In this study, cord blood CD34+ cells expressed CD81, a member of the transmembrane 4 superfamily, and were classified into 3 subpopulations on the basis of their expression levels: CD34+CD81+, CD34lowCD81+, and CD34+CD81high. The lymphohematopoietic activity of each subpopulation was then examined by using suspension and clonogenic cultures for hematopoietic potential, coculture with MS-5 cells for B-cell potential, organ cultures of thymus lobes from nonobese diabetic/severe combined immunodeficiency disease (NOD/SCID) fetal mice, coculture with stromal cells derived from NOD/SCID fetal-mouse liver tissue for natural killer (NK) cell and mast cell potentials, and xenotransplantation into NOD/SCID mice for long-term repopulating (LTR) ability. CD34+CD81+ cells represented a heterogeneous population that had all the lymphohematopoietic activities, including NOD/SCID mouse-repopulating ability. CD34lowCD81+ cells were enriched in erythroid, megakaryocytic, and NK lineage potentials but had lost T-cell and B-cell potentials and LTR ability. The CD34+CD81high fraction was depleted of most lymphohematopoietic potentials except NK cell and mast cell potentials. Thus, along the differentiation cascade from CD34+CD81+ lymphohematopoietic stem cells, an up-regulation of CD81 or a down-regulation of CD34 results in a change in lymphohematopoietic properties. CD81 may serve as a marker for defining developmental stages of lymphohematopoietic stem cells.
Introduction
Lymphohematopoietic stem and progenitor cells, which are common precursors of hematopoietic and lymphocytic cells, exist through clinical transplantation in humans as well as in laboratory animals.1-5 The differentiation cascade from lymphohematopoietic stem and progenitor cells toward a specific lineage can be defined by the expression of surface markers. In humans, CD34+ cells are capable of reconstituting human lymphohematopoiesis in scid mouse bone marrow (BM) in vivo6,7 and of differentiating into all lineages, including lymphoid lineages, of blood cells in vitro.8-11Evaluation of coexpression of a variety of markers with CD34 is useful for defining the events in a given pathway leading to a specific cell lineage. It was demonstrated that human lymphoid and myeloid cells can be derived in vitro from single cells defined by expression of CD34 and other surface markers.9-11 Other researchers also delineated a fraction phenotyping lineage marker–negative (Lin−) CD34+CD45RA+CD38+HLA-DR+that was capable of differentiating into T- and myeloid-lineage cells but depleted of erythroid and severe combined immunodeficiency disease (SCID) mouse-repopulating potentials.12 Thus, there seems to be a complex of classes in the development of lymphohematopoietic stem and progenitor cells.
CD81, a member of the tetraspanin or transmembrane 4 superfamily (TM4SF), is a 26-kd surface protein composed of 4 transmembrane and 2 extracellular domains and having an overall topology shared by other members of the TM4SF, such as CD19, CD37, CD53, CD63, CD82, and CD151.13,14 CD81 plays a role in a variety of biologic responses by associating with other proteins; regulating cell adhesion, migration, proliferation, and differentiation; and changing cell morphology and signal transductions.15-20 CD81 is expressed in most human tissues, including blood.21Studies of CD81 in blood cells, however, have been done mostly by using lymphoid cells, and the expression of CD81 on lymphohematopoietic stem and progenitor cells is not well understood. In this study, we delineated expression of CD81 on human lymphohematopoietic cells and revealed a possible role for CD81 (when costained with CD34) in the development of lymphohematopoietic stem and progenitor cells.
Materials and methods
Mice
Laboratory nonobese diabetic/Shi-scid (NOD/SCID) mice with dysfunctional mature lymphocytes and macrophages and lacking circulating complements22 were provided by the Central Institute for Experimental Animals (Kawasaki, Japan). The mice were kept in microisolator cages on a laminar-flow rack in a clean room and given irradiated, sterile food and autoclaved, acidified water. Mice used for transplantation experiments were all females aged 10 to 12 weeks. In some experiments, 16- to 20-week-old male and female mice were mated. Subsequently, female mice were checked for vaginal plugs (day 0 of gestation), and fetal livers and thymuses were removed on selected days.
Cell preparations
Human cord blood (CB) was obtained during normal full-term deliveries after informed consent was given. Mononuclear cells (MNC) were separated by Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden) density-gradient centrifugation after depletion of phagocytes with silica (Immuno Biological Laboratories, Fujioka, Japan).
Cytokines
Recombinant human interleukin 3 (IL-3), thrombopoietin (TPO), and erythropoietin (EPO) were donated by Kirin Brewery (Tokyo, Japan). Recombinant human stem cell factor (SCF) and IL-6 were provided by Amgen (Thousand Oaks, CA). Recombinant human granulocyte colony-stimulating factor (G-CSF) was provided by Chugai Pharmaceutical (Tokyo, Japan). Recombinant human IL-7 and IL-15 were purchased from R&D Systems (Minneapolis, MI). All cytokines were pure recombinant molecules and were used at concentrations that induced an optimal response in cultures of human cells. The concentrations used were 100 ng/mL for SCF and IL-6, 20 ng/mL for IL-3, 2 U/mL for EPO, and 10 ng/mL for TPO, G-CSF, IL-7, and IL-15.
