Abstract
The most common cause of an increase of the hematocrit is secondary to elevated erythropoietin levels. Erythrocytosis is assumed to cause higher blood viscosity that could put the cardiovascular system at hemodynamic and rheological risks. Secondary erythrocytosis results from tissue hypoxia, and one can hardly define what cardiovascular consequences are caused by chronic erythrocytosis or hypoxia. Herein, a novel transgenic (tg) mouse line is characterized that is erythrocytotic because of chronic overexpression of the human erythropoietin gene. These mice grow up well, reaching a hematocrit of about 0.80 in adulthood. Blood volume of adult tg mice was markedly increased by 75%. Unexpectedly, blood pressure was not elevated and cardiac output was not decreased. Still, the adult tg mice showed features of cardiac dysfunction with increased heart weight. In vivo, high-frequency echocardiography revealed marked ventricular dilatation that was confirmed by histologic examination. Furthermore, by transmission electron microscopy, a prominent intracellular edema of the cardiomyocytes was seen. Exercise performance of the tg mice was dramatically reduced, unmasking the severity of their compromised cardiovascular function. In addition, life expectancy of the tg mice was significantly reduced to 7.4 months. Our findings suggest that severe erythrocytosis per se results in cardiac dysfunction and markedly reduced life span.
Introduction
To maintain an adequate O2 capacity of the blood, erythropoiesis is adjusted to the O2 demand of the tissue by the glycoprotein hormone erythropoietin (EPO), which is produced in response to hypoxia predominantly in the kidney.1,2 EPO inhibits apoptosis of erythrocytic progenitors in the bone marrow and stimulates their proliferation and differentiation. Lack of EPO leads to anemia with fatigue and cellular hypoxia. Excess of EPO results in secondary erythrocytosis. A rise in hematocrit is associated with a greater blood viscosity that may cause hemodynamic and rheological problems.3 It is believed that hematocrit values above 0.50 increase the risk to acquire hypertension, heart failure, myocardial infarction, seizures, and thromboses. However, the cardiovascular consequences of an isolated chronic erythrocytosis have not been investigated in detail, because increased hemoglobin and red blood cell concentrations are generally induced by hypoxemia. Continued exposure to hypoxia causes vasoconstriction and vascular remodeling in the lung, hypertrophy of the right ventricle,4 and adaptive changes in the activity of the autonomous nervous system5 as well as in the formation of vasoactive hormones and other mediators.6 7
Constitutive, hypoxia-independent overexpression of the humanEPO gene in transgenic mice enabled us to investigate cardiovascular effects of an isolated, chronic erythrocytosis (mean hematocrit of 0.80 in adulthood). In these mice, we studied blood volume, mean arterial blood pressure, cardiac output, and exercise endurance. We applied light and electron microscopic techniques to study structural changes of heart and blood vessels. Our study sheds new light on the cardiovascular consequences of chronic erythrocytosis and reveals limits of compensatory mechanisms.
Materials and methods
Animals
The investigations were performed in strict accordance with theGuide for the Care and Use of Laboratory Animals (National Institutes of Health publication no. 85-23, revised 1996) and approved by the local Governmental Commissions for the Care of Animals. Erythrocytotic mice used in this study (line TgN [PDGFBEPO] 321 Zbz, also termed “tg6”) were transgenic (tg) for the humanEPO gene. The line was generated by microinjection ofEPO complementary DNA (cDNA) driven by the human platelet-derived growth factor B-chain promoter into pronuclei of B6C3F1 hybrid mouse fertilized oocytes.8 The transgene was transmitted in autosomal dominant fashion by mating heterozygous males (tg) with wild-type (wt) females. According to Mendelian rules, about 50% of the offspring carried the transgene. The nontransgenic offspring siblings were used as controls. Mice were kept in conventional housing. The adult mice studied had a weight of 27.1 ± 4.2 g at an age of 3.6 ± 1.8 months (means ± SD). For all experiments, animals were anesthetized with pentobarbital (30 mg/kg, 30 mg/mL) and ketamine (25 mg/kg, racemic mixture of S[+] and R[−] ketamine in a final concentration of 25 mg/mL) injected intramuscularly. Muscle relaxants or supplemental iron were not administered. Rectal temperature was 37°C to 38°C. Hematocrit was determined from tail blood by the microhematocrit technique. Because blood sampling without heavily squeezing the tail was impossible in mice with a body weight below 8 g, hematocrit values from such small animals were not included in the study.
