Abstract
The receptor for hepatocyte growth factor (HGF) is a transmembrane tyrosine kinase that is encoded by the proto-oncogene c-met. Recently, c-MET was detected in Reed-Sternberg (RS) cells from Epstein-Barr virus–positive (EBV+) Hodgkin disease (HD). The c-MET, EBER-1, and LMP-1 expression in 45 lymph node biopsies and 12 bone marrow biopsies obtained from patients with HD was analyzed. In addition, HGF levels in serum samples from 80 healthy individuals and 135 HD patients in different phases of disease. In all 45 lymph node and 12 bone marrow samples examined, RS cells expressed c-MET but not HGF+. These results were independent of the EBV infection. Interestingly, several HGF+ dendritic-reticulum cells were found scattered around c-MET+ RS cells. The mean ± SEM serum HGF levels in HD patients at diagnosis and at the time of relapse were 1403 ± 91 (95% confidence interval [CI], 1221-1585) and 1497 ± 242 pg/mL (95% CI, 977-2017), respectively. HGF values were significantly higher than those of healthy individuals (665 ± 28 pg/mL; 95% CI, 600-721; and P < .001 for both groups of patients) and of HD patients in remission (616 ± 49 pg/mL; 95% CI, 517-714; andP < .001 for both groups of patients). A significant correlation was found between serum HGF levels and B symptoms at diagnosis (P = .014). In conclusion, this study indicates that HGF and c-MET constitute an additional signaling pathway between RS cells and the reactive cellular background, thereby affecting adhesion, proliferation, and survival of RS cells. Furthermore, the serum concentration of HGF in HD patients may be a useful tool in monitoring the status of disease.
Introduction
Hepatocyte growth factor (HGF) is produced by various cells of mesenchymal origin and has pleiotropic functions in several cell types and organs.1-3 HGF is produced as a single-chain precursor, which is activated by proteolytic cleavage in pathologic conditions such as liver, lung, or kidney injuries.4 The active form is constituted by a disulfide-linked heterodimer consisting of a heavy subunit that is responsible for binding to the HGF receptor (c-MET) and a light subunit that contains a serine-protease–like structure similar to that of enzymes of the coagulation/fibrinolytic system.5Although first discovered as a liver-regeneration inducing factor,6 it has been shown that HGF acts on several cell types, particularly those of epithelial as well as endothelial origin.2 3
All the activities of HGF are mediated by binding to its receptor, a tyrosine kinase encoded by the proto-oncogene c-met.7 The HGF receptor, c-MET, is composed of an α-chain that is disulfide-linked to a β subunit in a complex of 190 kd. Both subunits are exposed on the cell surface, and the α subunit contains a membrane-spanning region and an intracellular domain that is responsible for the tyrosine kinase activity. After binding to HGF, c-MET undergoes autophosphorylation and activates several cellular targets involved in the signal transduction process.8 The c-met gene is predominantly expressed in epithelial cells and is overexpressed in several human and murine neoplastic tissues and cell lines. An increasing number of studies have demonstrated that several nonepithelial cells, such as hematopoietic, neural, and skeletal muscle cells, respond to HGF as well.9-14
In the hematopoietic system, HGF is produced by stromal bone marrow cells and synergizes with other growth factors (eg, granulocyte-macrophage colony-stimulating factor, interleukin 3 [IL-3], and erythropoietin), thereby inducing the proliferation and differentiation of a subset of CD34+/c-MET–expressing cells.15-18 The presence of HGF and/or HGF receptor has been extensively investigated in hematological malignancies. Myeloma cell lines and primary myeloma cell samples express c-MET and produce HGF,19,20 and a possible role for HGF has been suggested in the pathogenesis of osteolytic lesions.21 Furthermore, HGF promotes the proliferation and migration of myeloid leukemic cells.22,23 The c-MET/HGF network plays an important role in the lymphoid microenviroment as well by regulating the integrin-mediated adhesion of antigen-driven B cells to the germinal center.24,25 Weimar et al26 have recently demonstrated that c-MET is expressed by activated centroblasts in lymph nodes from healthy individuals and from patients with lymphoma. HGF was also shown to activate the α4β1 and α5β1 integrins involved in adhesion and migration of activated centroblasts and B-lymphoma cell lines.24 26
Among non-Hodgkin lymphomas, c-MET has been primarily detected in follicular lymphomas and CD30+ large B-cell lymphomas.26,27 In Hodgkin disease (HD), Weimar et al26 found c-MET expression only in Epstein-Barr virus–positive (EBV+) HD. However, another study on the expression of HGF and c-MET in several leukemia and lymphoma cell lines found that 5 of 6 Hodgkin lymphoma cell lines were c-MET+and that all 6 cell lines were also HGF+.28Although these studies of c-MET expression in HD are not in complete concordance, they raise the intriguing hypothesis that in this disease, the growth of c-MET+ Reed-Sternberg (RS) cells could be triggered or sustained by the autocrine or paracrine production of HGF.
