Abstract
We conducted a phase II randomized trial of recombinant granculocyte-macrophage colony-stimulating factor (GM-CSF) administered before topotecan chemotherapy to determine whether it could prevent myelosuppression and to determine the antitumor activity of this topoisomerase I inhibitor in 53 patients with metastatic malignant melanoma and renal cell cancer. All patients received GM-CSF after topotecan at a dose of 250 μg/m2 daily for at least 8 days. Patients randomly assigned to receive GM-CSF priming were treated with GM-CSF at 250 μg/m2 twice daily for 5 days before treatment. Twenty-five patients were randomly assigned to receive GM-CSF priming and 28 to receive topotecan without priming. The primary analysis was restricted to the protective effects seen during the first cycle of therapy. Grade 4 neutropenia occurred in 8 of 23 patients (35%) and grade 3 neutropenia in 5 of 23 patients (22%) randomized to GM-CSF priming, whereas 18 of 26 (69%) and 5 of 26 (19%) patients experienced grade 4 or 3 neutropenia, respectively, without GM-CSF priming (P = .0074). The mean duration of neutropenia was reduced by GM-CSF priming: grade 3 neutropenia from 5.2 ± 0.7 to 2.8 ± 0.7 days (P = .0232) and grade 4 neutropenia from 2.7 ± 0.6 to 1.1 ± 0.4 days (P = 0.0332). The protective effects of GM-CSF extended to the second cycle of treatment. The incidence of febrile neutropenia was also reduced. Chemotherapy-induced anemia and thrombocytopenia were similar in both groups. One partial response was seen in a patient with melanoma, and one patient with renal cell cancer had complete regression of pulmonary metastases and was rendered disease-free by nephrectomy.
Introduction
Administration of granulocyte-macrophage colony-stimulating factor before chemotherapy (GM-CSF priming) can expand the bone marrow myeloid mass, and its withdrawal can inhibit DNA synthesis in and thus possibly protect hematopoietic precursors.1,2 In that report GM-CSF administration increased the number of cycling bone marrow progenitors from a baseline of 18% to 51% during treatment, but within one day of stopping GM-CSF the number of cycling cells declined to a mean level of 2.9%, which is six-fold lower than baseline and remains depressed for as long as one week.1 Thus, GM-CSF priming may be effective in reducing the incidence and severity of chemotherapy-induced myelosuppression by increasing the number of myeloid precursors in the marrow before chemotherapy and decreasing the number of stem cells killed by chemotherapy.
Clinical trials evaluating GM-CSF priming have shown mixed results.1,3-7 In 2 prospective randomized trials, the pattern of hematopoietic recovery in patients treated with marrow-ablative chemotherapy regimens was not altered by GM-CSF priming.3,6 In a nonrandomized trial of high-dose chemotherapy, GM-CSF priming was similarly ineffective in restoring myelopoiesis compared with historical controls.7 A randomized study of a 5-day course of GM-CSF priming before a prolonged course of oral etoposide also was without benefit.5 A small randomized study of 12 patients with breast cancer also failed to document evidence of clinical benefit with GM-CSF priming.4
In contrast, a myeloprotective effect of GM-CSF priming was observed in a nonrandomized trial in patients with sarcoma receiving cyclophosphamide, doxorubicin, and dacarbazine.1 Patients who developed severe neutropenia (absolute granulocyte count less than 0.5 × 109/L [< 500/μL]) after their first cycle of chemotherapy became eligible for GM-CSF priming. The incidence and duration of neutropenia were significantly reduced with GM-CSF priming, and 41% of patients were able to receive a higher dose of chemotherapy in subsequent cycles. The positive results in this trial were achieved by selecting patients for prophylaxis who experienced neutropenia and by decreasing the duration of chemotherapy from 5 to 3 days.
Although ineffective in the setting of myeloablative chemotherapy, GM-CSF priming has not been tested adequately in a prospective fashion in moderate-dose outpatient chemotherapy regimens. In this study, we prospectively evaluated the efficacy of GM-CSF priming with the topoisomerase I inhibitor topotecan in chemotherapy-naive patients. Myelosuppression, the major dose-limiting toxicity of topotecan, causes reproducible neutropenia in patients treated without growth factor support; most studies show neutropenia occurring in 50% to 90% of patients treated with the dose of topotecan used in this study. A secondary end point of the study was to determine the antitumor activity of topotecan in patients with metastatic melanoma and renal cell cancer.