Flow cytometry and antibodies
For staining, CB MNC were suspended in phosphate-buffered saline (PBS) with 0.1% deionized fraction V bovine serum albumin (BSA) (Sigma Chemical, St Louis, MO) at a concentration of 5 × 105cells per tube. In all experiments, cell samples were preincubated in 0.1 mL PBS supplemented with 10 μL normal rabbit serum (Funakoshi, Tokyo, Japan) for 30 minutes to block nonspecific binding. After a wash with PBS, cells were stained for 30 minutes on ice with various monoclonal antibodies (mAbs) conjugated by fluorescein isothiocyanate (FITC), phycoerythrin (PE), or phycoerythrin-cyanin 5.1 (PC-5). In some experiments, cells were first stained with a biotin-coated mAb for 30 minutes, washed with PBS, and then stained with PC-5–conjugated streptavidin (Immunotech, Marseille, France). Stained cells were washed with PBS, and expression of cell-surface antigens specific for mAb was determined by using a fluorescence-activated cell-sorter scanner (FACS) flow cytometer and CellQuest software (Becton Dickinson, San Jose, CA).
For flow cytometric analysis, FITC-conjugated mouse immunoglobulin G1 (IgG1) and IgG2a (both used as negative isotype controls); mouse antihuman CD3 (SK7), CD4 (SK3), CD8 (SK1), CD10 (N8E7), CD13 (L138), CD16 (NKP15), CD19 (4G7), CD45 (2D1), and T-cell receptor (TCR) αβ (WT31); PE-conjugated mouse IgG1 (used as a negative isotype control); and mouse antihuman CD4 (SK3), CD13 (L138), and CD19 (4G7) antibodies (Abs) were purchased from Becton Dickinson. FITC-conjugated mouse antihuman glycophorin A (KC16), PC-5–conjugated mouse IgG1 (used as a negative isotype control), mouse antihuman CD34 (581) and CD45 (J.33), and streptavidin were purchased from Immunotech. PE-conjugated mouse antihuman CD81 (JS-81) and biotin-conjugated CD3 (UCHTI1) were purchased from Pharmingen (San Diego, CA). FITC-conjugated rabbit antihuman polyclonal Ab for IgM was purchased from Dako (Carpinteria, CA). FITC-conjugated mouse antihuman CD14 (CLB-Mon/1) and CD56 (NKI-nbl-1) were gifts from Nichirei (Toyko, Japan).
Alkaline phosphatase–anti-alkaline phosphatase staining
Mast cells were detected by means of a reaction with a mouse mAb against human tryptase by using the alkaline phosphatase–anti-alkaline phosphatase (APAAP) method (Dako) as described previously.23 24 Briefly, cytocentrifuged samples were fixed with buffered formalin and acetone, washed with Tris-buffered saline (TBS), and preincubated with normal rabbit serum to block the Fc receptors on the cell surface. After 3 washes with TBS, the samples were allowed to react with mouse antihuman tryptase mAb for 30 minutes at room temperature in a humidified chamber. After another 3 washes with TBS, the samples were incubated with rabbit antimouse IgG Ab, washed 3 more times, and then allowed to react with a calf intestinal alkaline phosphatase (AP)–mouse monoclonal anti-AP complex. Finally, AP activity was detected by using naphthol AS-MX phosphate, Fast Red TR, and levamisole to inhibit nonspecific AP activity. The specimens were counterstained with hematoxylin.
Cell sorting
Cells were sorted on a FACS Vantage sorter (Becton Dickinson) as described previously.25 26 Briefly, CB MNC were passed through a cell strainer (Falcon 2350; Becton Dickinson Labware, Lincoln Park, NJ) to remove cell aggregates. The cells were then stained with PC-5–conjugated CD34 and PE-conjugated CD81 as described above. A lymphocyte-sorting gate was established for both forward and side scattering. To provide a negative control, cells were stained with PC-5–conjugated or PE-conjugated mouse IgG1. Viability of the sorted cells was determined by dye exclusion.
Clonal cell cultures
Clonal cell cultures were done in triplicate as described previously.25,27 Briefly, 1-mL aliquots of a culture mixture containing 1 × 102 cells, α-minimum essential medium (α-MEM; Life Technologies, Grand Island, NY), 1.2% methylcellulose (Shinetsu Chemical, Tokyo, Japan), 30% fetal-calf serum (FCS) (Hyclone, Logan, UT), 1% deionized fraction V BSA, 10−4 M mercaptoethanol (Sigma), and a cocktail of cytokines (SCF, IL-6, IL-3, G-CSF, TPO, and EPO) were plated in 35-mm suspension culture dishes (1710099; Nunc, Naperville, IL). Cultures were then incubated at 37°C in a humidified atmosphere flushed with 5% carbon dioxide (CO2) in air. Except for megakaryocyte (Mk) colonies, all aggregates consisting of more than 50 cells were considered colonies. Colony types were determined on days 7 to 14 of the incubation by in situ observations using an inverted microscope, according to criteria established by Nakahata and Ogawa28and Nakahata et al.29 Colonies were considered Mk colonies if they contained at least 4 Mks.30 In experiments to analyze constituent cells, individual colonies were removed from the methylcellulose medium by using a 3-μL Eppendorf (Cologne, Germany) pipette under direct microscopical visualization and collected in Eppendorf microcentrifuge tubes containing 0.5 mL PBS. After 2 washes with PBS, samples were resuspended in 0.2 mL PBS containing 2.5% BSA and centrifuged in a cytocentrifuge (Shandon Southern, Elliott, IL) at 800 rpm for 5 minutes. To identify cell types, each smear was stained with May-Grünwald-Giemsa stain.
