Recent studies suggest that bone marrow (BM)–derived chemotactic mediators such as chemokines play key roles in hematopoietic stem cell trafficking. Lipid mediators, particularly leukotrienes, are involved in leukocyte chemotaxis during inflammation but have also been detected in the normal BM. Therefore, the effects of leukotrienes on hematopoietic progenitor cells were analyzed. Cysteinyl leukotrienes, particularly leukotriene D4 (LTD4), induced strong intracellular calcium fluxes and actin polymerization in mobilized and BM CD34+ progenitors. Chemotaxis and in vitro transendothelial migration of CD34+ and more primitive CD34+/CD38− cells were 2-fold increased by LTD4 at an optimum concentration of 25 to 50 nM. Accordingly, CD34+ cells expressed the 7-transmembrane LTD4 receptor CysLT1 by reverse transcriptase–polymerase chain reaction and Western blot. Effects of LTD4 were suppressed by the CysLT1 receptor antagonist MK-571 and reduced by pertussis toxin. In contrast, LTB4 induced strong responses only in mature granulocytes. LTD4-induced calcium fluxes were also observed in granulocytes but were not reduced by MK-571, suggesting that these effects were mediated by other receptors (eg, CysLT2) rather than by CysLT1. In addition, expression of 5-lipoxygenase, the key enzyme of leukotriene biosynthesis, was detected in both hematopoietic progenitor cells and mature leukocytes. The study concludes that the functionally active LTD4 receptor CysLT1 is preferentially expressed in immature hematopoietic progenitor cells. LTD4 released in the BM might regulate progenitor cell trafficking and could also act as an autocrine mediator of hematopoiesis. This would be a first physiologic effect of cysteinyl leukotrienes apart from the many known pathophysiologic actions related to allergy and inflammation.
Introduction
Mechanisms involved in hematopoietic stem cell trafficking have been largely unknown for a long time. During the past few years, the role of particular secreted (eg, cytokines) and cell-bound proteins (eg, adhesion molecules) in progenitor mobilization and homing has been recognized.1-4 More recently, it has been shown that cytokines with chemotactic effects (chemokines) may play a central role in progenitor cell trafficking, particularly in stem cell homing to the bone marrow (BM).2,5-7Interestingly, extravasation of mature leukocytes during inflammation and homing of immature progenitor and stem cells to the BM may at least partially depend on similar mechanisms.4 Inflamed tissues and the hematopoietic microenvironment share similarities, such as expression of particular adhesion molecules (E-selectin, vascular cell adhesion molecule-1) on microvascular endothelium.8 9
However, inflammatory chemotaxis of leukocytes is not only controlled by adhesion molecules and chemokines but also by nonpeptide mediators, particularly by lipid mediators such as leukotrienes.10One might therefore speculate that these mediators also play a role in progenitor cell trafficking. During inflammation, phospholipases release arachidonic acid from the cell membranes of activated leukocytes, which is metabolized to leukotrienes via the 5-lipoxygenase pathway.10,11 The initial product, leukotriene (LT)A4, is either hydrolized to LTB4 or conjugated with glutathione, providing the cysteinyl leukotriene LTC4, which is subsequently converted to LTD4 and LTE4. Specific functions of leukotrienes are mediated by receptors with distinct specificity, eg, the LTB4 receptor and the cysteinyl leukotriene receptor CysLT1, which have recently been identified.12,13 The finding that cysteinyl leukotrienes are produced in human BM supports their potential role in the hematopoietic microenvironment.14 Leukotrienes LTB4 and LTC4 have been shown to stimulate growth of colony-forming unit–granulocyte macrophage colonies in methylcellulose.15 However, the effect of leukotrienes on distinct progenitor cell populations (eg, immature progenitor cells) has not been studied.
Cysteinyl leukotrienes have a number of pathophysiologic activities, such as induction of proliferation and contraction of smooth muscle cells, promotion of eosinophil migration, and increases in vascular permeability.16-18 They are established as playing a major role in inflammation, allergy, and asthma,10,19,20 but no physiologic role has been described. Given the short half-life and range in vivo,10 LTD4 could act as a paracrine or autocrine mediator in the BM independent from its role in inflammation and allergy.
Many effects of LTD4, the most potent of the cysteinyl leukotrienes, are mediated through the recently cloned 7-transmembrane receptor CysLT1.12,21 This receptor shows structural similarities (7 transmembrane domains) to several chemokine receptors, including CXCR4, which is expressed on various cell types, including CD34+ hematopoietic progenitor cells.6,22,23The glycosylated receptor CysLT124 is coupled to pertussis toxin (PTX)-sensitive and -insensitive G proteins, indicating that different signal transduction pathways may mediate various activities.25 LTD4 activation results in a transient mobilization of intracellular calcium in the target cells.26,27 The LTD4-mediated effects can be inhibited by several specific CysLT1 receptor antagonists, such as montelukast, zafirlukast, pranlukast, and MK-571, which are successfully used in treatment of allergic asthma.28-32The functional role of additional cysteinyl leukotriene receptors (eg, CysLT2), which are not blocked by these antagonists, remains to be characterized.33
In the present study, we investigated the effect of leukotrienes on circulating CD34+ progenitors, mature granulocytes, and leukemic cell lines. In these cells, expression of the LTD4 receptor CysLT1 and postreceptor effects were measured, ie, intracellular calcium mobilization, actin polymerization, and chemotaxis. In addition, we determined expression of 5-lipoxygenase, the key enzyme of leukotriene biosynthesis, to evaluate whether progenitor cells could also contribute to leukotriene production in the hematopoietic microenvironment.
Materials and methods
Leukotrienes, CysLT1 antagonist
Leukotrienes were obtained from Paesel & Lorei (Hanau, Germany), with purity more than 90% by high-performance liquid chromatography. As controls, equal volumes of the carrier solution (methanol, final concentration < 0.5%) without leukotrienes were used. In some experiments, the CysLT1 receptor antagonist MK-571 (L-660711, 2 μM, kindly provided by Dr R. N. Young) was added to block receptor function.