To further elucidate the role of EBV infection in modulating c-MET expression in HD, we evaluated the expression of HGF and its receptor in lymph node and bone marrow biopsies from patients with either EBV+ or EBV− HD. Moreover, to study whether HGF could play a role in monitoring the disease status, we evaluated serum levels of HGF in a population of HD patients. We found that RS cells always express c-MET, independent of EBV infection, and that several HGF+ dendritic-reticulum cells are present in the reactive cellular background. In addition, we found that c-MET+ RS cells express α4β1 and α5β1 integrins, which suggests the existence of a HGF signaling pathway between RS cells and the reactive cellular background. Interestingly, serum HGF levels in HD patients at time of diagnosis and relapse were significantly higher than HGF levels in healthy individuals or in HD patients in remission following chemotherapy.
Materials and methods
Immunohistochemistry
Neoplastic samples.
This study was based on 57 samples of HD. The tumor panel included lymph node and bone marrow biopsies obtained from patients at the time of diagnosis or relapse. Altogether, 45 lymph node and 12 bone marrow biopsies were evaluated. Of these biopsies, 2 lymph node and 3 bone marrow samples were from human immunodeficiency virus (HIV)-infected patients. All samples were obtained as part of the diagnostic procedures, and in all patients a serum sample was collected for HGF level evaluation. Tissues were fixed in neutral-buffered formalin. In most cases a portion of unfixed tissue was snap-frozen in liquid nitrogen and stored at −80°C.
For immunophenotypic studies and lineage assignment of RS cells, the avidin-biotin-peroxidase complex (ABC-px) method was performed on paraffin sections using a commercially available kit (Dako LSAB 2; Dakopatts, Golstrup, Denmark) and the following commercially available monoclonal antibodies (mAbs) (Ylem, Rome, Italy): CD3, CD15, CD20, CD30, CD45, CD74, and epithelial membrane antigen (EMA). The CD30+, CD45−, CD15+, and EMA− diagnostic profile was required for the diagnosis of common HD. The revised European-American classification of lymphoid neoplasms (REAL)29 was used to classify the histologic subtypes of HD; the nodular sclerosis (NS) subtype was classified as NS I or NS II according to the British National Lymphoma Investigation.30
Immunostaining with c-MET rabbit polyclonal antibody (pAb) and with antihuman HGF goat antiserum was performed on frozen sections and on formalin-fixed paraffin-embedded tissues. The protocol that was used for each antigen tested is described below. Control experiments, which were invariably negative, consisted of omission of the primary antibody or staining using normal rabbit, goat, or mouse serum.
c-MET protein.
The c-MET protein was detected by the C-28 rabbit pAb raised against a peptide corresponding to the carboxy terminus of c-MET p140 of human origin (Santa Cruz Biotechnology, Santa Cruz, CA). Blocking peptides at 10-fold excess by weight relative to C-28 (sc-161 P, Santa Cruz) were used as competing compounds to assess the specificity of immunolabeling. Immunostaining for c-MET was performed on frozen sections and/or formalin-fixed, paraffin-embedded tissue sections using the alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP) method or the ABC-px method as previously described.31 In selected cases, the reactivity pattern of the C-28 rabbit pAb was compared with that of 8F-11, a mAb raised against the human c-MET protein (Novocastra Laboratories, Newcastle, England).