Patients and methods
Eligibility
Patients were required to have histologically confirmed and measurable metastatic melanoma or renal cell cancer, life expectancy more than 3 months, Karnofsky performance status of at least 70%, and normal cardiac function. The following clinical laboratory values were required: serum creatinine, 113 μM/L or less (≤ 1.5 mg/dL), or calculated creatinine clearance, 0.83 mL/s or more (≥ 50 mL/min); bilirubin, less than 25.7 μM/L (< 1.5 mg/dL); aspartate aminotransferase and alkaline phosphatase, less than 5 times the upper limit of normal; calcium, less than 2.75 mM/L (< 11.0 mg/dL), and albumin more than 30 g/L (> 3 g/dL); white blood cells, more than 4.0 × 109/L (> 4000/μL); granulocytes, more than 1.5 × 109/L (> 1500/μL); hemoglobin, 80 g/L or more (≥ 8 gm/dL); and platelet count, more than 100 × 109/L (> 100 000/μL). Patients were excluded for overt tumor involvement of the bone marrow, prior pelvic radiation therapy, or radiation therapy to more than 20% of the bone marrow. Patients were also excluded if they had received chemotherapy (other than immunomodulatory doses of cyclophosphamide or doxorubicin as part of immunotherapy regimens administered in other protocols conducted at the National Cancer Institute) and were not permitted to receive glucocorticoids. Patients were not eligible if they had a history of brain metastases, autoimmune diseases, serum positive for hepatitis B surface antigen, or antibodies to the human immunodeficiency virus. Patients with ocular melanoma were excluded. All patients voluntarily gave their written informed consent before therapy. The protocol was approved by the institutional review boards of the Frederick Cancer Research and Development Center and the National Cancer Institute.
Study design
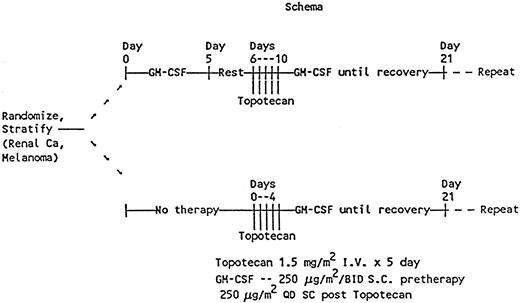
This study was designed as a prospective randomized phase II trial to evaluate GM-CSF priming to ameliorate neutropenia and a phase II trial to determine the response rate of chemotherapy-naive renal cell cancer and metastatic melanoma to topotecan. GM-CSF was provided by Immunex (Seattle, WA). Fifty-three patients—25 with renal cell cancer and 28 with melanoma—were enrolled between March 16, 1992, and August 23, 1994. The clinical characteristics of the patients are listed in Table 1. All patients were to receive topotecan at the recommended phase II dose of 1.5 mg/m2 daily for 5 days as a bolus infusion over 30 minutes, and GM-CSF was started on day 6 at a dose of 250 μg/m2daily for at least 8 days or until the neutrophil count was more than 1.5 × 109/L (> 1500/μL). Patients were stratified on the basis of tumor type and were randomly assigned to receive GM-CSF priming (250 μg/m2 twice daily for 5 days) before treatment with topotecan (Figure 1). GM-CSF was discontinued 36 hours before chemotherapy in patients randomly assigned to receive GM-CSF priming. Cycles were repeated at 21-day intervals. Twenty-nine patients received only 2 cycles of treatment. Seven patients received 1 or less than 1 cycle of treatment; 17 received more than 2 cycles; 13 received 4 cycles; 1 received 5 cycles; 2 received 6 cycles; and 1 received 14 cycles.
Schema for treatment with GM-CSF priming, topotecan, and postchemotherapy GM-CSF.
Schema for treatment with GM-CSF priming, topotecan, and postchemotherapy GM-CSF.