Blood volume determination
Blood volume was measured in spontaneously breathing anesthetized mice as described9 with the following modifications. In brief, 1 mL of freshly obtained heparinized mouse blood was labeled with 1.5 to 1.9 GBq 99mTc pertechnetate (UltraTag RBC kit, Mallinckrodt Medical, St Louis, MO) within 30 minutes. Radiochemical purity was 96% to 98% during repeated measurements. The activity of 200 μL 99mTc-RBC–labeled whole blood (activitypre) was counted and approximately 50 μL (30 to 45 MBq) was immediately injected via a jugular vein catheter. The catheter with the syringe still connected was removed, and the jugular vein tied during withdrawal of the catheter. The swabs for blotting dry any minor blood leakage that possibly occurred during withdrawal were carefully collected. Not injected radioactivity (activitypost) from the swaps, the catheter, the syringe, and any other material was counted. The activity administered (activityinj) was calculated (activitypre− activitypost). After 20 minutes, median laparotomy was performed. The caudal vena cava was exposed, cannulated with a 26-gauge catheter (Abbocath-T, Abbott Ireland, Sligo, Ireland), and 0.4 mL blood was drawn for measurement of radioactivity of 200 μL (activitydrawn). Measurements were performed in duplicate. Blood volume was calculated from the activitydrawn and the activityinj, including a half-life correction for decay of radioactivity (TH = 6 hours). The intravascular distribution of labeled red cells was visualized by planar scintigraphy (PRISM 3000 XP; Picker/Marconi, Cleveland Heights, OH).
Cardiovascular investigations
For measurements of the mean arterial pressure, the trachea was cannulated, and the animals were ventilated with oxygen-enriched air (Minivent Type 845, Hugo Sachs Elektronik–Harvard Apparatus, March, Germany) without positive end-expiratory airway pressure. The respiration rate was set between 130 and 180 breaths per minute. The tidal volume was 180 to 230 μL. Arterial pressure was measured in the common carotid artery via a 26-gauge catheter (Abbocath-T, Abbott Ireland) connected to an ISOTEC pressure transducer and a Plugsys amplifier system (Hugo Sachs Elektronik–Harvard Apparatus). Heart rate (HR) was calculated from the arterial pressure recording. Central venous pressure was measured in the cranial vena cava via a 26-gauge catheter (Abbocath-T, Abbott Ireland) in spontaneously breathing animals. The catheter was inserted into the external jugular vein and advanced into the cranial vena cava. The correct position of the catheter was verified by the typical tracing of the pressure curve. The pressure transducer P10EZ (nonlinearity and hysteresis ± 0.1% over 0-10 mm Hg, Ohmeda Singapore Pte, Singapore) was connected to an amplifier with the input circuit galvanically isolated from the output circuit and the power supply (Department of Engineering and Electronics, Medical University of Lübeck, Germany). This allowed for accurate measurement in the low-pressure range and eliminated source ground loops and line receiving of signals in the laboratory environment.
Cardiac output was measured by echocardiography with a 10 MHz phase array sector probe (ATL-5000 HDI, Advanced Technology Laboratories, Philips, Hamburg, Germany) in anesthetized mice breathing spontaneously. Pulsed Doppler measurements of the aortic blood flow were performed just above the aortic valve. Directed by color flow mapping, the sample volume of the pulsed wave Doppler was localized near an angle of 0° or 180° to the direction of blood flow. By measurement of the velocity time integral (VTI), aortic diameter and the HR cardiac output were calculated according to the formula π × d2/4 × VTI × HR. M-mode echocardiography in the long-axis view was applied to measure left ventricular systolic diameter, left ventricular end-diastolic diameter, and fractional shortening of the left ventricle. All measurements were performed from leading edge to leading edge, according to the guidelines of the American Society of Echocardiography.