HGF protein.
Antihuman HGF is a goat antiserum developed using recombinant human HGF as the immunogen. This antiserum shows no cross-reactivity with other cytokines (antihuman HGF ALP01; R&D Systems, Minneapolis, MN). Antihuman HGF was applied only to frozen sections because of its lack of reactivity in paraffin-embedded tissue sections. Briefly, 6-μm-thick cryostat sections were fixed for 10 minutes at room temperature (RT) in 4% paraformaldehyde and treated for 20 minutes with 0.3% hydrogen peroxide (H2O2) in methanol to block endogenous peroxidase activity. Sections were then incubated at 4°C overnight with antihuman HGF at a 1:40 final dilution, washed, and incubated with biotinylated horse antigoat immunoglobulin G (IgG) (Vector Laboratories, Burlingame, CA). Indirect immunostaining was achieved using the ABC-px method, Dako LSAB 2. Endogenous biotin was saturated by a biotin blocking kit (Vector). The peroxidase was developed with a DAB substrate kit (Vector).
Two-color staining
In selected HD cases, double-immunohistochemical stains were performed to detect (1) c-MET and HGF; (2) c-MET and one of the following antigens: CD30, α4β1 integrin (CD49d/VLA-4α [clone 15A8, Ylem]), or α5β1 integrin (CD49e/VLA-5α [clone SAM-1, Ylem]); or (3) HGF and one of the following antigens: CD3, macrophage (HAM 56, Dakopatts), CD20, CD21, and CD30. To detect c-MET and HGF, cryostat sections were first incubated at 4°C overnight with antihuman HGF goat antiserum and then immunostained with the ABC-px method developed with a DAB substrate kit (Vector) to produce a brown color. Subsequently, sections were incubated for 1 hour with c-MET rabbit pAb at RT and immunostained by the APAAP method using naphthol AS-MX phosphate along with fast-red TR salt (Sigma Chemical Co, St Louis, MO) for the development of alkaline phosphatase in order to produce a red color. To further assess coexpression of (1) CD30, CD49d, and CD49e and c-MET and (2) HGF and CD3, HAM 56, CD20, CD21, and CD30, double-immunofluorescence staining was also performed. Briefly, after incubation with antihuman HGF goat antiserum or with c-MET rabbit pAb, serial sections were first immunostained using rabbit antigoat or goat antirabbit tetraethylrhodamine isothiocyanate (Dakopatts). The same sections were subsequently incubated with CD3, HAM 56, CD20, CD21, CD30, CD49d, and CD49e and immunostained with horse antimouse fluorescein isothiocyanate. The specimens were observed and digitalized by a fluorescence Zeiss Axioskop (Zeiss; Jena, Germany) equipped with an intensified CCD camera system (series 200, Macintosh configuration; Photometrics, Tucson, AZ).
Analysis of viral infection
All HD samples included in this study were analyzed for EBV infection. In-situ hybridization of EBV-encoded small RNAs (EBERs) was performed using a cocktail of fluorescein-isothiocyanate–labeled oligonucleotides complementary to the 2 nuclear EBER (1/2) RNAs according to the manufacturer's instructions (Dakopatts). In all samples immunostaining for LMP1 was also performed with an LMP1-specific antibody (Dakopatts) on formalin-fixed, paraffin-embedded tissue sections as previously described.32
Serum HGF evaluation
Patients.
Serum samples were collected from 135 HD patients. Informed consent was obtained from all patients. Results from the patient samples were recorded between May 1995 and June 2000 at 3 different hematology centers in Italy: the Division of Hematology, Catholic University of Rome, Rome, Italy (66 patients); the Division of Hematology, Casa Sollievo della Sofferenza of S. Giovanni Rotondo, Italy (54 patients); and the Institute of Hematology Seragnoli, University of Bologna, Bologna, Italy (15 patients). Forty patients were studied at different phases of their disease. We evaluated 255 serum samples: 80 samples from healthy blood donors (43 males and 37 females; median age, 37 years; age range, 19-65 years); 106 samples from HD patients at the time of diagnosis; 15 samples from relapsing or resistant HD patients; and 54 samples from HD patients in remission. Five patients observed at the time of diagnosis were HIV-infected. Clinical findings and HD histotypes at diagnosis were balanced among the 3 groups of patients (Table 1).
