been associated with a shorter time to neutrophil recovery after transplantation by some21-23 but not all24,25 investigators. Rapid neutrophil recovery after allogeneic blood stem cell transplantation has been attributed to the higher number of CD34+ cells and the higher proportion of late committed progenitors in the stem cells in comparison to marrow allografts,26 and, as a result, some have suggested that the administration of hematopoietic growth factors after transplantation would not alter the rate of engraftment in these patients.27 We therefore conducted a randomized, controlled study to determine whether filgrastim administered after allogeneic blood stem cell transplantation shortened the time to neutrophil recovery.
Patients, materials, and methods
Patients
Forty-two adults were randomized in a prospective, controlled trial of filgrastim for the acceleration of hematopoietic recovery after HLA-matched blood stem cell transplantation at the M. D. Anderson Cancer Center. Inclusion criteria were that the patient have histologically proven malignancy or hematologic disorder appropriate for transplantation using a myeloablative preparative regimen; physiologic age 16 to 55 years; HLA-matched related donor; negative status for pregnancy; Zubrod performance status 1 or lower; life expectancy not severely limited by concomitant illness; adequate organ function for transplantation; no evidence of chronic active hepatitis or cirrhosis and more than 1 month since previous episode of hepatitis; no effusion or ascites of 1 L or more before drainage; no active infection; seronegative for human immunodeficiency virus; and patient or guardian able to sign informed consent. Patients who underwent minitransplantation or T-cell–depleted transplantation procedures were excluded. The protocol was approved by the Institutional Review Board, and written informed consent was obtained from each participant.
Filgrastim administration
Patients randomized to the filgrastim arm received 10 μg/kg per day filgrastim subcutaneously from day 1 after transplantation through the first day the absolute neutrophil count (ANC) was 1.0 × 109/L or greater and for a maximum of 28 days. Patients randomized to the control arm were to receive no growth factors before neutrophil recovery.
Blood stem cell collection and processing
Transplantation and supportive care
Patients received total body irradiation–based, busulfan-based, or carmustine-based myeloablative preparative regimens. The first 9 patients received tacrolimus and steroids to prevent GVHD. Because of a concern regarding fungal infections with the use of steroids, the protocol was revised to use tacrolimus and micromethotrexate (5 mg/m2 methotrexate intravenously on days 1, 3, and 6) thereafter. Details of the GVHD prophylaxis regimens, standard supportive care, prophylactic antibiotics, and transfusion support have been published.24 29 Patients were monitored prospectively for toxicity and GVHD.
Study definitions
The primary end point was time to neutrophil recovery, defined as the first of 3 consecutive days on which the ANC exceeded 1.0 × 109/L. Platelet recovery was defined as the day on which the platelet count exceeded the target number (20 or 50 × 109/L) with no platelet transfusions the following week. Transfusions were recorded as platelet transfusions (pooled or single-donor) or red blood cell (RBC) units administered from the day of transplantation to day 100 or death, whichever was earlier. The average number of transfusions per day was calculated by dividing the total number of transfusions by the number of days the patient was alive between day 0 and day 100. Early regimen-related toxicity (RRT) was graded according to the Seattle criteria,30 and GVHD was graded according to the consensus criteria.31Treatment-related mortality (TRM) was defined as death from any cause other than proximate relapse. Hospital charges were taken from actual billing records for day 0 to the day of discharge. Complete charges were unavailable for the 2 patients with the longest hospital stays. Estimates of missing charges for these patients were based on the services documented in the medical records and actual charges for similar services during subsequent days of hospitalization. Room charges and filgrastim charges were calculated based on rates at the time of transplantation.
Statistical considerations
The study was designed as a randomized, controlled trial with a 2-sided type 1 error of 0.05 and 85% power to detect a 4-day decrease in median time to ANC greater than 1.0 × 109/L. Because it could not be assumed that recovery times were normally distributed, a planned sample size of 60 was determined from computer simulations using historical data. Stopping criteria included grades 3 to 4 RRT greater than 20% or day 100 TRM greater than 20%. Analysis of the first 40 patients with 100-day follow-up revealed excess TRM in the control arm, and the trial was terminated. At the time of termination, 42 patients had been randomized. Systemic infection developed in one patient in the control arm early after transplantation, and he was given 5 μg/kg per day filgrastim beginning on day 5. The final study evaluation was performed on an intent-to-treat basis; hence, this patient remained in the control arm for the analysis. At the time of analysis, the median time from transplantation was 24 months (range, 8 to 48 months).