Histologic investigation
Paraffin specimens for light microscopy were cut in 7-μm–thick transversal sections and stained with hematoxylin-eosin (HE) and trichrome according to Masson Goldner. For transmission electron microscopy (TEM 400, Philips, Eindhoven, The Netherlands), specimens were immersed in 2.5% glutaraldehyde (in cacodylate buffer, pH 7.4), postfixed in 1% osmium tetroxide, and embedded in araldite (Fluka, Neu-Ulm, Germany). Ultrathin sections were contrastained with uranyl acetate and lead citrate.
Exercise endurance
Exercise endurance was measured as the time elapsed until the mice suffered from exhaustion when running on a belt treadmill. The treadmill (Department of Engineering and Electronics, Medical University of Lübeck, Germany) had an adjustable belt speed between 0 to 80 m/min. A shock grid with adjustable energy output (0.2 mA, 0 to 400 V DC) was situated at the lower end of the belt. Mice were adapted to running on the belt by placing them on the treadmill thrice weekly with the incline set to 0° and 3 m/min belt speed. After 1 week of adaptation, the exercise investigation was performed. The treadmill belt was set to a 20° incline with a speed of 20 m/min. Mice were forced to run on the belt until exhaustion by applying electric shocks (0.2 mA, 400 V, 1 Hz) from the shock grid. When mice stayed on the shock grid for more than 10 consecutive seconds, exhaustion was assumed.
Statistics
Data were analyzed by an unpaired t test or the Welch approximate test, if variances were not equal. Kaplan-Meier statistics were applied to survival data. Differences were considered significant at P < .05. Data are shown as means ± SD, unless otherwise specified.
Results
Growth and development
The average litter size of a breeding couple (heterozygous tg male × wt female) was 7.3 ± 2.8. At birth, there was no difference in weight between wt and tg siblings (Figure1). The weight gain of tg male and female mice was not different from that of their wt counterparts. Hematocrit gradually increased in the tg mice from 0.51 at 8 to 10 g body weight and 0.63 at 11 to 17 g (juvenile age) to 0.77 in early adulthood (17 to 20 g) and remained elevated at approximately 0.80 throughout life (Figure 1). There was a linear relationship between age, weight, and hematocrit in tg male and female mice between 3 to 10 weeks of age (Figure 2). In adult wt mice, hematocrit was 0.44 to 0.50 independent of their age. With respect to liveliness, no differences between wt and tg mice were recognized on regular observation of the animals in their cages. However, the life span of tg mice was markedly reduced. The number of tg animals suffering from premature death increased after 5.5 months of age (Figure 3). During 10.5 months of observation, of the 26 wt mice, 2 deaths occurred compared with 31 deaths of the 37 tg mice, resulting in a significantly reduced mean survival of 7.4 months for tg mice (P < .0001).
Growth and hematocrit increase of wt and tg mice.
Weight gain of wt and tg mice was not different for male and female mice ([A] 1526 determinations in 103 wt mice, 1207 determinations in 100 tg mice. [B] 1182 determinations in 85 wt mice, 1611 determinations in 109 tg mice). Hematocrit increased with age and weight in tg mice until adulthood both in male ([C] wt: n = 113; tg: n = 93) and female ([D] wt: n = 85; tg: n = 124) animals.
Growth and hematocrit increase of wt and tg mice.
Weight gain of wt and tg mice was not different for male and female mice ([A] 1526 determinations in 103 wt mice, 1207 determinations in 100 tg mice. [B] 1182 determinations in 85 wt mice, 1611 determinations in 109 tg mice). Hematocrit increased with age and weight in tg mice until adulthood both in male ([C] wt: n = 113; tg: n = 93) and female ([D] wt: n = 85; tg: n = 124) animals.
Relation between the increase of age, weight, and hematocrit.