Among patients in remission, 49 were in complete remission (defined as the complete disappearance of all abnormalities), and 5 were in partial remission (defined as the reduction of at least 50% in the sum of the products of the perpendicular diameters of all measurable masses, with no appearance of new lesions). In all cases, diagnosis was based on histological findings supported by immunohistochemical analysis, as reported previously. In 15 patients diagnosed by bone marrow biopsy, classification of the histological subtype could not be ascertained. In all patients, staging was performed according to the Cotswold meeting criteria33. Bulky disease was defined as a mediastinal mass exceeding 33% of the chest diameter or an adenopathy greater than 10 cm. In 14 patients a third serum sample was obtained in addition to those collected at the time of diagnosis and at the completion of chemotherapy in order to perform serial evaluation of HGF levels.
HGF enzyme-linked immunosorbent assay.
Serum HGF was measured using the Quantikine Human HGF immunoassay (R&D Systems) according to the manufacturer's instructions. In brief, standard dilutions or serum samples were incubated for 2 hours at RT in 96-well microplates coated with a mAb against HGF. Following incubation, samples were aspirated, and wells were carefully washed 4 times. Horseradish peroxidase–conjugated anti-HGF pAb was added to each well, and after a 2-hour incubation, the wells were washed 4 times, and a mixture of substrate solution (constituted by equal amounts of stabilized H2O2 and tetramethylbenzidine) was added. After a 30-minute incubation, reaction was stopped by adding 2 N sulfuric acid solution, and the optical density was determinated within 30 minutes at 450 nm. All samples were evaluated in duplicate.
Statistic methods
Because serum HGF levels were not normally distributed, results are expressed both as the mean ± SE and median (range). Serum HGF values were compared by the Kruskal-Wallis test, the Mann-Whitney test, and the paired t test when appropriate. In HD patients at diagnosis, serum HGF values were related to clinical and laboratory features both in univariate (Mann-Whitney test) and multivariate regression analyses: All evaluated variables were dichotomized, while HGF was evaluated as a continuous variable. Missing data were dealt with by excluding from particular analysis those patients lacking data for the required variable. Clinical findings in the different groups of HD patients were compared by the χ2 test.
Results
Detection of EBV infection in lymph node and bone marrow biopsies
The overall incidence of EBV infection in this series of patients was 30%. As expected, EBV+ HD resulted in both EBER+ at ISH and LMP-1+ at immunohistochemistry. EBER transcripts and LMP-1 viral protein were detected in all HIV+ HD cases studied independent of nodal or bone marrow involvement. In cases of HD occurring in the general population, 7 nodal biopsies and all bone marrow biopsies involved by tumor were found to be both EBER-1+ and LMP-1+.
Detection of c-MET and HGF in lymph node and bone marrow biopsies
RS cells and their variants expressed c-MET in all cases of HD, both in HIV-infected and immunocompetent patients. These cases were representative of the entire pathologic spectrum of common HD. The pattern of c-MET immunoreactivity in RS cells was both cytoplasmic and membranous, and its intensity varied from moderate to strong. The specificity of immune reaction with the C-28 antibody was demonstrated by competitive inhibition with the blocking peptide (Figure1A,B). Other than vascular endothelial cells and plasma cells, which appeared c-MET+, no c-MET+ elements were detected in the reactive cellular background. When residual germinal centers were present, they displayed weak c-MET positivity. Finally, there was no difference in the pattern of c-MET immunoreactivity between EBV+ and EBV− samples. To confirm our results, we compared the reactivity of the C-28 pAb with that of the 8F-11 mAb in 6 EBV− and 6 EBV+ samples. Both antibodies stained RS cells in both EBV+ and EBV− HD, confirming that c-MET expression in RS cells is independent of EBV infection.