Suspension cultures
Suspension cultures were done as described previously.30 Briefly, 2-mL aliquots of a culture mixture containing 5 × 103 cells, α-MEM, 20% FCS, 1% deionized fraction V BSA, and a cocktail of cytokines (SCF, IL-6, IL-3, G-CSF, TPO, and EPO) were plated in 12-well tissue-culture plates (Becton Dickinson) at 37°C in a humidified atmosphere flushed with 5% CO2 in air. The culture medium was refreshed every 4 days with the same mixture. Cells were harvested from each well at selected times.
Fetal-thymus organ cultures
T-cell differentiation was determined in NOD/SCID mouse fetal-thymus organ cultures (FTOC) with modifications as previously reported.31-33 Briefly, thymus lobes were dissected from NOD/SCID mouse fetuses on day 16.5 of gestation. The lobes were depleted of endogenous T-cell progenitors by incubation with 1.35 mM 2′-deoxyguanosine (Sigma) for 5 days and then individually plated with 5 to 10 × 103 cells in 30 μL FTOC medium in Terasaki plates (Sumitomo Bakelite, Tokyo, Japan). The plates were inverted immediately to allow the lobe and cells to make contact at the bottom of a hanging drop. After 48 hours of incubation, each lobe was transferred to filter membranes (Costar, Cambridge, MA; pore size, 8 μm) placed on sponges (Gelfoam, Upjohn, Kalamazoo, MI) that had been soaked in FTOC medium. The lobes were incubated for 4 to 5 weeks, with fresh FTOC medium added every 3 days. At selected times, lobes were pressed gently under a glass coverslip in 0.1 mL PBS containing 0.1% BSA to release cells, and the isolated cells were analyzed by flow cytometry for expression of CD3 and CD4. The FTOC medium used throughout the FTOC experiments consisted of RPMI-1640 medium (Sigma), 20% FCS, antibiotics, MEM nonessential amino acids solution ( × 100; Sigma), and MEM vitamin solution ( × 50; Sigma). FTOC cultures were maintained in 7% CO2 at 37°C in a humidified incubator. In all FTOC experiments, 2′-deoxyguanosine–depleted thymus lobes were incubated according to the same procedures, along with colonized lobes as negative controls.
Cocultures on MS-5 stromal cells
The B-cell potential of the cells was tested in a cocultivation system on a murine stromal cell line, MS-5 (provided by Dr K. J. Mori, Niigata University, Niigata, Japan), as reported previously.33 34 Briefly, 3 × 103 cells were cultured in α-MEM supplemented with 10% FCS, SCF, and G-CSF on confluently coated MS-5 cells in 12-well culture dishes. The cultures were incubated at 37°C in a humidified atmosphere flushed with 5% CO2 in air and were refreshed each week by renewing half of the medium. After 4 weeks, cells were harvested by using 0.05% trypsin (Life Technologies) and 0.02% EDTA (Wako, Osaka, Japan) and were analyzed by flow cytometry for expression of CD10 and CD19.
Cocultures on stromal cells derived from NOD/SCID fetal-mouse livers
Natural killer (NK) cell and mast cell potentials were determined by coculturing the cells on stromal cells derived from NOD/SCID fetal-mouse livers. Briefly, livers were dissected from NOD/SCID mouse fetuses on day 14.5 of gestation and torn with fine tweezers to release the cells. The isolated cells were cultured in α-MEM containing 10% FCS in a 75-cm2 culture flask (Falcon 353111; Becton Dickinson Labware) overnight. Nonadherent cells were removed by replacing the culture medium. After the fetal-liver stromal cells had become confluent, they were harvested by using trypsin and EDTA and frozen at −80°C. Before use, the frozen cells were thawed and plated in 12-well culture dishes at a concentration of 2 × 105 cells/well in 2 mL α-MEM medium containing 10% FCS. After the cells were confluent for 2 days of culture, they were exposed to 50 Gy γ-ray irradiation. The cells were cultured on these stromal cells at a concentration of 5 × 103 cells/well with a culture medium consisting of α-MEM, 10% FCS, 10−4 M 2-mercaptoethanol, and a cocktail of cytokines including SCF, IL-7, and IL-15. After 3 to 5 weeks of culture, nonadherent cells were harvested, washed, and stained with CD45 and CD56, CD16, or CD3. Preliminary experiments revealed that these nonadherent cells were 100% positive for an antihuman CD45 mAb. Cells that expressed CD56 but not CD3 were considered NK lineage cells. Mast cells were identified as the cells stained with antihuman tryptase when the APAAP method was used.