Interval data are reported as median and range. Censored data are reported as the Kaplan-Meier estimate and 95% confidence intervals, and comparisons between treatment groups were made using the log-rank test. Comparisons of times to neutrophil or platelet recovery were stratified for CD34+ cell dose above or below the median. There was no significant correlation between the CD34+ cell dose and total nucleated cell dose, nor was total nucleated cell dose predictive of hematopoietic recovery. Therefore, comparison of recovery times was not adjusted for total nucleated cell dose. Results are presented graphically by means of censored box plots32 in which quartiles are based on Kaplan-Meier estimates of the respective distributions. The Mann-Whitney U test was used to compare independent samples on an interval scale (age, graft characteristics, transfusions, hospital charges), and the χ2 test or Fisher exact test was used for categorical data (demographics, infections, RRT, and TRM). Two-sided P < .05 was considered significant.
Results
Study participants
Forty-two patients whose median age was 40 years (range, 15-52 years) were randomized and treated. There were no significant differences in baseline characteristics between the 2 groups, though the study arm had more patients with refractory disease and more cytomegalovirus-seronegative patient-donor pairs (Table1). Median age of the donors was 39 years (range, 8-56 years). One donor was the son of the recipient, and the other donors were siblings of the recipients.
Engraftment
Blood stem cell grafts contained a median 7.0 × 108TNC/kg and 4.7 × 106 CD34+ cells/kg. The median TNC dose was significantly higher for the control arm, but both groups received similar CD34+ cell doses (Table 1). The study group received filgrastim for a median 11 days after transplantation (range, 6-25 days). Life-threatening septic shock developed in one patient in the control arm shortly after transplantation, and he was given filgrastim starting on the fifth day. His ANC was 0.5 × 109/L or greater on day 18; neutropenia then recurred, and he died of graft failure and infection on day 31. All other evaluable patients achieved neutrophil recovery.
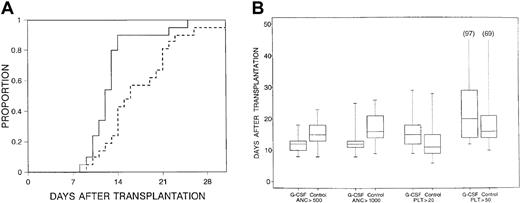
Median times to neutrophil recovery were 13 days (range, 8-23 days) after transplantation for an ANC 0.5 × 109/L or greater and 13 days (range, 8-31+ days) for an ANC 1.0 × 109/L or greater for all patients. Patients in the filgrastim arm had a significantly shorter time to neutrophil recovery (Figure1), even when adjusted for CD34+ cell dose (Table 2). For the subset of patients receiving methotrexate for GVHD prophylaxis, use of filgrastim was still associated with more rapid engraftment (P = .005) (Table 2).
Time to neutrophils.
(A) Time to neutrophils greater than 1.0 × 109/L for patients receiving filgrastim after tranplantation (solid line) versus those in control arm (dashed line) (P = .01). (B) Censored box plots for hematopoietic recoveries in the filgrastim (G-CSF) and control arms.
Time to neutrophils.
(A) Time to neutrophils greater than 1.0 × 109/L for patients receiving filgrastim after tranplantation (solid line) versus those in control arm (dashed line) (P = .01). (B) Censored box plots for hematopoietic recoveries in the filgrastim (G-CSF) and control arms.
Median times to platelet recovery were 13 days (range, 6-54+ days) for a platelet count of 20 × 109/L or greater, and 17 days (range, 10-100+ days) for a platelet count of 50 × 109/L or greater. There was a trend for more rapid recovery of platelets (P = .06 to platelets 20 × 109/L or greater), fewer platelet transfusions (P = .18), and fewer platelet transfusions per day (P = .20) in the control arm (Table 2). However, no trend was observed when only ABO-compatible pairs were assessed for platelet recovery and transfusion. There was no difference between study arms in RBC transfusions.