Baseline assessment and follow-up of response and toxicity
A complete history and physical examination and laboratory studies including a complete blood count, serum chemistry, coagulation profiles, and a urinalysis were obtained before therapy. Patients randomized to GM-CSF priming had a complete blood count before and on the third through fifth days. During topotecan treatment, complete blood counts and serum chemistries were obtained before treatment and on the final day of topotecan. A complete blood count was to be obtained at least 3 times weekly after topotecan or more often if clinically indicated. Radiologic imaging studies were obtained within 30 days of starting treatment and after every 2 cycles of therapy to assess disease response. A complete remission was defined as the disappearance of all clinical and laboratory signs and symptoms of disease for at least one month, a partial remission as a 50% or greater reduction in the size of measurable lesions lasting at least one month without any increase in size of a lesion or the appearance of any new lesions, and a minor response as a 25% to 49% reduction in the size of measurable lesions. Progressive disease was defined as an increase by 25% or more in the sum of the products of the longest perpendicular diameters of all measurable lesions or the appearance of any new lesions. Stable disease was defined as tumor measurements not satisfying any of the above criteria.
Statistical methods
As specified in the protocol, the grades of neutropenia toxicity produced by topotecan were compared by assigning to each patient a score of 0, 1, or 2 depending upon whether the patient experienced less than or equal to grade 2 neutropenia (absolute neutrophil count [ANC] ≥ 1.0 × 109/L [≥ 1000/μL]), grade 3 neutropenia (ANC 0.5. × 109/L to 1.0 × 109/L [500/μL to 999/μL]), or grade 4 neutropenia (ANC < 0.5 × 109/L [< 500/μL]), respectively. For neutropenia toxicity grades and for duration of neutropenia, comparisons between the patients receiving GM-CSF priming and those who were not primed were made using 2-sample permutation ttests. Comparisons between the toxicity of patients in cycle 1 versus cycle 2 were made using permutation paired t tests. All reported P values are 2-sided and were calculated using StatXact.8
Results
GM-CSF priming
The effects of GM-CSF priming on peripheral blood counts were evaluated in 23 patients. Treatment with GM-CSF twice daily for 5 days produced an increase in white blood cells: mean peak absolute white blood cell count (± SD), 21.3 × 109/L ± 4.1 × 109/L (21.3 × 103/μL ± 4.1 × 103/μL); range 14.8 × 109/L to 30.7 × 109/L (14.8 × 103/μL to 30.7 × 103/μL). Most of the increase was due to granulocytes. The mean peak absolute granulocyte count (± SD) was 17.2 × 109/L ± 3.8 × 109/L (17.2 × 103/μL ± 3.8 × 103/μL); range 12.4 × 109/L to 27.6 × 109/L (12.4 × 103/μL to 27.6 × 103/μL). Granulocyte counts returned to pretreatment baseline levels by the time of topotecan administration. Peak monocyte and eosinophil counts increased 4-fold and 7-fold, respectively, in response to GM-CSF priming. Platelet counts remained stable during GM-CSF priming.
Effects of GM-CSF on myelosuppression
Neutrophil effects.
Fifty-three patients were entered on study, 25 of whom were randomly assigned to GM-CSF priming and 28 of whom received topotecan without priming. Two patients in the GM-CSF priming group were not evaluable for hematologic recovery because one patient developed rapidly progressive disease and never received topotecan and the other patient did not comply with postchemotherapy blood count monitoring. In the control group, one patient experienced a stroke during postchemotherapy GM-CSF but before hematologic recovery, and the other patient withdrew from treatment before completion of topotecan and never received GM-CSF. Therefore, 23 patients assigned to GM-CSF priming and 26 patients without priming were available for the hematologic end points of the trial.