Cell lines and BM stromal cells
The CD34+ progenitor cell line KG1a, the promyelocytic cell line HL-60, the monocytic cell line THP-1, and the neuroblastoma cell line SK-N-LO34 were cultivated in RPMI 1640 medium (Seromed-Biochrom, Berlin, Germany) supplemented with 10% fetal calf serum. Primary BM stromal cells were isolated from BM aspirates and cultivated as described previously.35
Isolation of CD34+ hematopoietic progenitors
After informed consent, peripheral blood (PB) mononuclear cells (MNCs) were obtained from patients with nonhematologic malignancies or normal stem cell donors during PB progenitor cell mobilization with granculocyte colony-stimulating factor. BM aspirates from healthy donors served as a source for BM MNCs. MNCs were separated by Ficoll density gradient centrifugation. The CD34+ cells were isolated using immunomagnetic microbeads (MACS System, Miltenyi Biotec, Bergisch Gladbach, Germany). Samples with a purity of at least 98% (flow cytometry) were used for this study.
Isolation of normal MNCs and granulocytes
Isolation of PB MNCs and PB polymorphonuclear cells (PMNCs) was performed using dextran sedimentation and Ficoll density centrifugation as described previously.36 For the isolation of the granulocytes, the cell pellet was resuspended in 0.98% NH4Cl for lysis of residual erythrocytes37,38and washed in phosphate-buffered saline (PBS). In accordance with previously published data,36 the purity of the PMNCs was more than 98%.
Flow cytometry
A total of 105 cells were incubated for 30 minutes at 4°C with the fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies: CD34, CD38-PE, CD45 (Becton Dickinson, Heidelberg, Germany), and CD38-FITC (Coulter-Immunotech, Hamburg, Germany). Isotype-identical antibodies served as controls (immunoglobulin G1 and immunoglobulin G2, FITC/PE-conjugated; Becton Dickinson). Cells were analyzed using a FACSCalibur flow cytometer (Becton Dickinson). To calculate the percentage of positive cells, a proportion of 1% or fewer false-positive events was accepted in the negative control sample.
Reverse transcriptase–polymerase chain reaction analysis
Oligonucleotide primers for human CysLT1 were synthezised: 5′-GACAGCCATGAGCTTTTTCC-3′ (sense), 5′-ATGCAGCCAGAGACAAGGTT-3′ (antisense), resulting in a polymerase chain reaction (PCR) product of 514 base pairs (bp). For β-actin, the sense primer 5′-TCATGTTTGAGACCTTCAA-3′ and the antisense primer 5′-GTCTTTGCGGATGTCCACG-3′ were used (513 bp). For investigation of 5-lipoxygenase messenger RNA (mRNA) expression, 5′-ACTGGAAACACGGCAAAAAC-3′ served as sense and 5′-GTGCAGGGGTCTGTTTTGTT-3′ as an antisense primer (501 bp). Total RNA was isolated (Rneasy Mini Kit, Qiagen, Hilden, Germany) and transcribed to complementary DNA (murine leukemia virus reverse transcriptase, PerkinElmer, Langen, Germany). Complementary DNA PCR was performed as described previously.6 The PCR profile consisted of 1-minute initial denaturation at 94°C, followed by 30 to 36 cycles of 40-second denaturation at 94°C, 1-minute annealing (58°C for CysLT1 and 55°C for β-actin) or 30-second annealing (58°C for 5-lipoxygenase), 2-minute polymerization at 72°C and, finally, 10-minute extension at 72°C. As a control, each reaction was additionally performed without addition of reverse transcriptase.
Western blot analysis
Protein extracts were produced by dissolving the cellular proteins in Triton X-100 lysis buffer with protease inhibitors (leupeptin, aprotinin [Boehringer Mannheim, Mannheim, Germany]), 1 mM phenylmethylsulfonylfluoride (Sigma, St Louis, MO), and 1.8 mg/mL iodoacetamide (Sigma) for 45 minutes on ice. After centrifugation, the supernatants were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Hybond-P, Amersham Pharmacia Biotech, Freiburg, Germany). After blocking of the membranes with PBS-T (PBS plus 0.1% Tween 20) plus 5% dry milk powder at 4°C, the blots were incubated with a goat–anti-CysLT1 polyclonal antibody (1:3000, kindly provided by Dr J. F. Evans39) for 1 hour at room temperature. Following 5 wash steps with PBS-T, the membranes were incubated with a polyclonal, horseradish peroxidase–conjugated rabbit-antigoat antibody (1:4000; Dako, Hamburg, Germany) for 1 hour. After washing, bound antibodies were detected using the enhanced chemiluminescence plus Western blotting detection system (Amersham).
Measurement of intracellular calcium mobilization
Intracellular free Ca++ was measured using the fluorescent calcium indicator Fluo-3 (Molecular Probes, Leiden, Netherlands), which was loaded into the cells as described previously.40,41 Cells (107/mL) were incubated in Hanks balanced salt solution (Sigma) containing 10 μM Fluo-3 for 30 minutes at 37°C. After a 1:5 dilution in Hanks balanced salt solution/1% fetal calf serum and a further incubation for 40 minutes at 37°C, the cells were washed 3 times, resuspended in HEPES-buffered saline (137 mM NaCl, 5 mM KCl, 1mM Na2HPO4, 5 mM glucose, 1 mM CaCl2, 0.5 mM MgCl2, 1 g/L bovine serum albumin, 10 mM HEPES, pH 7.4), and incubated for at least 10 minutes at 37°C. After stimulation with leukotrienes (LTB4, LTC4, LTD4, or LTE4) or SDF-1 (recombinant human SDF-1β, R&D Systems, Wiesbaden, Germany), the fluorescence (FITC channel) was continuously analyzed using a FACSCalibur flow cytometer (Becton Dickinson) in 5-second acquisition intervals. As a control, fluorescence changes were measured after application of equal volumes of carrier solution. In some experiments, blocking of the LTD4 receptor CysLT1 was performed by adding MK-571, a specific CysLT1 receptor antagonist,28 at a final concentration of 2 μM, 2 minutes prior to leukotriene application. To analyze the effects of PTX on the CysLT1 signal transduction pathway, the cells were preincubated with 1 μg/mL PTX for 1.5 hours.