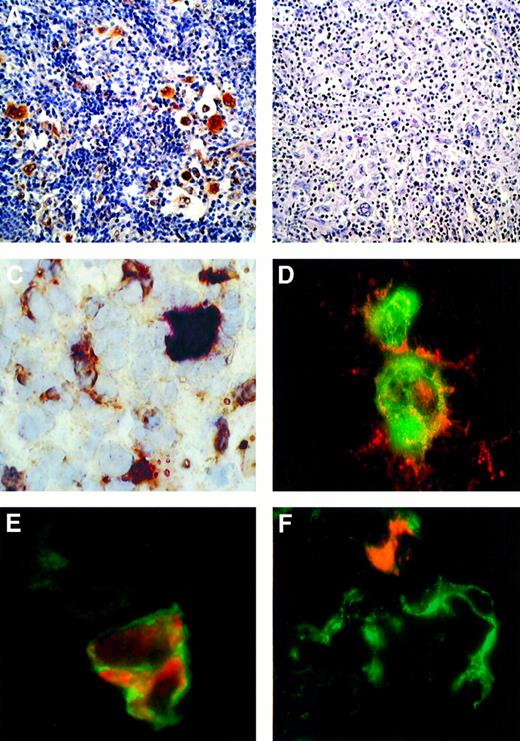
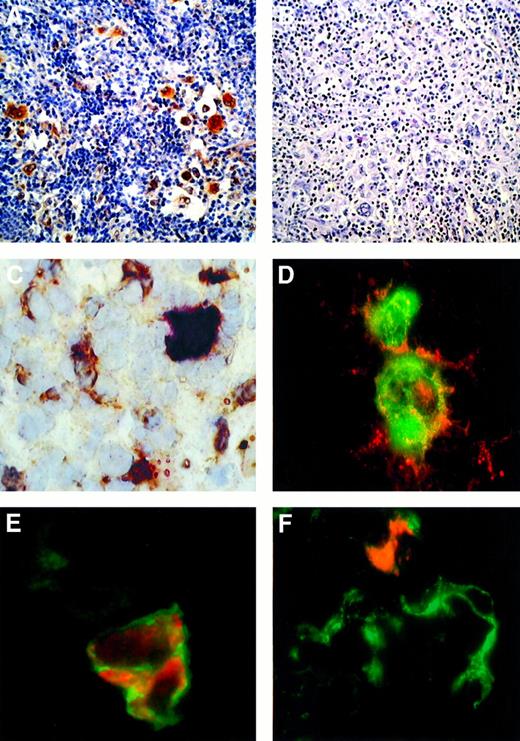
c-MET and HGF expression in Hodgkin disease.
(A,B) HD, NS subtype in an immunocompetent patient. Immunohistochemical detection of c-MET with the C-28 rabbit pAb (A) and competitive inhibition with molar excess of blocking peptide relative to C-28 (SC-161) (B). (C) HD, NS subtype in an immunocompetent patient. A frozen section was tested by 2-color staining with the C-28 rabbit pAb and antihuman HGF goat antiserum. A c-MET+ RS cell showing strong cytoplasmic and nuclear staining in red is surrounded by several HGF+ cells (cytoplasmic reaction in brown). (D) Double-labeled immunofluorescence showing detection of c-MET (red fluorescence) and CD30 mAb (green fluorescence). CD30+ RS cells exhibit membranous and cytoplasmic c-MET immunoreactivity. Yellow areas indicate colocalized CD30 and c-MET immunoreactivity. (E) Double-labeled immunofluorescence showing detection of c-MET (red fluorescence) and α5β1 integrin (green fluorescence). C-MET+ RS cell shows a membranous α5β1 immunoreactivity. In the same field a c-MET− stromal cell displays a weak α5β1 expression. (F) Double-labeled immunofluorescent detection of HGF (red fluorescence) and CD21 (green fluorescence). In a cluster of CD21+ dendritic-reticulum cells, one element exhibits both CD21 and cytoplasmic HGF immunoreactivity. ABC-px immunostaining with hematoxylin counterstaining is shown in panels A and B (original magnification × 100), and the immunostaining in panels C-F is described above (original magnification × 630).
c-MET and HGF expression in Hodgkin disease.