Infections
No significant differences occurred between study arms in the number of days on broad-spectrum antibiotics after transplantation during recovery from initial neutropenia (Table3), nor were there any differences in the proportion of patients with infection or in the types of infections in the early or late posttransplantation periods (Table 3).
Early posttransplantation outcomes
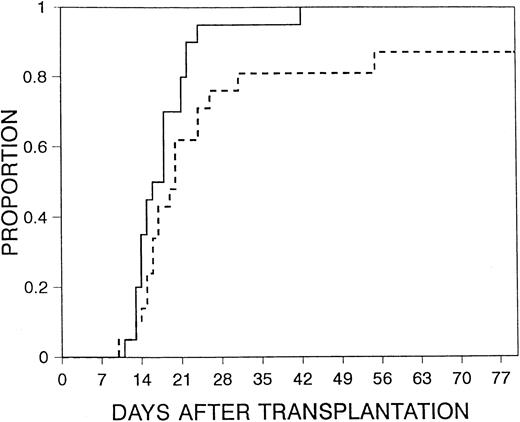
Mucositis was the most common RRT seen. There were no significant differences in mucositis between the 2 study arms (Table4), nor were there significant differences in moderate-to-severe or severe RRT between the 2 treatment arms. Median time to discharge was day 17 (range, 10-80 days) after transplantation. There was a trend for a shorter time to discharge for patients in the filgrastim arm (P = .05) (Figure2). There was no significant difference between the groups in the time from neutrophil recovery to discharge (median interval, 5 days in the filgrastim arm vs 4 days in the control arm; P = .16), suggesting that the earlier discharge with filgrastim resulted from more rapid hematopoietic recovery rather than prevention of incidents that prolonged hospitalization after hematopoietic recovery. No serious or unusual adverse effects attributable to filgrastim were reported.
Time to discharge.
Time to discharge for patients receiving filgrastim after transplantation (solid line) versus those in control arm (dashed line) (P = .05).
Time to discharge.
Time to discharge for patients receiving filgrastim after transplantation (solid line) versus those in control arm (dashed line) (P = .05).
Grades 2 to 4 GVHD occurred in 31% (95% CI, 16%-45%) and grades 3 to 4 in 16% (95% CI, 5%-27%) of patients. The risk for chronic GVHD at 1 year was 69% (95% CI, 49%-81%). Differences between arms in acute and chronic GVHD were not significant (Table 4).
Relapse and survival
The actuarial estimate of relapse at 1 year was 49% (95% CI, 33%-66%). There was a trend for less relapse in the control arm (P = .16) (Table 4). TRM was 2% at day 30 and 14% at day 100 after transplantation. In addition, there was a trend for increased TRM at day 100 after transplantation in the control arm (P = .18) (Table 4). Actuarial survival at day 100 was 90% (95% CI, 81%-100%) for the filgrastim arm and 76% (95% CI, 58%-94%) for the control arm. Overall survival at 1 year for all patients was 58% (95% CI, 42%-73%), and there was no significant difference in 1-year survival between arms (P = .94) (Table 4).
Analysis of charges
Comparisons of hospital charges from day 0 to the day of discharge are presented in Table 5. Total charges for each arm were comparable (median charge, $66,285 vs $65,021;P = .61). When comparing individual expense categories, there was a trend for lower cumulative room charges in the filgrastim arm (median, $15,200 vs $18,000; P = .09), but differences for the other categories were not significant (Table 5).
Discussion
In a retrospective analysis of a small series, Schmitz et al33 suggested that filgrastim accelerated engraftment in recipients of allogeneic blood stem cell transplants, but the efficacy of growth factor use in this setting had not been tested previously. We conducted a prospective, controlled trial in patients who underwent allogeneic blood stem cell transplantation, and we now report that the administration of filgrastim after transplantation significantly hastened neutrophil recovery. There was a 3-day reduction in time to ANC of 0.5 × 109/L or greater and a 4-day reduction to ANC of 1.0 × 109/L or greater. Our results confirmed those of a recent study by Bishop et al.34
We also noted a trend toward a delay in platelet recovery in filgrastim-treated patients, an observation not previously reported for filgrastim or lenograstim with autologous marrow or blood stem cell transplantation.5,7-9 The difference in time to platelet recovery between arms was not as apparent when only ABO-compatible patient-donor pairs were considered, so the difference seen in the whole group might have been due in part to iso-immunization. Additionally, there were no significant differences in numbers of transfusions between the 2 arms. Bishop et al34 also reported that filgrastim had no adverse impact on platelet recovery. In both studies, however, there might have been too few patients to allow for the detection of a small but significant difference in platelet recovery.