Measurement of polymerized F-actin
Cells were suspended in X-VIVO20 medium (Biowhittaker, Walkersville, MD), and LTD4 was added (final concentration 1 μM). By adding 1 volume of FITC-phalloidine (Sigma) working solution (18% formaldehyde, 50 mg/mL lysophosphatidylcholine, 0.4 μM FITC conjugated phalloidine) to 4 volumes of cell suspension, polymerized F-actin was fluorescence labeled, and the cells were fixed and analyzed by flow cytometry (FACSCalibur, Becton Dickinson) as described previously.42 43
Chemotaxis assay
Chemotaxis of CD34+ progenitors was analyzed in a modified Boyden chamber system using 30 μL Chemotx 96-well microplates (Receptor Technologies, Adderbury, United Kingdom) with 5-μm membrane pores. The membrane was coated with fibronectin (10 μg/mL, 1 hour) and positioned over the wells, which had been loaded with X-VIVO20 serum-free medium (Biowhittaker) containing chemoattractants or solvent (control). For each well, 50 μL serum-free medium containing 3 × 104 CD34+cells was pipetted onto the membrane. After a 14-hour incubation at 37°C and 5% CO2, the medium, including nonmigrated cells, was removed and the membrane was incubated with ethylenediaminetetraacetic acid solution (0.2%, 30 minutes, 4°C). After centrifugation (400g), the number of migrated cells was evaluated by colorimetric lactate dehydrogenase activity (Cytotox96, Promega, Mannheim, Germany).
Transendothelial migration
Migration across BM endothelial cells was quantitatively analyzed as described previously6 using BMEC-1 cells grown to confluency on 3-μm microporous Transwell inserts (Corning-Costar, Bodenheim, Germany) placed in 6-well tissue culture plates. LTD4 (50 nM) or SDF-1 (recombinant human SDF-1β, R&D Systems; 500 ng/mL) was added as a chemoattractant to the lower chamber and 5 × 105 CD34+ cells to the upper chamber of the transmigration system. Transmigrated cells were recovered and enumerated after 14 hours.
Statistical analysis
For statistical analysis of calcium mobilization, actin polymerization, chemotaxis, and transendothelial migration assays, data from at least 3 independent experiments were expressed as mean ± SEM. For the assessment of statistical significance, the Wilcoxon matched pairs signed rank test was applied.
Results
Expression of the LTD4 receptor CysLT1 in human CD34+progenitor cells
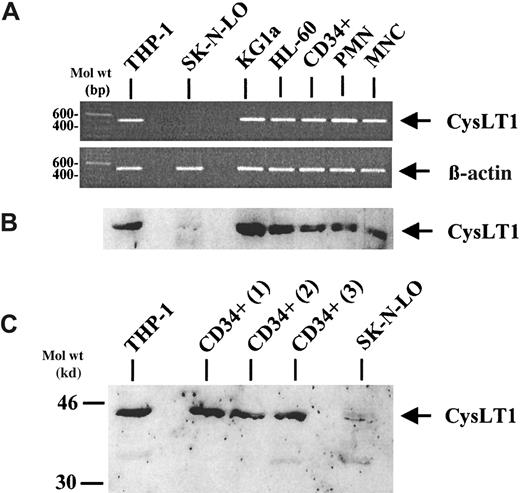
Expression of CysLT1 mRNA in CD34+ cells, mature leukocytes, and in cell lines was analyzed by reverse transcriptase–polymerase chain reaction (RT-PCR) (Figure1A). Corresponding to indirect evidence from previously published data,25 the CD34-monocytic cell line THP-1 expressed CysLT1 mRNA and was therefore used as a positive control. In contrast, no expression of the specific CysLT1 mRNA was observed in the neuroblastoma cell line SK-N-LO, serving as a negative control. By RT-PCR, PB CD34+ hematopoietic progenitor cells, PMNCs, and MNCs expressed the CysLT1 mRNA, as well as the CD34+ progenitor cell line KG1a and the CD34-myeloid cell line HL-60. Analysis of β-actin mRNA expression served as an internal control. In accordance with the RT-PCR data, Western blot analysis revealed a single band between the 30- and 46-kd weight markers in all samples, except the negative control cell line SK-N-LO (Figure 1B), which was consistent with previously published results using the same polyclonal antibody39 and corresponds to the calculated molecular mass of CysLT1 (38.5 kd).12 The strongest expression of CysLT1 protein was observed in the CD34+ cell line KG1a. In mobilized, circulating CD34+ cells, the signal was at least as strong as in mature leukocytes (PMNCs, MNCs). Because expression of CysLT1 in circulating CD34+ cells may show interindividual differences, additional samples from 3 different donors were investigated as shown in Figure 1C. All CD34+cell samples analyzed were brightly positive for CysLT1 expression at a similar level.
CysLT1 expression in CD34+ hematopoietic progenitors, mature leukocytes, and cell lines.