(A,B) HD, NS subtype in an immunocompetent patient. Immunohistochemical detection of c-MET with the C-28 rabbit pAb (A) and competitive inhibition with molar excess of blocking peptide relative to C-28 (SC-161) (B). (C) HD, NS subtype in an immunocompetent patient. A frozen section was tested by 2-color staining with the C-28 rabbit pAb and antihuman HGF goat antiserum. A c-MET+ RS cell showing strong cytoplasmic and nuclear staining in red is surrounded by several HGF+ cells (cytoplasmic reaction in brown). (D) Double-labeled immunofluorescence showing detection of c-MET (red fluorescence) and CD30 mAb (green fluorescence). CD30+ RS cells exhibit membranous and cytoplasmic c-MET immunoreactivity. Yellow areas indicate colocalized CD30 and c-MET immunoreactivity. (E) Double-labeled immunofluorescence showing detection of c-MET (red fluorescence) and α5β1 integrin (green fluorescence). C-MET+ RS cell shows a membranous α5β1 immunoreactivity. In the same field a c-MET− stromal cell displays a weak α5β1 expression. (F) Double-labeled immunofluorescent detection of HGF (red fluorescence) and CD21 (green fluorescence). In a cluster of CD21+ dendritic-reticulum cells, one element exhibits both CD21 and cytoplasmic HGF immunoreactivity. ABC-px immunostaining with hematoxylin counterstaining is shown in panels A and B (original magnification × 100), and the immunostaining in panels C-F is described above (original magnification × 630).
Double-staining experiments on frozen sections demonstrated the presence of several HGF+ elements with a dendritic appearance scattered around the c-MET+ RS cells (Figure1C). To simultaneously demonstrate the presence of 2 antigens, we used a double-immunofluorescence staining technique. These experiments showed that CD30+ RS cells coexpress c-MET but not HGF (Figure 1D and data not shown). In addition, we found that c-MET+ RS cells coexpress α4β1 (data not shown) and α5β1 integrin (Figure 1E). Finally, CD21+dendritic-reticulum cells displayed a cytoplasmic staining pattern for HGF, while T lymphocytes, B lymphocytes, and macrophages did not express HGF (Figure 1F and data not shown).
HGF serum levels in HD patients
Serum HGF values in healthy individuals and the different groups of HD patients are shown in Table 2. No difference was found between HD patients in remission and controls (P = .1). In contrast, patients at diagnosis and in relapse showed higher HGF levels (P < .001) than healthy individuals and patients in remission. Notably, HGF levels were similar in patients studied at the time of diagnosis and in relapse (P = .62). Moreover, in 35 patients whose serum samples were collected at diagnosis and in remission, a significant decrease in HGF levels was observed at the time of remission (mean ± SEM: 678 ± 77 pg/mL; 95% CI, 520-837; and 1220 ± 107 pg/mL; 95% CI, 1000-1441, respectively; P = .0009). No differences were found in the HGF values of HIV-infected and immunocompetent patients (data not shown) or EBV+ and EBV− patients (Table 3). We correlated HGF levels with disease characteristics at the time of diagnosis (Table4): The HGF value was evaluated as a continuous variable, while all the other variables were dichotomized. The cut-off level for each variable was chosen on the basis of its prognostic value, as reported by Hasenclever and Diehl.34Histological subtypes were grouped by “good” or “poor” prognosis: The former group consisted of lymphocyte-rich (LR) and NS I subtypes, and the second group consisted of NS II, mixed cellularity (MC), and lymphocyte-depletion (LD) subtypes. As shown in Table 4, in univariate analysis, HGF levels closely correlated with low levels of hemoglobin (P = .007) and with increased ESR (P = .0002). Moreover, although no differences were found between early and advanced stages, patients with B symptoms presented with higher levels of HGF compared to patients without symptoms (P < .0001). Multivariate analysis of these parameters (Table 5) demonstrated that only the presence of B symptoms was independently associated with high HGF values at diagnosis (P = .014).
