Abstract
Fractalkine displays features that distinguishes it from the other chemokines. In particular, besides its chemoattractant action it promotes, under physiologic flow, the rapid capture and the firm adhesion of a subset of leukocytes or intervenes in the neuron/microglia interaction. This study verified that indeed the human monocytic MonoMac6 cell line adheres to fibronectin-coated filters in response to soluble fractalkine (s-FKN). s-FKN stimulates, with distinct time courses, extracellular signal-related kinases (ERK1 and ERK2) and stress-activated protein kinases (SAPK1/JNK1 and SAPK2/p38). Both p60 Src and p72 Syk were activated under s-FKN stimulation with a rapid kinetic profile compatible with a downstream regulation on the mitogen-activated protein kinase (MAPK) congeners. The use of specific tyrosine kinase inhibitors revealed that the ERK pathway is strictly controlled by Syk, whereas c-Src up-regulated the downstream SAPK2/p38. In contrast, the SAPK1/JNK1 pathway was not regulated by any of these nonreceptor tyrosine kinases. The s-FKN–mediated increased adherence of MonoMac6 cells was partially inhibited by SB202190, a broad SAPKs inhibitor, PD98059, an MEK inhibitor, LY294002, a phosphatidyl inositol 3-kinase inhibitor, and a pertussis toxin-sensitive G protein. These data highlight that the integration of a complex array of signal transduction pathways is necessary to complete the full s-FNK–dependent adherence of human monocytic cells to fibronectin.
Introduction
Chemokines are small secreted proteins that stimulate the directional migration of leukocytes and mediate inflammation.1 This superfamily of chemotactic peptides falls into at least 4 subfamilies principally based on the relative position of highly conserved cysteine residues in their amino acid sequences.1,2 Fractalkine is so far the only member of the δ-chemokine subclass.3 Its recent discovery has revealed distinctive structural features in this gene family including a CX3C motif, a mucin-like stalk tethering the chemokine domain to transmembrane spanning, and short intracellular domains.3,4 Fractalkine thus exists in 2 forms, as a membrane-anchored proadhesive protein (m-FKN) or as a soluble chemotactic peptide (s-FKN), the effects of which remain poorly documented. Furthermore, unlike most chemokine peptides, fractalkine expression can be observed on interleukin (IL)-1 and tumor necrosis factor (TNF)-activated primary endothelial cells to promote strong adhesion of T cells and monocytes,3,5 and in nonhematopoietic tissues including brain, kidney, lung, and heart.3,6 7
Recently, Imai et al have identified and characterized a high-affinity receptor for fractalkine,8,9 CX3CR1, corresponding to a classical heptahelical chemokine receptor. This receptor was shown to mediate both the adhesive and migration functions of fractalkine. Chemokine receptors identified to date on leukocytes all present a 7-transmembrane G protein-linked structure.10,11 They have been shown to transduce signals that lead to cytoskeletal reorganization, integrin activation, and other functions required to increase adhesion and migration of the cells. These effects are frequently, but not exclusively, mediated through pertussis toxin-sensitive G protein-coupled pathways. Nevertheless, it has been demonstrated that the association of chemokine receptor to different G proteins depends on the receptor and the cell line studied.12
Fractalkine displays distinctive biologic activities compared to the other members of the chemokine family. Under soluble form, this unique CX3C chemokine was first reported to exert, in vitro, a chemotactic effect on monocytes, natural killer (NK) cells, and T lymphocytes and to induce cellular adhesion.3,4,8 However reports suggested that s-FKN behaves through its receptor CX3CR1, as an adhesion molecule, able to promote under physiologic flow, the firm adhesion of a subset of leukocytes. This adhesive function is not inhibited by pertussis toxin and is integrin independent.5,13 However, it has recently been demonstrated that s-FKN can induce adhesion by up-regulating integrin avidity in a pertussis toxin-sensitive manner.14
To date, information on the cellular signaling triggered by chemokine is scarce. Chemokines have been shown to trigger biochemical events such as increased intracellular cyclic adenosine monophosphate (cAMP) level,15 phospholipase activation,16increased tyrosine phosphorylation particularly with the activation of FAK and ZAP-70,17 stimulation of 2 phosphoinositide-3 kinase (PI3-kinase) isoforms (p85/p110 and C2α),18activation of the JAK2/STAT3 pathway,19 and activation of the ERKs/MAPK cascade.20 21
In this study, we present evidence that SAPKs, ERKs, and PI3K pathways have to act in conjunction for the mediation of s-FKN–dependent adhesion of MonoMac6 cells to fibronectin.