(A) CysLT1 mRNA was expressed in CD34+ hematopoietic progenitor cells, mature PMNCs and MNCs, as well as in leukemic cell lines including the CD34+ progenitor cell line KG1a, as assessed by RT-PCR. The cell line THP-1 served as a positive control and the neuroblastoma cell line SK-N-LO as a negative control. Expression of β-actin mRNA was analyzed as a control for RNA amounts in the RT reaction. (B) Western blot analysis of CysLT1 protein expression confirmed the RT-PCR results using a polyclonal CysLT1 antibody.39 The strongest CysLT1 expression was observed in the CD34+ cell line KG1a. In CD34+ cells, CysLT1 expression was at least as strong as in mature leukocytes. (C) Circulating CD34+ progenitors from 3 different, additional individuals showed CysLT1 expression at a similar level.
CysLT1 expression in CD34+ hematopoietic progenitors, mature leukocytes, and cell lines.
(A) CysLT1 mRNA was expressed in CD34+ hematopoietic progenitor cells, mature PMNCs and MNCs, as well as in leukemic cell lines including the CD34+ progenitor cell line KG1a, as assessed by RT-PCR. The cell line THP-1 served as a positive control and the neuroblastoma cell line SK-N-LO as a negative control. Expression of β-actin mRNA was analyzed as a control for RNA amounts in the RT reaction. (B) Western blot analysis of CysLT1 protein expression confirmed the RT-PCR results using a polyclonal CysLT1 antibody.39 The strongest CysLT1 expression was observed in the CD34+ cell line KG1a. In CD34+ cells, CysLT1 expression was at least as strong as in mature leukocytes. (C) Circulating CD34+ progenitors from 3 different, additional individuals showed CysLT1 expression at a similar level.
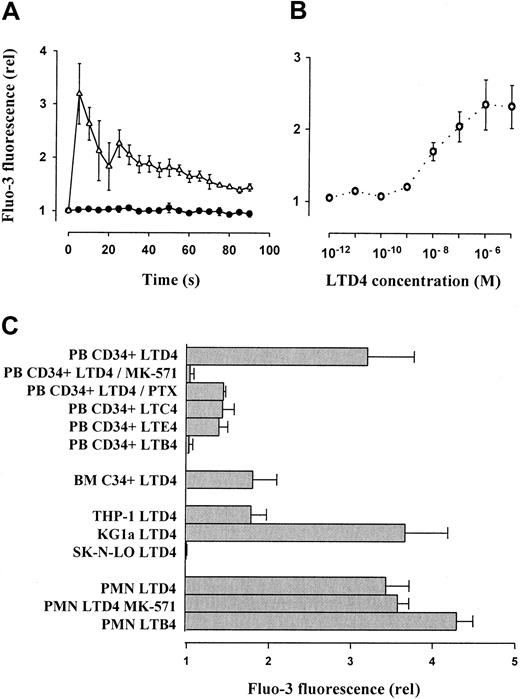
Leukotriene-mediated mobilization of intracellular free calcium in progenitor cells
LTD4 (1 μM) induced a significant, transient increase of intracellular free calcium in PB CD34+ cells (Figure2A), which was measured semiquantitatively as relative Fluo-3 mean fluorescence. Concentrations as low as 1 nM resulted in significant calcium mobilization (dose-response curve, Figure 2B). The cells showed weaker effects in response to LTC4 and LTE4 given in an equimolar concentration (1 μM). Calcium mobilization was not observed after application of LTB4 (Figure2C). Interestingly, the effect of LTD4 was more pronounced in PB than in BM-derived CD34+ progenitors. Calcium fluxes were completely blocked by the specific CysLT1 receptor antagonist MK-571.28 Only partial inhibition was observed after preincubation of the cells with PTX (1 μg/mL) for 1.5 hours. Similarly, LTD4-induced calcium fluxes were observed in the control cell line THP-1 but even stronger in the CD34+ cell line KG1a. In contrast, LTB4 induced intracellular calcium fluxes in PMNCs as described previously,44 whereas the effect of LTD4 was less pronounced and not suppressed by MK-571. In SK-N-LO cells, none of the tested leukotrienes caused an intracellular calcium mobilization.
Leukotriene-induced mobilization of intracellular free calcium in CD34+ hematopoietic progenitors, mature leukocytes, and cell lines.
(A) Mobilization of intracellular calcium in isolated PB CD34+ cells was analyzed by flow cytometry using the green fluorescent calcium indicator Fluo-3. After addition of LTD4 (1 μM), time-dependent, relative (compared to baseline level = 1) changes of the mean fluorescence were recorded. Control, ●; LTD4, ▵. (B) LTD4-induced intracellular calcium mobilization (expressed as relative Fluo-3 fluorescence 5 seconds after addition of LTD4) in primary PB CD34+ progenitors was dose dependent, with an optimum concentration of 1 μM. (C) The LTD4-induced calcium release in PB CD34+ cells (expressed as relative Fluo-3 fluorescence 5 seconds after addition of LTD4) was completely blocked by the CysLT1 receptor antagonist MK-571 and partially reduced by PTX. Other cysteinyl leukotrienes (LTC4, LTE4 at 1 μM) were less effective. The noncysteinyl leukotriene LTB4 (1 μM) did not induce calcium fluxes in CD34+ cells. The response of BM-derived CD34+progenitors was similar to THP-1 cells, whereas the strongest calcium fluxes were observed in the KG1a cell line. LTD4 induced calcium mobilization also in mature PMNCs, which, however, was not suppressed by MK-571 and was less efficient than the noncysteinyl leukotriene LTB4.
Leukotriene-induced mobilization of intracellular free calcium in CD34+ hematopoietic progenitors, mature leukocytes, and cell lines.