The horizontal and vertical scatter of the data pairs for age versus hematocrit and body weight versus hematocrit in tg male (A) and tg female (B) mice demonstrated a linear correlation between the increase of age, weight, and hematocrit (n = 217).
Relation between the increase of age, weight, and hematocrit.
The horizontal and vertical scatter of the data pairs for age versus hematocrit and body weight versus hematocrit in tg male (A) and tg female (B) mice demonstrated a linear correlation between the increase of age, weight, and hematocrit (n = 217).
Kaplan-Meier plot of survival for wt and tg mice.
Mean survival of tg mice (n = 26) was significantly reduced to 7.4 months, whereas wt mice (n = 37) had a mean survival of 10.0 months. The observational period was 10.5 months. Cumulative survival was 0.92 ± 0.05 (mean ± SEM) for wt mice in contrast to 0.34 ± 0.08 for tg mice. Survival distribution was significantly different (P < .0001, log-rank test).
Kaplan-Meier plot of survival for wt and tg mice.
Mean survival of tg mice (n = 26) was significantly reduced to 7.4 months, whereas wt mice (n = 37) had a mean survival of 10.0 months. The observational period was 10.5 months. Cumulative survival was 0.92 ± 0.05 (mean ± SEM) for wt mice in contrast to 0.34 ± 0.08 for tg mice. Survival distribution was significantly different (P < .0001, log-rank test).
Blood volume
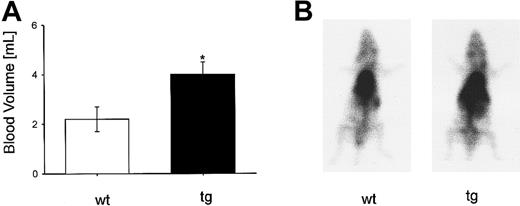
The thoracic aorta appeared dilated in tg animals, and the large veins and the subcutaneous tissue were markedly hyperemic and congested. In adult tg mice, blood volume (Figure4A) was increased by 75% (P < .05) compared with that of wt mice. On the planar99mTc-scintiscan (Figure 4B), an increase of activity is shown in tg mouse in the thoracic and upper abdominal region corresponding to the heart, lung, liver, and on the left side, the spleen. In tg mice, a blood volume of 4.0 mL was measured. The hematocrit of the tg mice was about 0.80. Thus, plasma volume was 0.8 mL and red cell volume was 3.2 mL. For wt mice, blood volume was 2.2 mL with a hematocrit of 0.50, resulting in a plasma and a red cell volume of 1.1 mL each. Overall, the increased blood volume in tg mice was predominantly because of a higher red cell volume.
Blood volume determination and visualization using99mTc-labeled red blood cells.
(A) Adult tg mice had a 75% increase of blood volume (wt: n = 4; tg: n = 5), *P < .05. (B) Planar scintiscan showing an increased radioactivity of the tg mouse in the areas of the heart, lung, liver, and left abdomen, where the more than 4-fold (data not shown) enlarged spleen was situated.
Blood volume determination and visualization using99mTc-labeled red blood cells.
(A) Adult tg mice had a 75% increase of blood volume (wt: n = 4; tg: n = 5), *P < .05. (B) Planar scintiscan showing an increased radioactivity of the tg mouse in the areas of the heart, lung, liver, and left abdomen, where the more than 4-fold (data not shown) enlarged spleen was situated.
Cardiovascular data
Invasively measured mean arterial pressure (Figure5A) did not differ between wt and tg mice. HR (wt: 529 ± 49 beats per minute; tg: 497 ± 56 beats per minute) as well as arterial pO2 (wt: 158 ± 23 mm Hg; tg: 146 ± 74 mm Hg) in mice spontaneously breathing room air supplemented with O2 were also similar. Central venous pressure was significantly (P < .05) higher in tg compared with wt mice, indicating an increased cardiac preload in the tg animals (Figure 5B). Pulsed Doppler measurements by noninvasive high-frequency echocardiography revealed that there was no significant difference of cardiac output between wt and tg mice (Figures 5C,6A). In the echocardiographic M-mode investigation (Figure 6B,C), left ventricular systolic diameter and left ventricular end-diastolic diameter (data not shown) were significantly increased in tg animals. Fractional shortening of the left ventricle in tg mice was reduced by 25%.