Transplantation into NOD/SCID mice
Xenotransplantation of human lymphohematopoietic cells was done with modifications as described previously.7 35 Briefly, 1 × 104 human cells were injected through a tail vein into NOD/SCID mice irradiated with 2.4 Gy (cobalt 60) total-body irradiation. To reduce NK cell activity, the recipient mice were injected intraperitoneally with 400 μL PBS containing 20 μL anti-asialo GM1 antiserum (Wako) immediately before the transplantation. Identical treatments were done on days 11, 22, and 33 after transplantation. Mice were killed in a CO2 chamber 12 to 16 weeks after transplantation, and BM cells were harvested with PBS containing 5% FCS. Cell suspensions were filtered through a cell strainer (pore size, 40 μm; Falcon 352340; Becton Dickinson Labware) to remove clumps and debris and were then processed for flow cytometric analysis.
Statistical analysis
Data are presented as mean ± SD values. Statistical significance was determined by using the Student ttest.
Results
Expression of CD81 on human CB cells
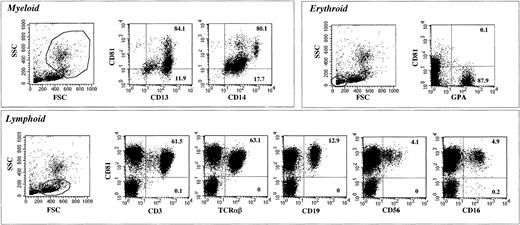
We first investigated expression of CD81 and hematopoietic lineage–specific antigens on CB cells. As shown in Figure1, more than 80% of CD13+ or CD14+ myeloid cells expressed low to medium levels of CD81, whereas all the lymphoid cells, including CD3+ or TCRαβ+ T cells, CD19+ B cells, and CD56+ or CD16+ NK cells were 100% positive for CD81. The level of expression of CD81 was highest in the CD19+ B cells. On T and NK cells, the intensity of expression was somewhat lower, although still high. Erythroid cells positive for glycophorin A (GPA) were negative for CD81. Thus, consistent with observations in a previous study,21 we found that CD81 was highly expressed on B, T, and NK lymphocytes, weakly or moderately expressed on myeloid cells, and not expressed on erythroid cells.
Flow cytometric profiles of expression of CD81 and lineage-specific surface markers on CB cells.
CB cells were stained with CD81 and various lineage-specific markers. Gates for different lineages were determined by their forward scatter (FSC) and side scatter (SSC) distributions. Cells gated for specific lineages are shown in the profiles. The numbers in each graph are the percentages of expression in a quadrant area.
Flow cytometric profiles of expression of CD81 and lineage-specific surface markers on CB cells.
CB cells were stained with CD81 and various lineage-specific markers. Gates for different lineages were determined by their forward scatter (FSC) and side scatter (SSC) distributions. Cells gated for specific lineages are shown in the profiles. The numbers in each graph are the percentages of expression in a quadrant area.
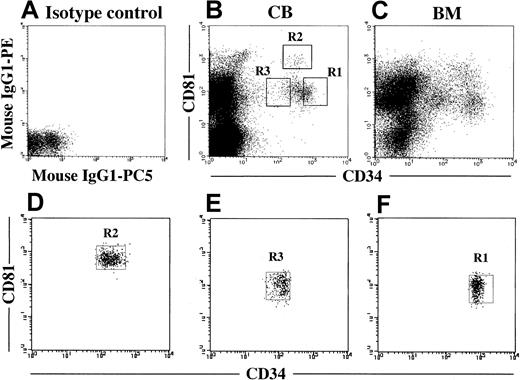
We next analyzed coexpression of CD81 on CD34+ cells. Figure 2B shows a representative flow cytometric profile for CB MNC. Most of the CB CD34+ cells expressed CD81 (97.4% ± 3.1%; n = 6). Among them, 85.9% ± 7.9% expressed a medium level and 11.9% ± 6.2% a high level of CD81. As shown in Figure 2C, BM MNC and CB MNC were similar with respect to the pattern of expression of both markers. According to the flow cytometric profile for coexpression of CD81, CD34+cells could be divided into 3 fractions: CD34+CD81+ (56.1% ± 6.2%), CD34+CD81high (7.5% ± 4.6%), and CD34lowCD81+ (21.2% ± 5.1%; fractions R1, R2, and R3, respectively, in Figure 2B).
Coexpression of CD81 on CD34+ cells in CB and BM MNC.
(A) Isotype control. (B) Expression of CD34 and CD81 on CB MNC. CD34+CD81+, CD34+CD81High, and CD34LowCD81+ cells were gated as shown in R1, R2, and R3, respectively. These gates were used for cell sorting. (C) Expression of CD34 and CD81 on BM MNC. (D-F) Cytometric reanalysis of sorted fractionated cells in each gate shown in Figure 2B.
Coexpression of CD81 on CD34+ cells in CB and BM MNC.
(A) Isotype control. (B) Expression of CD34 and CD81 on CB MNC. CD34+CD81+, CD34+CD81High, and CD34LowCD81+ cells were gated as shown in R1, R2, and R3, respectively. These gates were used for cell sorting. (C) Expression of CD34 and CD81 on BM MNC. (D-F) Cytometric reanalysis of sorted fractionated cells in each gate shown in Figure 2B.