(A) Mobilization of intracellular calcium in isolated PB CD34+ cells was analyzed by flow cytometry using the green fluorescent calcium indicator Fluo-3. After addition of LTD4 (1 μM), time-dependent, relative (compared to baseline level = 1) changes of the mean fluorescence were recorded. Control, ●; LTD4, ▵. (B) LTD4-induced intracellular calcium mobilization (expressed as relative Fluo-3 fluorescence 5 seconds after addition of LTD4) in primary PB CD34+ progenitors was dose dependent, with an optimum concentration of 1 μM. (C) The LTD4-induced calcium release in PB CD34+ cells (expressed as relative Fluo-3 fluorescence 5 seconds after addition of LTD4) was completely blocked by the CysLT1 receptor antagonist MK-571 and partially reduced by PTX. Other cysteinyl leukotrienes (LTC4, LTE4 at 1 μM) were less effective. The noncysteinyl leukotriene LTB4 (1 μM) did not induce calcium fluxes in CD34+ cells. The response of BM-derived CD34+progenitors was similar to THP-1 cells, whereas the strongest calcium fluxes were observed in the KG1a cell line. LTD4 induced calcium mobilization also in mature PMNCs, which, however, was not suppressed by MK-571 and was less efficient than the noncysteinyl leukotriene LTB4.
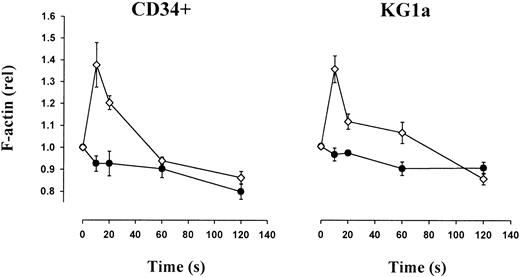
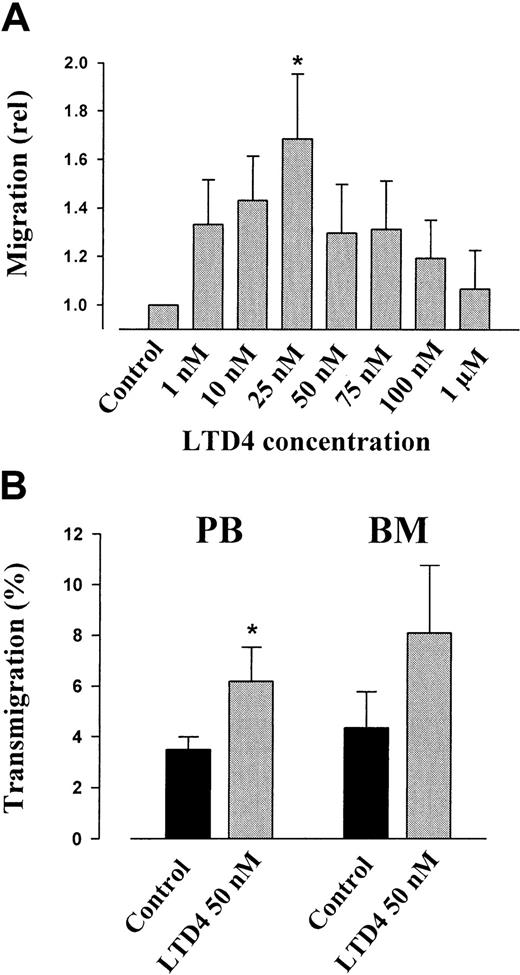
LTD4-induced actin polymerization, chemotaxis, and transendothelial migration of CD34+ hematopoietic progenitor cells
Actin polymerization in response to LTD4 as an initial step in cell locomotion was analyzed (Figure 3). In both mobilized PB CD34+ progenitors and KG1a cells, application of 1 μM LTD4 immediately resulted in an approximately 40% increase of polymerized F-actin, which depolymerized to the baseline level within 2 minutes. Using a modified Boyden chamber chemotaxis assay, the chemotactic activity of LTD4 on CD34+progenitor cells was analyzed. Figure 4A shows the mean relative migration of PB CD34+ progenitor cells in response to different LTD4 concentrations. The greatest migration rate was observed in response to 25 nM LTD4. However, the optimum concentrations were different in individual CD34+samples, ranging from 10 to 75 nM. As shown in Figure 4B, LTD4 at a concentration of 50 nM added to the lower chamber of the in vitro assay of transendothelial migration increased the number of transmigrating PB- and BM-derived CD34+ cells approximately 2-fold.
LTD4-induced actin polymerization in CD34+progenitors and KG1a cells.
Polymerization of cellular actin was assessed by flow cytometry using FITC-conjugated phalloidin. In both CD34+ and KG1a cells, an increase (40%) of polymerized F-actin was observed after addition of 1 μM LTD4; the level of F-actin returned back to the baseline level within 2 minutes. Control, ●; LTD4, ⋄.
LTD4-induced actin polymerization in CD34+progenitors and KG1a cells.
Polymerization of cellular actin was assessed by flow cytometry using FITC-conjugated phalloidin. In both CD34+ and KG1a cells, an increase (40%) of polymerized F-actin was observed after addition of 1 μM LTD4; the level of F-actin returned back to the baseline level within 2 minutes. Control, ●; LTD4, ⋄.
Chemotaxis and in vitro transendothelial migration of hematopoietic progenitor cells in response to LTD4.
(A) The chemotactic effect of LTD4 on CD34+ hematopoietic progenitor cells at different concentrations was analyzed in a modified Boyden chamber assay. Addition of LTD4 to the lower chamber of the chemotaxis assay stimulated migration of CD34+ cells at an average optimum concentration of 25 nM/L. (B) Using an in vitro assay of transendothelial migration across BM endothelium,6 the percentage of migrating CD34+ progenitor cells (14 hours) was approximately 2-fold increased by LTD4 at 50 nM. Both mobilized PB and BM-derived progenitors responded to the cysteinyl leukotriene. *P < .05, LTD4 vs control.
Chemotaxis and in vitro transendothelial migration of hematopoietic progenitor cells in response to LTD4.