Cardiovascular data.
(A) Mean arterial pressure measured in the carotid artery was not significantly different between wt and tg mice (wt: n = 12; tg: n = 8). (B) Central venous pressure measured in the cranial vena cava was significantly increased in tg mice (wt: n = 3; tg: n = 5, *P < .05). (C) Cardiac output determined by echocardiography was similar in wt and tg mice (wt: 14.4 ± 4.0 mL/min; tg: 14.8 ± 3.1 mL/min).
Cardiovascular data.
(A) Mean arterial pressure measured in the carotid artery was not significantly different between wt and tg mice (wt: n = 12; tg: n = 8). (B) Central venous pressure measured in the cranial vena cava was significantly increased in tg mice (wt: n = 3; tg: n = 5, *P < .05). (C) Cardiac output determined by echocardiography was similar in wt and tg mice (wt: 14.4 ± 4.0 mL/min; tg: 14.8 ± 3.1 mL/min).
Representative transthoracic high-frequency echocardiograms.
(A) Pulsed Doppler tracing of the left ventricular outflow tract measured just above the aortic valve without angle correction. (B) M-mode echocardiogram in the parasternal long-axis view. (C) Systolic diameter of the left ventricle (M-mode long-axis view, wt: 11 measurements in 6 mice; tg: 10 measurements in 5 mice) was significantly increased in tg mice, *P < .05.
Representative transthoracic high-frequency echocardiograms.
(A) Pulsed Doppler tracing of the left ventricular outflow tract measured just above the aortic valve without angle correction. (B) M-mode echocardiogram in the parasternal long-axis view. (C) Systolic diameter of the left ventricle (M-mode long-axis view, wt: 11 measurements in 6 mice; tg: 10 measurements in 5 mice) was significantly increased in tg mice, *P < .05.
Anatomic studies
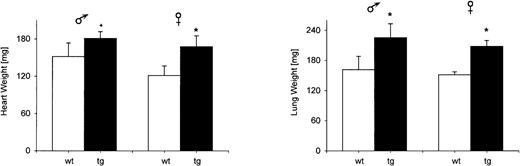
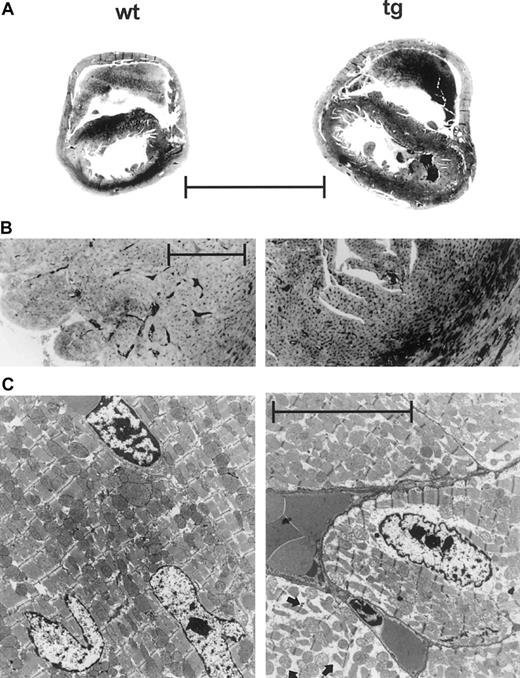
Macroscopically, the hearts of tg mice were enlarged. Heart weight was significantly increased in adult tg animals (Figure7), as was the weight of the lung. A cross section (wt: n = 3; tg: n = 3) at the level of the pars muscularis of the interventricular septum showed dilatation of both ventricular cavities without preference for either side of the heart (Figure 8A). The free wall of the right and left ventricle was hypertrophied by about 30%. The hypertrophy of the interventricular septum was less pronounced.
Organ weights.