Materials and methods
Cell culture
MonoMac6 cells (DSM ACC124) originally established from a patient with monoblastic leukemia22 were obtained from the German Collection of Microorganisms. The cell cultures (Braunschweig, Germany) were grown in suspension in RPMI 1640 (Gibco, Cergy-Pontoise, France) supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco) l-glutamine (2 mM), penicillin (100 U/mL), and streptomycin (100 μg/mL). Cells were passaged every 3 to 6 days using a split ratio of 1:3.
Products
Human s-FKN was purchased from R&D Systems (Abington, UK). Pertussis and cholera toxins were obtained from Sigma (Saint Louis, MO). PP2, piceatannol, PD98059, SB202190, and LY294002 were purchased from Calbiochem (Meudon, France).
Cell stimulation and lysis
MonoMac6 cells (106 cells/mL) were starved 16 hours in RPMI 1640 medium and harvested by centrifugation for 5 minutes at 1000g before being resuspended in RPMI at a concentration of 2 × 107 cells/mL. Cells (5 × 106) were treated at 37°C with or without the effectors for the indicated times and lysed 30 minutes at 4°C in a buffer containing 150 mM NaCl, 0.8 mM MgCl2, 5 mM EGTA, 1% NP-40, 1 mM phenylmethylsulfonyl fluoride (PMSF), 15 μg/mL leupeptin, 1 μM pepstatine, and 1 mM Na3VO4 and 50 mM Hepes at pH 7.5. The crude lysates were centrifuged at 18 000gfor 10 minutes at 4°C, the supernatants (lysates) were removed, and the protein concentration was assayed using the Bradford method (Biorad, Hercules, CA).
Immune-complex kinase assays
ERKs and SAPK2/p38 MAPK activities.
Cell lysates (100 μg) were boiled after addition of 9 × Laemmli sample buffer prior to being resolved on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto Immobilon-P membranes (Millipore, Bedford, MA) as detailed previously.23 The immunoblots were incubated overnight at 4°C with rabbit polyclonal antiactive ERKs antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) or antiactive p38 antibodies (Promega, Madison, WI). After 3 washes with buffer, 10 mM Tris at pH 7.4, 150 mM NaCl, 1% NP-40, the antibody binding was detected using horseradish peroxidase (HRP)-conjugated goat antirabbit (GAR) antibodies (Dako, Botany, Australia) with the enhanced chemiluminescence (ECL) system with autoradiography hyperfilms MP. The amount of ERKs and SAPK2/p38 was evaluated on each lane after stripping for 30 minutes at 50°C in 67 mM Tris at pH 6.7, 2% SDS, 100 mM 2-mercato-ethanol, by Western blotting using anti ERK2 or SAPK2/p38 antibodies (Santa Cruz Biotechnology) and revealed by chemiluminescence using the ECL kit (Amersham, Arlington Heights, IL) and hyperfilms MP (Amersham).
SAPK1/JNK1 activity.
Cell lysates were precleared with rabbit nonimmune serum prebound to protein A-Sepharose (Pharmacia-LKB Biotechnologies, Uppsala, Sweden) and JNK1 kinase was immunoprecipitated from precleared lysates by incubation at 4°C for 16 hours with 1 μg polyclonal anti-JNK1 antibodies (Santa Cruz Biotechnology) bound to protein A-Sepharose. Immunopellets were washed twice with lysis buffer and twice with MAP kinase buffer (30 mM NaCl, 0.1% NP-40, 10% glycerol, 200 μM Na3VO4, 30 mM Hepes at pH 7.5) before being resuspended in 50 μL MAP kinase buffer containing 30 mM Mg-acetate in the presence of 0.5 mg/mL GST-ATF2, which was used as exogenous substrate. The kinase assay was initiated by addition of 25 μM adenosine triphosphate (ATP) and 20 μCi/mL γ32P]ATP (370 MBq/mL, ICN). After incubation at 30°C for 30 minutes, the reactions were stopped by addition of 9 × Laemmli sample buffer and boiled for 3 minutes.
Src kinase activity.