(A) The chemotactic effect of LTD4 on CD34+ hematopoietic progenitor cells at different concentrations was analyzed in a modified Boyden chamber assay. Addition of LTD4 to the lower chamber of the chemotaxis assay stimulated migration of CD34+ cells at an average optimum concentration of 25 nM/L. (B) Using an in vitro assay of transendothelial migration across BM endothelium,6 the percentage of migrating CD34+ progenitor cells (14 hours) was approximately 2-fold increased by LTD4 at 50 nM. Both mobilized PB and BM-derived progenitors responded to the cysteinyl leukotriene. *P < .05, LTD4 vs control.
Effect of LTD4 on primitive and more differentiated hematopoietic progenitor cells
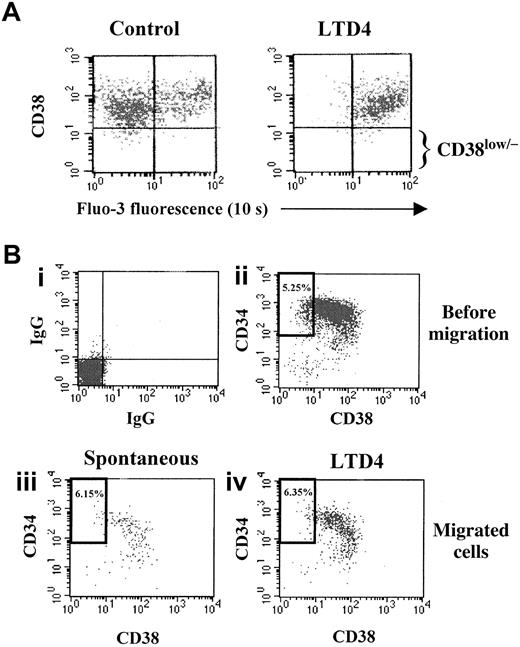
Expression of the surface molecule CD38 is associated with lineage commitment of hematopoietic progenitors, whereas absence or low expression of this antigen on CD34+ cells is characteristic for more primitive progenitor cells.45-48 Therefore, we analyzed whether the effects of LTD4 on CD34+ cells are related to the level of CD38 expression. Particularly, the response of more primitive CD34+/CD38low/− cells to this cysteinyl leukotriene was assessed by counterstaining of isolated, Fluo-3–loaded progenitors with CD38-PE and analysis of the LTD4-mediated intracellular calcium mobilization and CD38 expression by flow cytometry. The transient shift of the Fluo-3 fluorescence, caused by the intracellular calcium flux, was observed in both the CD38+ and the CD38low/− cells (Figure5A). Corresponding results were obtained by fluorescence-activated cell sorter analysis of CD34+cells that migrated in response to LTD4 (chemotaxis assay) compared with spontaneously migrated cells (Figure 5B). The percentage of more immature CD34+/CD38low/− cells was similar in both populations (6.35% and 6.15%) as well as in the CD34+ population initially used for the assay (5.25%). Similar results were obtained in the transmigration assay. The percentage of CD34+/CD38low/− cells among the PB CD34+ progenitors migrating in response to LTD4 (7.1% ± 1.7%) tended to be even greater compared with the spontaneously migrating cells (5.4% ± 2.0%) or initially isolated CD34+ cells (6.4% ± 2.9%).
Effects of LTD4 on immature CD34+/CD38low/− hematopoietic progenitor cells.
(A) Isolated PB CD34+ progenitor cells were loaded with the calcium indicator Fluo-3 and stained with a CD38-PE antibody. Ten seconds after addition of LTD4, a shift of the green Fluo-3 fluorescence was observed due to intracellular calcium mobilization (right panel), which occurred in both CD38+ and more primitive CD38low/− progenitor cells. (B) Analysis of isolated PB CD34+ cells by flow cytometry before and after chemotaxis showed a similar percentage of more immature CD34+/CD38low/− cells (bold rectangular gate) in the starting population (ii, before migration, 5.25%), in spontaneously migrating cells (iii, 6.15%), and in migrated cells attracted by LTD4 (iv, 6.35%). In this particular experiment, a more than 2-fold increase of the migration rate in response to LTD4 (50 nM) was observed. The isotype-specific control is shown in panel i.
Effects of LTD4 on immature CD34+/CD38low/− hematopoietic progenitor cells.
(A) Isolated PB CD34+ progenitor cells were loaded with the calcium indicator Fluo-3 and stained with a CD38-PE antibody. Ten seconds after addition of LTD4, a shift of the green Fluo-3 fluorescence was observed due to intracellular calcium mobilization (right panel), which occurred in both CD38+ and more primitive CD38low/− progenitor cells. (B) Analysis of isolated PB CD34+ cells by flow cytometry before and after chemotaxis showed a similar percentage of more immature CD34+/CD38low/− cells (bold rectangular gate) in the starting population (ii, before migration, 5.25%), in spontaneously migrating cells (iii, 6.15%), and in migrated cells attracted by LTD4 (iv, 6.35%). In this particular experiment, a more than 2-fold increase of the migration rate in response to LTD4 (50 nM) was observed. The isotype-specific control is shown in panel i.
Comparison of the effects of LTD4 and SDF-1 on hematopoietic progenitor cells
We compared the effects of LTD4 on calcium mobilization and transendothelial migration with the chemokine SDF-1 in parallel experiments. Calcium fluxes (relative Fluo-3 fluorescence 5 seconds after addition of the ligand) in response to LTD4 (1 μM) were 3.0-fold ± 1.0-fold stronger than fluxes induced by saturating doses of SDF-1 (500 ng/mL), but the opposite result was observed regarding transendothelial migration. SDF-1 (500 ng/mL) augmented in vitro transendothelial migration of mobilized CD34+ cells 3.8-fold ± 0.7-fold more efficiently than LTD4 (50 nM).