The organ weights of heart and lung were significantly increased in adult tg mice (wt: n = 8; tg: n = 8);+P = .057; *P < .05.
Organ weights.
The organ weights of heart and lung were significantly increased in adult tg mice (wt: n = 8; tg: n = 8);+P = .057; *P < .05.
Representative histology of the adult heart.
(A) Cross section at the level of the pars muscularis of the interventricular septum. Tg hearts were enlarged with biventricular dilatation and hypertrophy of the free walls. (Hematoxylin-eosin stain; magnification ×3.5, scale 3 mm.) (B) Prominent hyperemia of the capillaries packed with erythrocytes in the tg heart. (Trichrome; magnification ×106, scale 200 μm.) (C) Transmission electron micrograph showing marked intracellular edema in cardiomyocytes of the tg heart (arrows, lower right part). (Magnification ×8300, scale 5 μm.)
Representative histology of the adult heart.
(A) Cross section at the level of the pars muscularis of the interventricular septum. Tg hearts were enlarged with biventricular dilatation and hypertrophy of the free walls. (Hematoxylin-eosin stain; magnification ×3.5, scale 3 mm.) (B) Prominent hyperemia of the capillaries packed with erythrocytes in the tg heart. (Trichrome; magnification ×106, scale 200 μm.) (C) Transmission electron micrograph showing marked intracellular edema in cardiomyocytes of the tg heart (arrows, lower right part). (Magnification ×8300, scale 5 μm.)
At a higher magnification, all cardiac vessels, including the venous and the arterial system as well as the capillaries, were hyperemic and congested with erythrocytes in tg mice (Figure 8B) but no thromboembolism was found. This histologic appearance was confirmed in lung, liver, kidney, and spleen. In transgenic mice, the lung showed no intra-alveolar microhemorrhage or pulmonary fibrosis. On transmission electron microscopy, some cardiomyocytes exhibited a marked intracellular edema with spaces and gaps between the bundles of contractile proteins, the mitochondria, and toward the cellular wall (Figure 8C). Between the cardiomyocytes, only occasionally edematous separation of cellular walls was observed.
Exercise endurance
On observation of physical activities like moving, climbing, or interaction with littermates in cage housing, no obvious difference between wt and tg mice was noted by 3 independent investigators. To measure the physical endurance during exercise, mice were subjected to belt treadmill exercise after adaptation to the treadmill. The treadmill test (Figure 9) showed a prominent reduction of the exercise endurance of tg mice. Although wt mice were able to run on the belt for 74.5 ± 30 minutes, the tg mice suffered from exhaustion after only 6.3 ± 2.9 minutes.
Exercise testing unmasked the cardiovascular impairment of the tg mice.
During the belt treadmill stress test the time until exhaustion (10 consecutive seconds on the shock grid) was drastically reduced in tg mice (wt: n = 3; tg: n = 4), *P < .05.
Exercise testing unmasked the cardiovascular impairment of the tg mice.
During the belt treadmill stress test the time until exhaustion (10 consecutive seconds on the shock grid) was drastically reduced in tg mice (wt: n = 3; tg: n = 4), *P < .05.
Discussion
The increase in blood viscosity associated with abnormally high hematocrits is thought to put the cardiovascular system at hemodynamic and rheological risks. Other than in the rare polycythemia, rubra vera erythrocytosis is secondary to hypoxemia. Apart from erythrocytosis, hypoxemia itself may cause additional pathology and blur the picture of what is caused by high red cell counts or by tissue hypoxia. So far, animal experiments have not overcome these limitations. An animal model was needed to study chronic erythrocytosis independent of the confounding coexisting disease or manipulation. We have used tg mice with chronic EPO overexpression that results in extremely high hematocrits without hypoxemia. Although the blood volume of the wt mice was well within the range of other studies,10 11 tg animals had a 75% increase in blood volume. This was caused by an increase in red cell volume, while plasma volume in tg mice was reduced. In line with this finding was the observation that the capillaries were packed with erythrocytes in every organ examined, including the myocardium. Large abdominal veins appeared heavily filled on inspection. Consistently, central venous pressure was significantly increased in tg mice. Most surprisingly, however, despite a hematocrit of 0.80, no increase in blood pressure and/or a reduction of cardiac output in the erythrocytotic mice was observed. In fact, both mean arterial pressure and cardiac output were normal and exactly within the range of the wt, normocythemic control littermates.