Although rhGM-CSF also reduces the duration of neutropenia after allogeneic marrow transplantation,35-37 its effect is abrogated when methotrexate is used for GVHD prophylaxis.38,39 By contrast, even when methotrexate was used, the time to neutrophil recovery after allogeneic marrow transplantation was significantly shorter with filgrastim or lenograstim in comparison to historical controls.15-17,19 40 We here confirm that low-dose methotrexate does not abrogate the effect of filgrastim on neutrophil recovery in recipients of allogeneic blood stem cells.
Rapid engraftment after allogeneic marrow transplantation has been associated with an increased risk for acute GVHD,41raising questions regarding the safety of filgrastim for allogeneic marrow or blood stem cell transplantation. However, studies42-44 in murine allogeneic marrow transplant models showed that filgrastim improved hematopoietic recovery without an increase in GVHD. In addition, neither we in this study nor others13-20 found that the use of filgrastim increased the rate of acute or chronic GVHD after allogeneic blood stem cell or marrow transplantation. It should be noted, however, that to avoid potential cytokine reactions from excessively high white blood cell counts, we here refrained from administering filgrastim once the ANC exceeded 1.0 × 109/L.
In our study, patients receiving filgrastim had a slightly higher relapse rate than patients in the control arm. Although some types of leukemia display receptors for filgrastim, a substantial body of literature supports the contention that filgrastim does not promote leukemia growth in a clinically meaningful way; therefore, direct stimulation of the underlying malignancy was not expected. However, whether filgrastim alters the graft-versus-leukemia (GVL) effect after allogeneic transplantation is unknown. Filgrastim-mobilized blood stem cells reduce natural killer activity and Th2 polarization of lymphocytes compared with peripheral blood mononuclear cells,45,46 characteristics potentially associated with loss of GVL effect. Despite this, preclinical studies demonstrate that filgrastim-mobilized blood stem cells still mediate GVL in vivo47 and that filgrastim potentiates the antitumor effects of IL-12.48 In addition, filgrastim has been used successfully as therapy for relapse after allogeneic transplantation,49 and no impact of filgrastim on long-term survival has been seen in comparative or randomized studies of filgrastim in allogeneic marrow transplanation.16-19Consequently, we believe that the higher rate of relapse in the study arm occurred because a higher proportion of patients in that arm had refractory disease before transplantation.
A cost savings has been reported for the use of filgrastim with autologous marrow or blood stem cell transplantation and with allogeneic marrow transplantation,8,10,19 largely as a result of shorter hospital stays.16-19 The magnitude of the savings varied with the dose and schedule of filgrastim. We here have shown the efficacy of filgrastim 10 μg/kg per day beginning on the first day after transplantation. Others have evaluated different doses and schedules. In nonrandomized comparisons, the rate of engraftment after allogeneic transplantation was similar with 5 or 10 μg/kg per day12,13,40 or when the initiation of filgrastim therapy was delayed to the fifth or seventh day after transplantation.13,14,50 51
Overall, the use of filgrastim after allogeneic blood stem cell transplantation led to earlier hematopoietic recovery and discharge. The heterogeneity of the patient population and the number of patients enrolled precluded exclusion of a small but significant difference between arms in other transplantation outcomes or hospital charges.
Supported in part by Amgen and by the National Institutes of Health (grant CA-16672).
P.A. is a paid consultant for Amgen, and R.C. is a member of the Oncology Advisory Board for Amgen.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Donna Przepiorka, Center for Cell and Gene Therapy, Baylor College of Medicine, 6565 Fannin St, M964, Houston, TX 77030; e-mail: donnap@bcm.tmc.edu.