Serial evaluation of HGF levels during follow-up
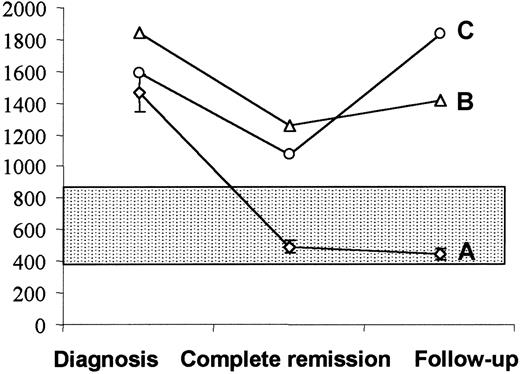
Fourteen patients with high HGF levels at diagnosis were further evaluated after the completion of chemotherapy and during follow-up (Figure 2). All 14 patients achieved complete remission after chemotherapy, and all but 2 patients had normal HGF levels at this time and during follow-up. Whereas all 12 patients who had normal HGF values after chemotherapy remain in complete remission (mean follow-up, 16 months; follow-up range, 8-52 months), the 2 patients with high HGF levels after chemotherapy relapsed at 3 and 5 months, respectively.
Serum HGF in 14 patients evaluated at diagnosis, at the completion of chemotherapy, and during the follow-up.
(A) Twelve patients showing normal HGF values (mean ± SEM) after chemotherapy were in continuing complete remission at a median follow-up of 16 months (range, 8-52 months). On the contrary, 2 patients with high levels of HGF after chemotherapy, despite achievement of clinical remission, relapsed 3 (B) and 5 (C) months later. The shadowed area indicates HGF values (mean ± SD) in healthy individuals.
Serum HGF in 14 patients evaluated at diagnosis, at the completion of chemotherapy, and during the follow-up.
(A) Twelve patients showing normal HGF values (mean ± SEM) after chemotherapy were in continuing complete remission at a median follow-up of 16 months (range, 8-52 months). On the contrary, 2 patients with high levels of HGF after chemotherapy, despite achievement of clinical remission, relapsed 3 (B) and 5 (C) months later. The shadowed area indicates HGF values (mean ± SD) in healthy individuals.
Discussion
Hodgkin disease is characterized by the presence of malignant RS cells, which constitute only 1% to 2% of total tumor cell mass. Most of the neoplastic tissue consists of reactive elements including lymphocytes, histiocytes, eosinophils, plasma cells, and stromal cells. Despite this, RS cells evade immunosurveillance, in part due to the interaction between malignant and reactive components of HD that promote their survival in a potentially hostile environment.35 Typically, members of the tumor necrosis factor receptor (TNFR) and of the TNF ligand superfamilies play an important role in RS cell survival, proliferation, and cytokine production.36,37 In this study we demonstrated that an additional possible mechanism of signaling between RS cells and the reactive milieu may involve the c-MET/HGF pathway. We found that in all HD cases evaluated, RS cells showed a clear expression of c-MET, while several dendritic-reticulum cells from the reactive cellular background produced HGF. Moreover, we showed that c-MET+RS cells coexpress α4 and α5 integrins which are specifically activated by HGF in order to promote adhesion to extracellular matrix molecules.24-26 These findings suggest that HGF may constitute an additional signal between RS cells and the reactive cellular background, thereby affecting the adhesion of RS cells.