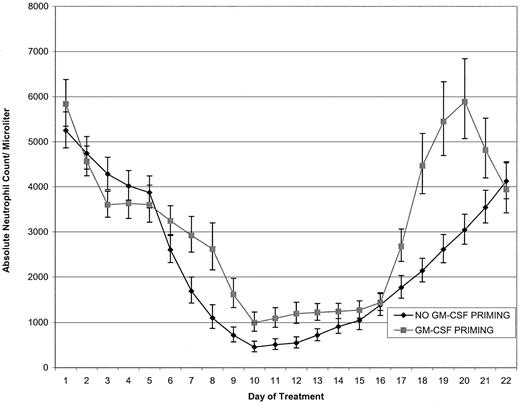
The primary analysis of the first course data is given in Table2. GM-CSF priming significantly reduced the incidence of grades 3 and 4 neutropenia (P = .0074). Five patients in each group had grade 3 neutropenia, but only 8 of 23 (35%) treated with GM-CSF priming had grade 4 neutropenia, whereas 18 of 26 (69%) had grade 4 neutropenia without GM-CSF priming. To investigate whether this difference might be due to the priming for the second course, which occurred during the end of the first course, the grade of toxicity was re-examined, restricting attention to the first 15 days of course 1. The results were unchanged; the worst grade of neutropenia always occurred during the first 15 days of course 1. Figure 2 shows the geometric mean granulocyte count during cycle 1 of treatment for both groups.
Granulocyte counts in randomized patients treated with and without GM-CSF priming.
Geometric mean curves (with approximate 66% confidence limits) of individual patient granulocyte counts for patients treated with topotecan with (n = 23) and without (n = 26) GM-CSF priming. Individual patient curves were linearly interpolated on a log scale for days with missing values.
Granulocyte counts in randomized patients treated with and without GM-CSF priming.
Geometric mean curves (with approximate 66% confidence limits) of individual patient granulocyte counts for patients treated with topotecan with (n = 23) and without (n = 26) GM-CSF priming. Individual patient curves were linearly interpolated on a log scale for days with missing values.
As a secondary end point, the duration of neutropenia, both grades 3 and 4, was examined. The median number of days that patients experienced grade 3 or greater neutropenia was significantly reduced in patients primed with GM-CSF. The duration of neutropenia following priming declined from (mean ± SE) 5.2 ± 0.7 to 2.8 ± 0.7 days (P = .0232) and from 2.7 ± 0.6 to 1.1 ± 0.4 (P = .0332) days for grades 3 and 4 neutropenia, respectively.
Interpretation of the hematologic recovery data from the second course was complicated by the requirement for protocol-specified dose reductions of GM-CSF and topotecan and patient withdrawals; however, the protective effects of GM-CSF priming extend to the second cycle (Table 3). The analysis was restricted to the first 15 days of the second course. Nine patients were not evaluable for hematologic recovery in the second cycle of treatment: 2 who received GM-CSF priming and 7 patients who did not. Grade 3 or 4 neutropenia occurred in 5 of 23 (22%) and 4 of 23 (17%), respectively, of patients treated with GM-CSF priming, whereas 8 of 21 (38%) experienced grade 3 and 8 of 21 (38%) experienced grade 4 toxicity without priming. Neutropenia was significantly worse (P = .0284) without GM-CSF priming in the second cycle.
The postchemotherapy GM-CSF in cycle 1 can be considered as GM-CSF priming for the second cycle of treatment. It is of interest to compare the severity of toxicity seen in cycles 1 and 2 of treatment. In patients randomly assigned to GM-CSF priming, there was less toxicity in the second cycle, but the difference was not statistically significant (P = .2461; 22 patients evaluable for both cycles). In contrast, nonprimed patients showed a significant reduction in second cycle hematologic toxicity (P = .0283; 21 patients evaluable for both cycles), presumably based on the postchemotherapy GM-CSF of cycle 1. Of the 18 patients who experienced grade 4 neutropenia in the first cycle, only 6 experienced a similar degree of neutropenia after the same dose of topotecan in the second cycle, 7 had grade 3 neutropenia, and 2 had grade 2 or less toxicity. Three patients were not evaluable for second-cycle hematologic recovery, which may have favorably biased the results.
Neutropenic fevers occurred less often in patients who received GM-CSF priming during the first cycle. One patient primed with GM-CSF had a neutropenic fever of 3 days' duration, whereas 4 unprimed patients had fevers that lasted 3, 4, 8, and 9 days while neutropenic. There were no documented infections in any of the febrile neutropenic patients. The only other episode of neutropenic fever occurred in the third cycle of treatment in an unprimed patient.