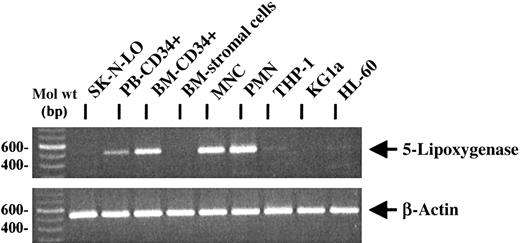
RT-PCR analysis of 5-lipoxygenase expression in hematopoietic progenitor cells
Because 5-lipoxygenase is the key enzyme of leukotriene biosynthesis,10,11 RT-PCR analysis was performed to investigate the expression of 5-lipoxygenase mRNA in hematopoietic progenitor cells, cell lines, mature leukocytes, and primary human BM fibroblast-like stromal cells (Figure 6). As expected, mature PMNCs and MNCs were positive for the specific mRNA.10 Expression of 5-lipoxygenase mRNA was also observed in PB CD34+ and BM CD34+ progenitor cells, whereas primary BM fibroblast-like stromal cells as well as the neuroblastoma cell line SK-N-LO were negative for the specific PCR product. Analysis of CD34+ and CD34− leukemic cell lines revealed only weak or negative PCR signals. As a control for equal RNA amounts in the RT-reaction, β-actin mRNA expression was analyzed.
RT-PCR analysis of 5-lipoxygenase mRNA expression in primary hematopoietic cells and cell lines.
By RT-PCR, 5-lipoxygenase mRNA was expressed in PB and BM CD34+ progenitors, PB PMNCs, MNCs, and at a low level in the cell lines THP-1 and HL60. No specific PCR signal could be observed in the negative control cell line SK-N-LO, in BM stromal cells, and in KG1a cells. Similar RNA amounts were used in the reactions, as demonstrated by RT-PCR for β-actin expression.
RT-PCR analysis of 5-lipoxygenase mRNA expression in primary hematopoietic cells and cell lines.
By RT-PCR, 5-lipoxygenase mRNA was expressed in PB and BM CD34+ progenitors, PB PMNCs, MNCs, and at a low level in the cell lines THP-1 and HL60. No specific PCR signal could be observed in the negative control cell line SK-N-LO, in BM stromal cells, and in KG1a cells. Similar RNA amounts were used in the reactions, as demonstrated by RT-PCR for β-actin expression.
Discussion
In this study, we demonstrate that CD34+ hematopoietic progenitor cells express the transmembrane receptor CysLT1, which mediates the effects of the lipid mediator LTD4. LTD4 induced calcium fluxes and actin polymerization in CD34+ progenitors and increased progenitor cell chemotaxis and in vitro transendothelial migration. Both PB- and BM-derived progenitors responded to LTD4. The specifity of these effects was demonstrated by abrogation of the calcium fluxes in the presence of the specific CysLT1 receptor antagonist MK-571.28 Until now, only CXCR4 has been described as a functionally active 7-transmembrane receptor expressed in CD34+ progenitors. However, CXCR4 is present on circulating progenitors at a relatively low level compared with mature leukocytes, and it is not detectable in most CD34+progenitor cell lines such as KG1a.6 In contrast, CysLT1 is expressed in CD34+ cells at least as strong as in PMNCs or MNCs and in high levels in the CD34+ progenitor cell line KG1a. KG1a therefore represents a suitable positive control for the study of CysLT1 expression and may be a useful cell line for analysis of LTD4-mediated signal transduction pathways in hematopoietic progenitors, similar to the successful use of CXCR4-expressing progenitor cell lines in the investigation of SDF-1–mediated signal transduction.49
Chemotactic effects of LTD4 have previously been observed only in mature eosinophils and in neutrophils at high concentrations (10 μM).17,18 Our finding that MK-571 did not reduce the effects of LTD4 on calcium fluxes in mature granulocytes suggests that other LTD4 receptors, such as the recently identified CysLT2 receptor,33 mediate these effects, despite the fact that CysLT1 protein expression was also detected in granulocytes. However, circulating CD34+ cells respond to LTD4 with increased chemotaxis and transendothelial migration at an optimum concentration as low as 25 to 50 nM. Interestingly, in mature granulocytes, noncysteinyl leukotrienes such as LTB4, which were inactive in CD34+ progenitors, were more efficient than LTD4. These results demonstrate that during myeloid differentiation, a functionally active CysLT1 receptor is preferentially expressed in immature progenitors. The particular role of LTD4 for early myeloid development is supported by the fact that the key enzyme of leukotriene biosynthesis, 5-lipoxygenase, has been detected in the hematopoietic microenvironment,10,11 and production of cysteinyl leukotrienes has been found in human BM.14
CysLT1 belongs to the same group of G protein–coupled 7-transmembrane domain receptors12 as the SDF-1 receptor CXCR4. This receptor type is characteristic for a variety of chemokine receptors that share similar G protein–coupled signal transduction pathways resulting in a chemotactic response.22,23,50 Furthermore, LTD4 was able to induce a reorganization of the cytoskeleton by actin polymerization, which is an early step in cellular migration. The narrow bell-shaped LTD4 concentration optimum of the chemotactic effect (Figure 4) is in contrast with the sigmoidal dose-response relation of the calcium mobilization (Figure 2). Maximal intracellular calcium fluxes were observed at LTD4 concentrations more than one log step higher than required for optimum chemotactic responses of the progenitors. Interestingly, the intensity of the calcium-mobilizing effect of LTD4 on CD34+ cells at an optimum concentration (1 μM) was even stronger than that induced by optimal amounts of SDF-1, which has previously been described to induce intracellular calcium release.5 In contrast, transendothelial migration was more efficiently stimulated by SDF-1, which, however, could also be due to the short half-life and degradation of LTD4 in the transmigration assay.