The proto-oncogene c-met plays an important role in the regulation of adhesion and migration of normally activated B cells. In normal lymphatic tissues (ie, lymph node, tonsil, or spleen), c-MET is expressed in activated centroblasts of germinal centers and, to a lesser extent, in B cells from the marginal zone of secondary follicles, which reflects the interaction of these cells with antigen-specific T lymphocytes.24,25 Different mechanisms have been demonstrated to elicit c-MET expression in B cells. van der Voort et al24 demonstrated that the ligation of CD40 by CD40 ligand induces a transient up-regulation of c-MET expression, in particular when concomitant B-cell receptor activation occurs. Furthermore, Weimar et al26 found that circulating B lymphocytes, c-MET−, express c-MET after infection by EBV. Accordingly, the authors found c-MET expression in EBV-related HD only. In our experience, c-MET is expressed in lymph nodes from HD patients independent of the presence of EBV, and this finding is confirmed by similar results obtained with the mAb 8F-11. The discrepancy between our observations and those of Weimar et al26 may be due to different technical procedures. These authors used the same C-28 rabbit pAb, but at a higher dilution and for a different incubation time.26 Our findings agree with the reported expression of c-MET in almost all HD cell lines studied28 and suggest that in RS cells, c-MET expression is due to mechanisms other than EBV infection such as the CD40/CD40-ligand interaction38 or cytokine stimulation. In fact, HGF itself, as well as other cytokines such as IL-6 and TNF-α, can induce c-MET expression in hepatocellular carcinoma lines through activation of the c-metpromoter.39 In conclusion, c-MET expression may be induced by RS cells secreting several cytokines40 or dendritic-reticulum cells secreting HGF.
The role of the c-MET/HGF pathway in the interaction between stromal cells and hematopoietic progenitors has been highlighted in the hematopoietic microenvironment.16-18 Although it is not clear which of the stromal elements (fibroblasts, endothelial cells, or adipocytes) produces HGF, it has been shown that stromal-derived HGF promotes adhesion, proliferation, and survival of hematopoietic progenitors.9 16-18 Similarly, our findings that c-MET+ RS cells expressing α4- and α5-integrins are surrounded by HGF-producing dendritic-reticulum cells suggests a role for the c-MET/HGF pathway in sustaining adhesion, proliferation, and survival of RS cells.
Previous studies have shown that HGF serum values in patients with multiple myeloma are significantly related to the status of the disease. Similarly, we found that serum level values of HGF are significantly higher in HD patients with active disease compared to patients in remission. Among patients observed at the time of diagnosis, higher serum HGF levels were associated with the presence of B symptoms, typically reflecting the immune activation induced by the disease more than the extent or burden of disease (advanced stage and bulky disease). Many clinical and laboratory features of HD, such as fever, weight loss, and alteration of inflammatory parameters, have been typically ascribed to enhanced cytokine secretion. We hypothesize that HGF/c-MET interaction stimulates cytokine release by c-MET+ RS cells, as recently shown in multiple myeloma where IL-11 secretion is induced by c-MET+osteoblasts.21 Alternatively, cytokines such as IL-1 and IL-6, transforming growth factor β, and TNFα could up-regulate c-MET, as described in hepatocellular carcinoma and pancreatic cancer cells.39,41 Interestingly, c-MET could also have an anti-apoptotic role due to its association with BAG-1, an anti-apoptotic protein and functional partner of Bcl-2.42
Our findings show that HGF levels, high at diagnosis, significantly decrease after chemotherapy in patients achieving clinical remission, whereas persistent high HGF values after chemotherapy predict early relapse. Although these observations are obtained from a small number of patients, they suggest that the serial determination of serum HGF levels could represent an important clinical tool in monitoring the response to chemotherapy. A longer follow-up might assess whether serum HGF level at diagnosis could represent a new prognostic factor in HD.
In conclusion, c-MET/HGF interaction may constitute an additional pathway of signaling between RS cells and the reactive cellular background in Hodgkin disease and, like the TNF ligand/TNFR family members, HGF and its receptor c-MET may be important elements in the unbalanced cytokine network typical of HD.
Acknowledgments
The authors thank Dr Raffaella Urbano, Alessandro Rinelli, and Rosella Matera for excellent technical assistance and Dr Maurizio Martini and Dr Maria Luigia Vigliotti for the useful suggestions.
Supported in part by a grant from the Associazione Italiana per la Ricerca sul Cancro, Milan, Italy, and by grant RF 1999 ICS120.5/RF99.98 from the Ministero della Sanità Rome, Rome, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Luigi Maria Larocca, Institute of Pathology, Università Cattolica del Sacro Cuore, Largo F. Vito 1, 00168, Rome, Italy; e-mail: llarocca@rm.unicatt.it.