Precleared lysates were incubated at 4°C for 4 hours with 2 μg polyclonal anti- c-Src antibodies (Argëne Biosof, Varilhes, France) followed by the addition of protein A/G Sepharose (Santa Cruz Biotechnology), and then incubated for 1 additional hour at 4°C. The immunopellets were washed twice with lysis buffer, followed by 2 washes with the tyrosine kinase buffer (10 mM MnCl2, 20 mM Hepes at pH 7.5). Samples were then resuspended in 50 μL tyrosine kinase buffer supplemented with 1 mM DTT and 0.1 mg/mL acid-denatured enolase, which was used as an exogenous substrate. The kinase assay was started by addition of 3.75 μM ATP and 20 μCi/mL γ32P]ATP. After 15 minutes at 30°C the reactions were stopped by addition of 25 μL of 9 × Laemmli sample buffer and boiled for 3 minutes.
For SAPK1/JNK1 and c-Src kinase activities, immunocomplex reactions were fractionated by SDS-PAGE (10%-15% gel) followed by blotting onto Immobilon-P-membrane (Millipore) and autoradiography using Biomax ML (Kodak, Rochester, NY). The total amount of immunoprecipitated SAPK1/JNK1 and c-Src was evaluated on each lane by Western blotting using the appropriate antibodies and revealed by chemiluminescence using the ECL kit (Amersham) and hyperfilms MP (Amersham).
Syk phosphorylation.
The Syk kinases were immunoprecipitated from precleared lysates by incubation at 4°C for 3 hours with polyclonal anti-Syk antibodies (Santa Cruz Biotechnology) bound to protein A-Sepharose. Immunopellets were washed twice with lysis buffer, twice with lysis buffer in the presence of 0.25% sodium deoxycholate, and resuspended in 50 μL of 3 × Laemmli sample buffer. Proteins resolved by SDS-PAGE were electrophoretically transferred onto Immobilon-P membrane and tyrosine phosphorylated Syk kinases were detected using 4G10 monoclonal antiphosphotyrosine antibodies (Upstate Biotechnology, Lake Placid, NY). The total amount of immunoprecipitated Syk was evaluated on each lane, after stripping as described above and reprobed with monoclonal anti-Syk antibodies (Santa Cruz Biotechnology) and revealed by chemiluminescence using the ECL kit and hyperfilms MP.
PI kinase assays
Cleared whole lysates from untreated or s-FKN–stimulated MonoMac6 cells were precipitated with an antiphosphotyrosine monoclonal antibody (Upstate Biotechnology) for 2 hours at 4°C, then protein A-Sepharose beads were added for 1 hour. Immunopellets were treated and subjected to PI kinase assay in the absence or in the presence of either 0.2% Nonidet-P40 or 200 μM adenosine to discriminate between PI3K and PI4K, as previously described.23 Phosphorylated reaction products were extracted by methanol-chloroform and then separated by thin layer chromatography onto silica gel plates (Whatman 60A LK6D). The spots corresponding to the phosphorylated products were visualized by autoradiography.
Cell adhesion assays
The response of MonoMac6 cells to s-FKN was evaluated by using 24-well migration chambers and polyethylene terephtalate (PET) inserts with 8 μm pores filters (Becton Dickinson, San Jose, CA) coated with 6.5 μg/mL fibronectin (Sigma, St Louis, MO) on both sides. MonoMac6 cells, incubated for 16 hours with [3H]-methyl thymidine (ICN, 2.5 μCi/mL) were placed, in presence or in absence of 60 nM s-FKN, in the upper well (106 cells/100 μL) and RPMI 1640 medium was added in the lower well. Plates were incubated for 3 hours at 37°C in 5% CO2 atmosphere. At the end of the migration assays, fibronectin-coated filters were collected and washed twice in phosphate-buffered saline (PBS) buffer, and the adhesion was evaluated by the measure of the amount of [3H]-methyl thymidine-labeled cells by scintillation spectroscopy.
Results
s-FKN stimulates ERKs and SAPKs activities in human MonoMac6 cells following distinct time courses
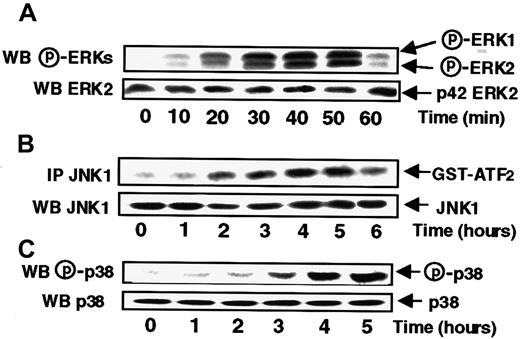
To study the effects of s-FKN on ERK activities, MonoMac6 cells were treated with s-FKN (60 nM) for various periods of time before being lysed. The activation status of ERK1 and ERK2 was then evaluated by measuring the extent of p42/p44 ERKs phosphorylation with appropriate antibodies. As shown in Figure1A, s-FKN induced a rapid increase in the level of ERK1 and ERK2 phosphorylation starting at 20 minutes before declining to the basal level by 1 hour.
s-FKN stimulates with distinct time courses ERKs, JNK1, and p38 activities in MonoMac6 cells.