Hematopoietic potential of CB cells defined by expression of CD81 and CD34
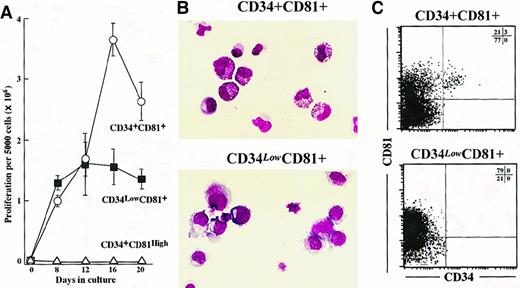
We created suspension cultures of CD34+CD81+, CD34+CD81high, and CD34lowCD81+ cells sorted from CB MNC to explore their hematopoietic potential. Sorting gates were determined as shown in Figure 2B, and sorted cells were reanalyzed cytometrically to confirm purity (Figures 2D-2F). Five thousand cells in each fraction were cultured with SCF, IL-3, IL-6, G-CSF, TPO, and EPO (Figure3A). CD34+CD81high cells could not proliferate in the culture. The time-course study revealed that CD34+CD81+ cells reached a proliferative peak on day 16 of culture, whereas CD34lowCD81+cells did so 4 days earlier. Moreover, the peak number of CD34+CD81+ cells was 2 times as large as that of CD34lowCD81+ cells (Figure 3A). Cytospin preparations of the cultured cells on day 12 showed that the differentiation potential of the CD34+CD81+ and CD34lowCD81+ fractions was quite different (Figure 3B). Most of the cells cultured from the CD34+CD81+ fraction were myeloid cells (86.6% ± 2.1%; n = 4), whereas most of the cells from the CD34lowCD81+ fraction were erythroid cells (86.9% ± 3.3%) and only a few myeloid cells were present (7.3% ± 0.8%). Interestingly, flow cytometric analysis of the cultured cells on day 15 showed that those derived from the CD34+CD81+ population contained a substantial number of CD34+ cells, whereas cells from the CD34lowCD81+ fraction did not (Figure3C).
Proliferation and differentiation potentials for CD34+CD81+, CD34lowCD81+, and CD34+CD81high cells in suspension cultures.
(A) Proliferation of 1 × 103CD34+CD81+ (○), CD 34lowCD81+ (▪), and CD34+CD81high cells (▵) in a time-course experiment. (B) Photographs of progenies derived from CD34+CD81+ and CD34lowCD81+ cells in suspension cultures on day 12. A tendency for CD34+CD81+ cells to generate myelocytes and for CD34lowCD81+ cells to generate erythrocytes is shown (× 400). (C) Low cytometric profiles of expression of CD81 and CD34 on progenies derived from CD34+CD81+ and CD34lowCD81+ cells in suspension cultures on day 14. CD34+ cells were obtained only from the CD34+CD81+ fraction.
Proliferation and differentiation potentials for CD34+CD81+, CD34lowCD81+, and CD34+CD81high cells in suspension cultures.
(A) Proliferation of 1 × 103CD34+CD81+ (○), CD 34lowCD81+ (▪), and CD34+CD81high cells (▵) in a time-course experiment. (B) Photographs of progenies derived from CD34+CD81+ and CD34lowCD81+ cells in suspension cultures on day 12. A tendency for CD34+CD81+ cells to generate myelocytes and for CD34lowCD81+ cells to generate erythrocytes is shown (× 400). (C) Low cytometric profiles of expression of CD81 and CD34 on progenies derived from CD34+CD81+ and CD34lowCD81+ cells in suspension cultures on day 14. CD34+ cells were obtained only from the CD34+CD81+ fraction.
To examine the proliferation and differentiation potentials of the cells in the 3 fractions more closely, methylcellulose clonal cultures were done with the same cytokine combinations used in the suspension cultures (Table 1). CD34+CD81high cells gave rise to no colonies in 6 experiments, whereas CD34+CD81+, and CD34lowCD81+ cells did produce colonies, although the numbers and types of colonies were different. The observations regarding CD34+ cell colonies (as expected by the estimation from the proportion of each fraction in CD34+ cells) and colony formation indicated that binding of CD81 did not affect colony formation. CD34+CD81+ cells generated the most colonies, among which were all the types of hematopoietic colonies except for Mk colonies. Mk colonies were generated only from CD34lowCD81+ cells, but few myeloid colonies were produced from that fraction. Erythroid bursts were produced from both CD34+CD81+ and CD34lowCD81+ cells. Mixed colonies were also generated from both fractions, but those derived from CD34lowCD81+ cells were greatly enriched (78.3% ± 7.6%; range, 65.2%-87.5%; P < .001 when compared with the CD34+CD81+ fraction). The cytospin preparations of individual colonies chosen randomly on day 10 of culture (10 colonies for each fraction) showed that the mixed colonies derived from CD34lowCD81+ cells contained a higher proportion of erythroid cells and a lower proportion of myelocytic cells than those from CD34+CD81+cells (Table 2). Colonies containing Mks were found more frequently in mixed colonies derived from CD34lowCD81+ cells. These results indicate that most of the myeloid and megakaryocytic progenitors were in the CD34+CD81+ and CD34lowCD81+cell fractions, respectively, and that erythroid and multipotential progenitors were shared by both fractions but that multipotential progenitors in the CD34lowCD81+ fraction had lost most myeloid potential.