The LTD4 concentration required for optimal migration in the chemotaxis assay varied interindividually, which could indicate differences in the CysLT1 expression similar to expression of the chemokine receptor CXCR4 6 or might be due to partial down-regulation or receptor internalization dependent on the amount of cysteinyl leukotrienes actually present in the PB and bound to the receptor, which has similarly been described for other 7-transmembrane receptors.51
Expression of a functionally active LTD4 receptor on CD34+cells suggests a role of this inflammatory lipid mediator in hematopoiesis, particularly when considering the finding that already low concentrations of LTD4 are sufficient to induce a chemotactic response of progenitor cells. The concept that similar mechanisms control inflammatory leukocyte extravasation and progenitor cell trafficking is supported by the fact that other factors participating in inflammation are also involved in progenitor cell homing to the BM.1,3,4 For example, the adhesion molecule E-selectin responsible for leukocyte rolling during inflammation is known to contribute to chemokine-induced transendothelial migration of CD34+ cells in vitro, which is prevented by an E-selectin antibody.2 Furthermore, the integrin VLA-4 provoking the firm adhesion of leukocytes to vascular endothelium at sites of inflammation plays an important role in progenitor cell homing, as shown in vivo using the fetal sheep model of in utero transplantation.1 In the absence of inflammation, both E-selectin and vascular cell adhesion molecule-1 (ligand of VLA-4) are present preferentially on microvascular endothelium of the BM.8,9 Thus, our results demonstrating interaction of leukotrienes with their receptors on hematopoietic progenitor cells indicate also that chemotaxis of progenitors might be controlled by mechanisms that have previously been shown to regulate extravasation of mature leukocytes during inflammation. It is unclear whether LTD4 and CysLT1 are involved in stem cell homing, but the fact that transplantation of fetal liver cells from CXCR4−/− mice into lethally irradiated recipients still results in engraftment to the BM52,53 indicates that the homing process does not solely depend on CXCR4 as a chemotactic receptor, although the chemokine SDF-1 and its receptor CXCR4 seem to play a major role.6,7 54
Subset analysis of the LTD4-induced effects on CD34+ cells was performed to determine whether more primitive progenitors also respond to LTD4, because most CD34+ cells represent committed progenitors rather than immature, pluripotent cells with repopulation capacity, and CysLT1 mRNA expression has also been described in mature PB leukocytes.12,55 By costaining with a PE-labeled CD38 antibody during the analysis of the calcium mobilization, we could show that more primitive CD34+/CD38low/− progenitor cells45-48 were also activated by LTD4. According to these results, flow cytometry of the migrated cells following chemotaxis or in vitro transendothelial migration revealed that the chemotactic effect of LTD4 is not restricted to committed progenitors, which implies a role of LTD4 in the trafficking of more primitive hematopoietic progenitors, including true stem cells. However, CD34+/CD38low/− progenitor cells were not selectively attracted by LTD4, indicating a potential role for migration and spatial distribution of more committed progenitors.
Until now, cysteinyl leukotrienes have only been reported to play a role in the pathophysiology of inflammatory and allergic disorders, particularly in bronchial asthma.10,19,20 A physiologic role of these lipid mediators has not been established yet. The potential importance of cysteinyl leukotrienes as regulators of hematopoiesis is supported by the finding that mRNA expression of the CysLT1 receptor is greater in leukocytes and hematopoietic organs such as spleen than in lung tissue.12 Very short half-lives, particularly of LTD4,10 make them ideal candidates for short-range control of hematopoietic cell functions.
RT-PCR analysis demonstrated that the key enzyme of leukotriene biosynthesis, 5-lipoxygenase, is expressed not only in mature leukocytes but also in PB and particularly in BM CD34+cells. This raises the possibility that CD34+ cells not only respond to LTD4 but also produce this mediator in an autocrine or paracrine way. Signal transduction via CysLT1 involves both PTX-sensitive and -insensitive G proteins,25 which is in accordance with our finding that PTX only partially inhibits LTD4-induced calcium fluxes. Distinct signal transduction pathways could result in LTD4 effects on CD34+ cells other than chemotaxis, ie, effects on cell proliferation and differentiation,10 which might be controlled in an autocrine way. This idea is supported by the finding that inhibition of leukotriene biosynthesis after irradiation suppresses hematopoietic recovery.55 However, further studies are required to identify the source and regulation of leukotrienes produced in the BM.14 A recent study suggests that hematopoietic progenitor cells are involved in the early steps of leukotriene biosynthesis such as the release of arachidonate, but further metabolism to leukotrienes is probably mediated by other cell types.56
It is still an open question how the specificity of the homing process is realized, because a specific homing factor for stem cells has yet not been found. Adhesion molecules known to participate in the homing process are not exclusively expressed on BM endothelium or CD34+ cells. Also SDF-1 and its receptor CXCR4 are more broadly expressed and are also involved in embryogenesis, heart development, neuronal cell migration, and vascular development.54,57,58 Similarly, the expression of LTD4 and its receptor is not restricted to the hematopoietic microenvironment.12 However, the short in vivo range supports the idea that LTD4 is an ideal candidate mediator for the regulation of the spatial distribution of cells in the BM. The homing of CD34+ progenitor cells could therefore be mediated by a BM–specific combination of nonspecific factors, such as SDF-1, adhesion molecules, and other paracrine factors, including cysteinyl leukotrienes, rather than by a single factor alone.
We thank Christine Zimmermann for excellent techical assistance; Dr J. F. Evans, Department of Human Genetics, Merck Research Laboratories, West Point, PA, for generously providing the CysLT1 polyclonal antibody; and Dr R. N. Young, Merck Frosst Centre for Therapeutic Research, Quebec, Canada, for kindly providing MK-571.
Supported by grants from Deutsche Forschungsgemeinschaft (SFB 510).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert Möhle, Dept of Medicine II, University of Tübingen, Otfried-Müller-Str 10, 72076 Tübingen, Germany; e-mail:robert.moehle@med.uni-tuebingen.de.