One patient in the control group but none in the GM-CSF–primed cohort had a reduction in the dose of topotecan in the second cycle because of myelosuppression. Two patients in each group had a delay in the start of the second cycle. In the GM-CSF–primed group, the delay in one patient was related to surgery (resection of an ulcerated and bleeding skin lesion between the first and second cycle) and in the other to thrombocytopenia. In the unprimed group, one patient had a central-line infection and one had prolonged myelosuppression necessitating removal from the study.
Platelet and red blood cell effects.
Few patients in either arm of the trial experienced significant thrombocytopenia. Three patients, all in the group treated without GM-CSF priming, received platelet transfusions during the first cycle. Platelets were transfused for a count of less than 20 × 109/L (< 20 000/μL). One GM-CSF–primed patient received a platelet transfusion in the second cycle of treatment. Red cell transfusions, administered for hemoglobin below 80 g/L (< 8 g/dL), were given to 3 GM-CSF–primed and 2 unprimed patients in the first cycle. In the second cycle, 8 GM-CSF–primed patients and 4 unprimed patients were transfused with red blood cells.
Toxicity of therapy
We found that, similar to other trials, GM-CSF therapy was well tolerated in most patients,9 with toxicity graded at below 3 in most cases. Grade 3 hypotension requiring phenylephrine support occurred in one patient during GM-CSF priming, but further treatment at a reduced dose was administered without toxicity. Two patients experienced a stroke with motor weakness while receiving GM-CSF after topotecan; symptoms resolved completely in one case, but the other patient had mild residual weakness. Both patients had insulin-dependent diabetes mellitus. No abnormalities were detected on an enhanced computed tomography scan of the brain in one patient or on magnetic resonance imaging of the brain in the other. Carotid Doppler and echocardiograms were also normal. One of the patients had the same symptoms several years earlier, which resolved completely.
Antitumor activity
Twenty-five patients with renal cell cancer were entered in our study, with 24 evaluable for response. No complete or partial responses were seen (12%, upper 95% confidence interval for the response rate); one patient had complete regression of multiple small pulmonary parenchymal nodules without response in the primary tumor and was rendered disease-free by resection of the primary kidney tumor. He remains free of disease 7 years after treatment. Twenty-eight patients with melanoma were entered, including 14 each with visceral and nonvisceral metastases. Twenty-seven are evaluable for tumor response. No responses were seen in patients with visceral metastases. One patient with multiple subcutaneous and nodal metastases achieved a partial response (17%, upper 95% confidence interval for the response rate).
Discussion
The purpose of this prospective randomized trial was to determine whether GM-CSF priming reduced the incidence and severity of neutropenia induced by topotecan chemotherapy. A secondary end point was to determine the response rate of topotecan in patients with advanced melanoma and renal cell cancer. Our data support the notion that pretreatment with GM-CSF reduces the neutropenia following subsequent therapy with topotecan. We observed a statistically significant 50% reduction in grade 4 neutropenia in patients treated with GM-CSF priming (69% vs 35%). In addition, the duration of grades 3 and 4 neutropenia was reduced by GM-CSF priming. Fewer patients treated with GM-CSF priming developed neutropenic fevers although, because of the small numbers, this difference did not achieve statistical significance. In contrast to the protective effects of GM-CSF priming on neutrophils, no difference in the degree or severity of thrombocytopenia or anemia was noted.
There are 2 potential mechanisms for the reduction in neutropenia in GM-CSF–primed patients. First, the number of CSF-responsive progenitors could be increased; if the same proportion of cells were killed by chemotherapy, the number of stem cells surviving therapy would be greater than if the progenitor number were not expanded. Determination of bone marrow progenitor number is imprecise because of variability in sampling and qualitative changes in bone marrow composition. In a phase I trial of interleukin-1 alpha, bone marrow cellularity in biopsy specimens increased but stem cell numbers in bone marrow aspirates declined; this was interpreted as a dilutional effect due to the expansion of non-CD34 myeloid mass.10Development of measures that accurately assess bone marrow mass are needed and may help to clarify the protective effect of priming. Second, GM-CSF withdrawal may induce a state of chemotherapy resistance by reducing stem cell cycling. A small study that used the interleukin-3/GM-CSF fusion protein, PIXY321, to induce chemotherapy resistance showed no reduction in toxicity associated with a decreased fraction of cells in S and G2/M compared with patients who did not have a decrease in cycling cells.11 In Murren's study, half of the patients showed a decrease in the number of cycling cells in peripheral blood, whereas cycling increased in the others. Cell cycle status was not measured in our study. Additional studies of bone marrow and peripheral blood progenitor cell-cycle status are needed to understand the role cell-cycle arrest plays in the protective effect of GM-CSF priming. The results of our study do not allow us to distinguish between increased myeloid mass and cell-cycle arrest as the protective mechanism of GM-CSF priming seen in this study.