B-cell potential of cells defined by expression of CD81 and CD34
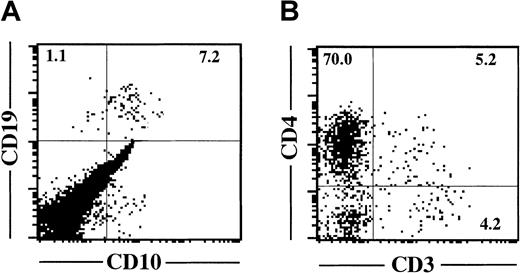
To examine the potential to commit to a B-cell lineage, 3 × 103 cells from each fraction were plated on MS-5 stromal cells in the presence of SCF and G-CSF.34CD34+CD81high cells could not grow on MS-5 cells and disappeared within a week. CD34lowCD81+ cells also could not proliferate on MS-5 and disappeared gradually during 2 to 3 weeks. B cells expressing CD19 or CD10 were generated only from the CD34+CD81+ fraction (Figure4A). Similar results were obtained in 3 independent experiments. Thus, B-lymphoid lineage potential was observed only in the CD34+CD81+ cell population.
B- and T-lineage potentials of CD34+CD81+ cells.
(A) Flow cytometric profile shows expression of CD19 and CD10 on lymphocyte-gated cells derived from 3 × 103CD34+CD81+ cells cocultured with MS-5 cells for 4 weeks. (B) Flow cytometric profile shows expression of CD3 and CD4 on lymphocyte-gated cells derived from 5 × 103CD34+CD81+ cells recovered in FTOC for 4 weeks.
B- and T-lineage potentials of CD34+CD81+ cells.
(A) Flow cytometric profile shows expression of CD19 and CD10 on lymphocyte-gated cells derived from 3 × 103CD34+CD81+ cells cocultured with MS-5 cells for 4 weeks. (B) Flow cytometric profile shows expression of CD3 and CD4 on lymphocyte-gated cells derived from 5 × 103CD34+CD81+ cells recovered in FTOC for 4 weeks.
T-cell potential of cells defined by expression of CD81 and CD34
T-cell potential was determined in FTOC as reported previously.33 When 5 × 103CD34+CD81+ cells were cocultured with NOD/SCID fetal-mouse thymus lobes, the number of cells recovered in FTOC 4 weeks later was 7.42 ± 0.38 × 104/lobe (n = 10). Flow cytometric analysis revealed that 75.2% of the cells recovered were CD4+ cells and 9.4% were CD3+ T cells (Figure4B). On the other hand, thymus lobes cocultured with neither CD34lowCD81+ nor CD34+CD81high cells were enlarged and few cells were recovered in FTOC. To eliminate the possibility that committed T-cell progenitors that could develop into mature T cells in a shorter period existed in the CD34lowCD81+ or CD34+CD81high fractions, we also harvested cells in FTOC on days 14 and 21 of culture. Cells from these 2 fractions did not generate lymphoid cells. Similar results were obtained in 3 independent experiments. Thus, the T-lymphoid potential existed exclusively in the CD34+CD81+cell fraction.
NK cell and mast cell potentials of cells defined by expression of CD81 and CD34
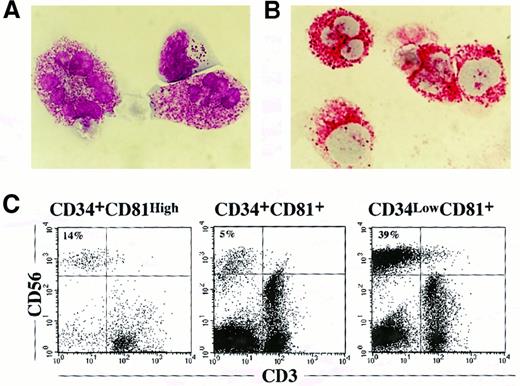
Human NK cells have been reported to be induced in vitro from CD34+ cells in many different culture systems.10,36-38 One study found that a stromal cell line derived from murine fetal-liver tissue supported generation of NK cells from human Lin−CD34+CD38−cells.38 In our preliminary experiments, CD34+cells from human CB MNC could generate CD56+CD3− NK cells when cultured on stromal cells derived from NOD/SCID fetal-mouse liver with a cytokine cocktail of SCF, IL-7, and IL-15 for 4 to 6 weeks (data not shown). Human tryptase–positive mast cells were also found in the cocultures (data not shown). Using this culture system, we investigated NK cell and mast cell potentials in the 3 cell fractions. In 5 weeks of coculture with stromal cells derived from NOD/SCID fetal-mouse liver, 5 × 103 CD34+CD81+, CD34lowCD81+, and CD34+CD81high cells yielded 5.17 ± 0.96, 6.11 ± 0.36, and 0.29 ± 0.06 × 105 cells, respectively. CD34+CD81high cells, which had low proliferation potential on NOD/SCID fetal-mouse liver stroma, generated large granular lymphocytes and mast cells that stained with antihuman tryptase when the APAAP method was used (Figures 5A and5B). CD34+CD81+and CD34lowCD81+ cells also generated NK cells and mast cells in addition to myeloid cells. Flow cytometric analysis revealed that CD56+CD3− NK cell and CD56−CD3low mast cell fractions were present in the coculture of CD34+CD81high cells on day 28 (Figure 5C). These cells were also produced from CD34+CD81high and CD34+CD81+ fractions, but the most pronounced production of CD56+CD3− NK cells was observed in the CD34lowCD81+ population (39% of total cell recovery).