In contrast, when hematocrit was raised in dogs to 0.68, cardiac output fell by 51%.12 For those experiments, the regression line between cardiac output and hematocrit indicated that cardiac output would have been close to zero at a hematocrit of 0.78. Thus, differences between species or in the experiments should be responsible for the discrepant results. Possibly the acute rise in hematocrit in the dogs let whole blood viscosity increase too rapidly and could not be compensated for. Our tg mice developed erythrocytosis gradually while growing up over several weeks, which may have provided sufficient time for the cardiovascular system of the tg mice to adapt. Although, as we show later, this was only for a very limited time.
In rats, when hematocrit was raised over a 3-week period to a value of 0.63 by the administration of EPO 500 units thrice weekly,13 mean arterial pressure, left ventricular end-diastolic pressure, right ventricular peak pressure, and cardiac output did not differ from the values in control animals (hematocrit 0.47). This supports the hypothesis that a gradual increase of hematocrit does not lead to a reduction of cardiac output as observed for acutely raised red cell volume. However, it should be kept in mind that the hematocrit of 0.63 in the experiments of Petit et al13 was much lower than the hematocrit of our tg mice studied. Blood viscosity increases exponentially with hematocrit. Our data clearly indicate that there are mechanisms to compensate even very high hematocrits, at least for a limited time. Cardiac output of the wt mice was comparable with data from the literature for the C3 mouse line.10 In addition, cardiac output of the tg, erythrocytotic mice, herein determined by high frequency echocardiography, was not different from wt mice.
For daily life activities in cage housing like moving, climbing, or interaction with littermates, no obvious difference between tg and wt animals was observed. But it has long been recognized that cardiac performance assessed at basal resting states does not reflect cardiac limits.14 Therefore, mice were submitted to belt treadmill exercise where the strain of running would lead to exhaustion. For this purpose, speeds up to 27 m/min have been applied previously.15 Under these exercise conditions, we observed a profound difference in endurance, which clearly demonstrates that erythrocytosis with a hematocrit of 0.80 is detrimental during physical exercise. We have no data that indicate which organ system limits exercise performance of the tg mice, but the numerous pathologic findings of the tg heart make it likely that the cardiovascular system is the limiting factor.
Cardiac hypertrophy and degeneration of the vascular endothelium was also observed by Semenza et al16 in tg mice with high hematocrit (0.69) that overexpressed the complete human EPOgene. These animals were generated to study 5′- and 3′-flanking regions of the EPO gene in search of regulatory DNA elements. However, the hemodynamic consequences for these transgenic animals were not further studied. In contrast, in rats in which the hematocrit was raised to 0.63 by EPO administration,13,17 no hypertrophy of the right or left ventricle was observed. Intraperitoneal injection of packed red cells into mice (hematocrit of 0.67 to 0.80) led to moderate right ventricular hypertrophy without left ventricular hypertrophy in one study18 but right and left ventricular hypertrophy in another (hematocrit 0.70 to 0.80).19 These data indicate that cardiac alterations are more likely to be found at hematocrit values above 0.70 with right ventricular hypertrophy first, followed by left ventricular hypertrophy later on. Our tg mice followed that pattern with the right and left heart being equally involved.