A protective effect of GM-CSF priming was seen in a randomized trial in patients with early-stage breast cancer receiving cyclophosphamide and doxorubicin.12 In this study, patients were randomly assigned to receive GM-CSF at a dose of 250 μg/m2 for 5 days before treatment. The neutrophil nadir was similar with and without priming, but the proportion of patients with a neutrophil nadir below 0.5 × 109/L (500/μL) was lower in GM-CSF–primed patients, and the day 16 neutrophil counts were higher. In the positive trial of GM-CSF priming conducted by Vadhan Raj et al, it was necessary to shorten the duration of chemotherapy from 5 to 3 days for the positive effects of GM-CSF priming to be observed.1Interestingly, both of these positive trials administered a brief chemotherapy regimen. Our trial differs from both of these studies in that topotecan was administered for 5 days. The negative trials of GM-CSF priming used chemotherapy regimens administered for longer periods.
There was a trend toward an increase in red blood cell transfusion requirement in patients treated with GM-CSF priming. Similar numbers of patients were transfused with red cells in the first cycle, but twice as many transfusions were given in the second cycle to GM-CSF–primed patients. The difference did not achieve statistical significance, but these results raise the possibility that enhanced myeloid growth may occur at the expense of red cell precursors. Few patients in this study received more than 2 cycles of treatment but, if this effect is real, it could result in greater transfusion requirements in patients treated with longer courses of GM-CSF priming.
Topoisomerase inhibitors produce irreversible DNA strand breaks during DNA replication or RNA transcription. The model of topoisomerase inhibitor–mediated cell death proposes that a collision between a moving replication fork and a topoisomerase-cleavable complex produces an irreversible DNA strand break that leads to cell death.13,14 DNA synthesis is required for topoisomerase I inhibitor–mediated cell killing, as shown by the protective effect of aphidicolin, a reversible inhibitor of DNA polymerase on cell death induced by topoisomerase I inhibitors. Pretreatment of cells with aphidicolin inhibits both DNA synthesis and cell death induced by topoisomerase I inhibitors.13 GM-CSF withdrawal may function like aphidicolin through inhibition of DNA synthesis in hematopoietic progenitors and thereby protect these cells from topotecan-mediated killing.
The importance of DNA synthesis in topotecan-induced killing has been demonstrated clinically. The concurrent administration of G-CSF and topotecan increased myelosuppression compared with historical controls treated with topotecan alone.15 Both neutropenia and thrombocytopenia were accentuated during concurrent therapy. Similar results were seen with concurrent administration of 5-fluorouracil/leucovorin and G-CSF.16 It will be important to determine whether GM-CSF withdrawal will be protective in other moderate-dose chemotherapy regimens or whether its effects are limited to topoisomerase I inhibitors and to determine whether the myelotoxicity of high-dose but nonablative chemotherapy can be reduced with this approach.
This study was conducted using the recommended phase II dose of topotecan.17,18 Topotecan administered as a bolus infusion daily for 5 days does not have significant antitumor activity in patients with melanoma or renal cell cancer. These results are comparable to those observed in other phase II trials of topotecan administered at a similar dose and schedule. No responses were seen in 15 patients with renal cell cancer19 or in 17 patients with melanoma.20 Topotecan has activity in ovarian cancer and small cell carcinoma of the lung but is primarily used as a salvage treatment in patients whose tumors have progressed on previous chemotherapy regimens.21-23 GM-CSF priming may be useful in maintaining dose intensity in responding patients who experience significant myelosuppression that compromises continued treatment.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John E. Janik, Metabolism Branch, Division of Clinical Sciences, Bldg 10, Rm 4N115, 10 Center Dr, Bethesda, MD 20892-1374; e-mail: janikj@mail.nih.gov.