NK cell and mast cell production from CD34+CD81+, CD34lowCD81+, and CD34+CD81high cells cultured on stroma derived from NOD/SCID fetal-mouse liver.
(A) Appearance of NK and mast cells derived from the CD34+CD81high fraction in 4-week culture with NOD/SCID fetal-mouse liver stroma. The photograph shows 2 mast cells and a large granular NK cell stained with May-Grünwald–Giemsa solution (× 1000). (B) The presence of mast cells were confirmed by the reaction with a mAb specific to human tryptase on APAAP staining. The photograph shows positive staining for tryptase in mast cells derived from the CD34+CD81high fraction (× 1000). (C) Flow cytometric profiles of expression of CD56 and CD3 on cells cultured from CD34+CD81+, CD34lowCD81+, and CD34+CD81high cells on NOD/SCID fetal-mouse liver stroma for 4 weeks.
NK cell and mast cell production from CD34+CD81+, CD34lowCD81+, and CD34+CD81high cells cultured on stroma derived from NOD/SCID fetal-mouse liver.
(A) Appearance of NK and mast cells derived from the CD34+CD81high fraction in 4-week culture with NOD/SCID fetal-mouse liver stroma. The photograph shows 2 mast cells and a large granular NK cell stained with May-Grünwald–Giemsa solution (× 1000). (B) The presence of mast cells were confirmed by the reaction with a mAb specific to human tryptase on APAAP staining. The photograph shows positive staining for tryptase in mast cells derived from the CD34+CD81high fraction (× 1000). (C) Flow cytometric profiles of expression of CD56 and CD3 on cells cultured from CD34+CD81+, CD34lowCD81+, and CD34+CD81high cells on NOD/SCID fetal-mouse liver stroma for 4 weeks.
LTR ability of the cells defined by expression of CD81 and CD34
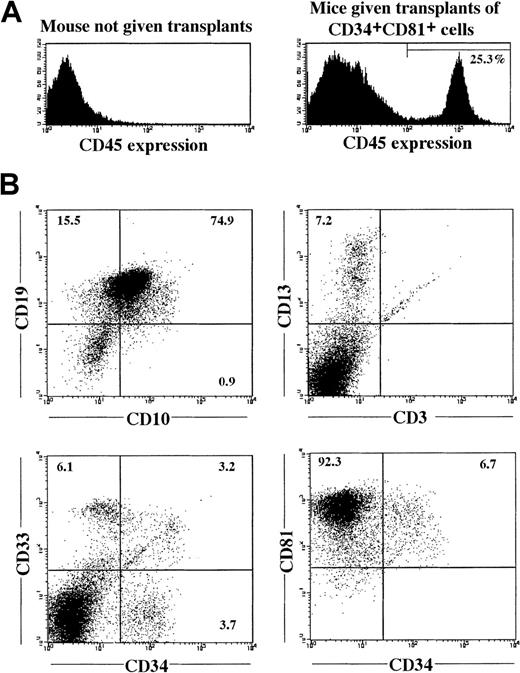
To examine the LTR ability of the 3 fractions, cells of each fraction were transplanted into NOD/SCID mice (Table3). When CD34lowCD81+ or CD34+CD81high cells were transplanted into 9 mice (1 × 104 cells in each recipient), none of the mice had a successful engraftment. However, when 1 × 104CD34+CD81+ cells were transplanted into 13 mice, 9 of the mice had human CD45+ cells in BM 12 to 16 weeks after transplantation. A representative BM profile of a mouse that had successful engraftment with CD34+CD81+cells is shown in Figure 6. The CD45+ cells contained CD13+ or CD33+ myeloid cells and CD10+ or CD19+ B cells (or both) but no or few CD3+ T cells (Figure 6B and Table 3). The CD45+ cells also contained CD34+ immature cells, most of which coexpressed CD81. This result indicates that LTR hematopoietic stem cells exist in the CD34+CD81+ population.
Reconstitution of lymphohematopoiesis in a NOD/SCID mouse by CB CD34+CD81+ cells.
(A) Human CD45+ cells in BM of NOD/SCID mice not given transplants and in NOD/SCID mice given transplants of 1 × 104 CD34+CD81+ cells, 16 weeks after transplantation. (B) Expression of various lineage-specific markers on human CD45+ cells shown in Figure 6A.
Reconstitution of lymphohematopoiesis in a NOD/SCID mouse by CB CD34+CD81+ cells.