In contrast, if hematocrit increases because of chronic hypoxia, ventricular hypertrophy is predominantly seen on the right side.19,20 EPO treatment in combination with hypobaric hypoxia (0.5 ATM) does not further increase pulmonary arterial pressure and right ventricular mass to a greater extent than hypoxia alone.13 This EPO-independent effect is in line with our data, where we did not observe an increase in mean arterial pressure, despite continuously elevated plasma EPO levels. Moreover, this observation strongly argues against a direct hypertensive effect of EPO.21,22 In contrast, a recent study has revealed a direct activating effect of EPO on nitric oxide-synthase in endothelial cells.23,24 Indeed, we recently showed that increased amounts of endothelial nitric oxide could compensate for the increase in the resistance because of the elevated whole blood viscosity, providing an explanation for the lack of increase in blood pressure in our animals.8
Few controlled studies have been carried out on the relationship between hematocrit and arterial blood pressure in healthy humans. As recently pointed out,25,26 hemorheology and hypertension may be linked, but details of this association and its cause-effect relation remain unclear. Patients with primary erythrocytosis generally do not have hypertension,27 and earlier reports indicate no significant change in blood pressure after repeated therapeutic venesections to reduce hematocrit.28 Hence, such patients may develop similar compensatory mechanisms as our mouse model to prevent hypertension. The recent association between hypertension and hematocrit may just be due to defects in these mechanisms.25 26
The life span was markedly reduced in the tg mice investigated. Mean survival of tg mice was 7.4 months, and the difference of the distribution of the mean survival was highly significant. Villeval et al29 induced erythrocytosis in mice by transplanting bone marrow cells with a retroviral vector carrying a monkey EPOcDNA into lethally irradiated mice. These mice died at a mean of 71 days, but the individual effect of the lethal irradiation, separate from the high hematocrit (0.90 ± 0.05) on mortality, could not be discriminated. In our study, cardiac preload was increased, echocardiography revealed a dilated left ventricle, and the histologic alterations of the heart indicate cardiac dysfunction. Cardiac reserve, typically found to be decreased in clinical cardiac insufficiency,30 was reduced, which became evident from the markedly lowered exercise endurance capacity. Finally, the premature death of tg animals was obvious from 5.5 months onward. High hematocrit without accompanying hypoxemia appears sufficient to damage the heart, most likely because of the increased blood viscosity. As such, these mice may provide a useful animal model to study the adaptive changes to erythrocythemia early in life as well as the exact reasons for cardiac dysfunction later on.
Acknowledgments
Dr Hasegawa is a research fellow from the Department of Anesthesiology and Resuscitation, Shinshu University School of Medicine, Matsumoto City, Nagano, Japan. We thank Frank T. Ruschitzka, Roland H. Wenger, and Christian Bauer for helpful discussions of the manuscript.
Supported by grants from the Faculty of Medicine of the Medical University of Lübeck, Germany, and the Swiss National Science Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Klaus F. Wagner, Department of Anesthesiology, Medical University Lübeck, Ratzeburger Allee 160, D-23538 Lübeck, Germany; e-mail: wagner@physio.mu-luebeck.de.

![Fig. 1. Growth and hematocrit increase of wt and tg mice. / Weight gain of wt and tg mice was not different for male and female mice ([A] 1526 determinations in 103 wt mice, 1207 determinations in 100 tg mice. [B] 1182 determinations in 85 wt mice, 1611 determinations in 109 tg mice). Hematocrit increased with age and weight in tg mice until adulthood both in male ([C] wt: n = 113; tg: n = 93) and female ([D] wt: n = 85; tg: n = 124) animals.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/2/10.1182_blood.v97.2.536/5/m_h80210592001.jpeg?Expires=1766194812&Signature=0xnYTD-umwGIdvNUFovkVAEf5w-QFuU3E0rY7kmvRbs1xqW8lTzM9n-Lr-u-4YIXZV-CnPOFqzI352MHrzsAouduMx9v0BTI6byPxAI0lT~b9XnxzXzQ0x4M91mQz1d0aZJSikBrPgVpIrajoFKDaYIYNKY9GnIRSQPFaVxDrSpvKYRlh-bfYUY7hkNJrXbesaDC1lyAPYi9dB0i1LfRE9Gkz4dzp6dWRKuMioj1oDRolzttMmR8Iq0To31pDroAy~xgUHJ36UU~dNsHhbpZbX3h5otn~ObFRx-ZwjU39AXSeRWpP0N~1GqRtjyoVNltjGfviFtuYOHxRG~9bDcNRA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)