(A) Human CD45+ cells in BM of NOD/SCID mice not given transplants and in NOD/SCID mice given transplants of 1 × 104 CD34+CD81+ cells, 16 weeks after transplantation. (B) Expression of various lineage-specific markers on human CD45+ cells shown in Figure 6A.
Discussion
In this study, we found that CD81 is expressed on the majority of human blood cells except mature erythrocytes. In particular, human CD34+ cells from either CB or BM were mostly positive for CD81. The CD34+ cells could be divided into 3 populations—CD34+CD81+, CD34lowCD81+, and CD34+CD81high—according to the coexpression of CD81. The suspension culture results and clonogenic assays revealed that the CD34+CD81+ cells were a heterogeneous population composed of myeloid, erythroid, and multipotential—but not megakaryocytic—progenitors, and most of the myeloid progenitors were in this fraction. On the other hand, the CD34lowCD81+ fraction had less proliferative ability and was more likely to be enriched with erythroid and Mk progenitors accompanied by a low number of myeloid progenitors. Multipotential progenitors were also numerous in the CD34lowCD81+ population, but although these progenitors were able to generate erythroid cells and Mks, they produced few myeloid cells, unlike those in CD34+CD81+ population. This observation indicates that multipotential progenitors are heterogeneous in terms of hematopoietic potential and that when multipotent progenitors down-regulate CD34 expression at the CD81+ cell stage, their myeloid potential is exhausted.
Stem cell activity was evaluated by the ability to reconstitute human lymphohematopoiesis in NOD/SCID mouse BM and was present exclusively in the CD34+CD81+ cell fraction. This may be why CD34+ cells were retained after 15 days of suspension culture of CD34+CD81+ cells. LTR ability was not detected in the CD34lowCD81+ or CD34+CD81high cell fraction. Therefore, down-regulation of CD34 or up-regulation of CD81 on CD34+CD81+ lymphohematopoietic stem cells may indicate loss of stem cell activity.
The divergence of lymphoid potential in the development of lymphohematopoietic stem and progenitor cells has not been well described. We previously showed that three fourths of mouse Lin−c-Kit+Sca-1+ multipotential progenitors had no T-cell potential, suggesting that the divergence of T-cell potential occurs at an early stage of the development of lymphohematopoiesis.39 In the current study, T- and B-cell potentials were found only in the CD34+CD81+cell fraction, in association with LTR ability. We also observed that CD34lowCD81+ multipotential progenitors with erythroid and megakaryocytic potentials but little myeloid potential had already lost T- and B-cell potentials. These observations may support the idea that T- and B-lymphoid potentials diverge early during the development of lymphohematopoietic progenitor and stem cells. In contrast to T and B cells, NK cells and mast cells were generated from all 3 fractions (CD34+CD81+, CD34lowCD81+, and CD34+CD81high cells) independently of hematopoietic potential or LTR ability, indicating that NK cell and mast cell potentials are more widely distributed than T- and B-cell potentials in CD34+ progenitors. CD34+CD81high cells constitute a unique population whose potentials are restricted to NK cell and mast cell lineages.
Overall, the current study indicates that there is a complex of classes in the hierarchy of lymphohematopoietic progenitor and stem cells and that they may have unique types of expression of surface markers according to their potentials. Galy et al8,12 showed that human BM CD34+ cells could be fractionated into lymphohematopoietic stem and progenitor cells with various potentials by using Thy-1, CD45RA, and CD10. Another study showed that T-lymphoid potential is quantitatively different among CD34+Lin−, CD34+CD38+, and CD34++CD38− subpopulations,40suggesting a sequential loss of stem cell features and an enhancement of T-lineage potential in the CD34+ compartment. There appear to be several developmental classes for divergence of lymphoid, myeloid, erythroid, and megakaryocytic lineages from each other in the CD34+ pool.
In summary, the current study delineated expression of CD81 on human lymphohematopoietic stem and progenitor cells. The ubiquitous expression on these cells indicates the existence of functional roles for CD81 in the development of lymphohematopoiesis. According to our findings, along the differentiation cascade from CD34+CD81+ lymphohematopoietic stem cells, there appear to be pathways to CD34lowCD81+ or CD34+CD81high cells, even if they are indirect. CD34lowCD81+ pathways define the loss of LTR ability, T- and B-cell potentials, and myeloid potentials, whereas CD34+CD81high pathways represent the exclusive commitment to NK cells and mast cells. Thus, CD81 may serve as a marker for separating human lymphohematopoietic stem and progenitor cells in their developmental stages. This research may increase understanding of the characteristics of human lymphohematopoietic stem and progenitor cells and benefit clinical use of fractionated lymphohematopoietic stem and progenitor cell transplantation.
Supported by the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Pharmaceutical Safety and Research of Japan, and a Grant-in Aid for the High-Tech Research Center from the Japanese Ministry of Education, Science, Sports, and Culture to Nihon University.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kohichiro Tsuji, Division of Cellular Therapy, Advanced Clinical Research Center, Institute of Medical Science, University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108-8639, Japan; e-mail: tsujik@ims.u-tokyo.ac.jp.