MonoMac6 cells were incubated with 60 nM s-FKN for the indicated times and extracted proteins from lysates were resolved by SDS-PAGE and electrophoretically transferred to Immobilon-P membrane before being detected by immunoblotting with antiactive ERKs (A) or p38 (C) antibodies. JNK1 activity (B) was performed, from cell lysates, by in vitro kinase assay using GST-ATF2 as substrate after immunoprecipitation (IP) with specific antibodies as described in “Materials and methods. Amounts of ERKs, JNK1, or p38 in immunoprecipitates were assessed by Western blotting (WB) analysis with the appropriate antibodies. The results are representative of 3 experiments.
s-FKN stimulates with distinct time courses ERKs, JNK1, and p38 activities in MonoMac6 cells.
MonoMac6 cells were incubated with 60 nM s-FKN for the indicated times and extracted proteins from lysates were resolved by SDS-PAGE and electrophoretically transferred to Immobilon-P membrane before being detected by immunoblotting with antiactive ERKs (A) or p38 (C) antibodies. JNK1 activity (B) was performed, from cell lysates, by in vitro kinase assay using GST-ATF2 as substrate after immunoprecipitation (IP) with specific antibodies as described in “Materials and methods. Amounts of ERKs, JNK1, or p38 in immunoprecipitates were assessed by Western blotting (WB) analysis with the appropriate antibodies. The results are representative of 3 experiments.
To further characterize the different signaling pathways triggered in response to s-FKN, we investigated the level of activation of the 2 SAPKs, namely JNK1 and p38. JNK1 activity was assayed in JNK1 immunoprecipitates from lysates of s-FKN–treated MonoMac6 cells, by assaying their ability to phosphorylate GST-ATF2 as substrate. As shown in Figure 1B, s-FKN stimulated JNK1 activity with a kinetic profile markedly delayed compared to the time course observed for ERKs, being detectable only by 2 hours and lasting for up to 5 hours. Similarly, s-FKN stimulated p38 activity with a lag period of 3 hours and persisting for up to 5 hours.
Syk- and Src-like protein tyrosine kinases are involved in s-FKN–induced MAPKs activation in MonoMac6 cells
We examined the possible involvement of protein tyrosine kinases, often described as implicated in signal transduction from G protein-coupled receptors (GPCRs).24 MonoMac6 cells were preincubated in the presence of either PP2, a potent inhibitor of the Src kinase family,25 or piceatannol, a specific inhibitor of the Syk tyrosine kinase family26 before being exposed to s-FKN.
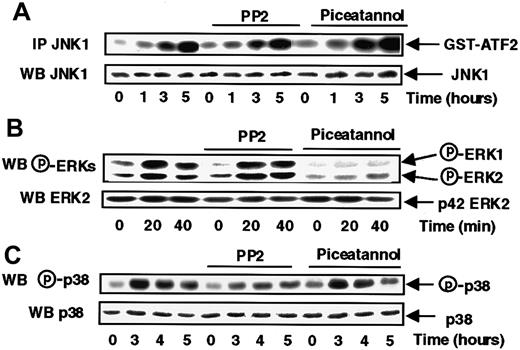
The data presented in Figure 2A show that these 2 tyrosine kinase inhibitors were without effect on JNK1 activation in response to s-FKN. Instead, s-FKN–induced ERK1 and ERK2 activation was abrogated by piceatannol, with no effect of PP2 suggesting that Syk, but not Src, controlled the s-FKN–mediated activation of both types of ERKs (Figure 2B).
Syk and Src-like tyrosine kinases are respectively involved in the s-FKN–induced ERKs and p38 activations.
MonoMac6 cells, untreated or preincubated 2 hours with 10 μM PP2 or 5 μg/mL piceatannol, were exposed following different time courses to 60 nM s-FKN. The activity and the amount of each MAPK were evaluated as described in “Materials and methods.” The results are representative of 3 experiments.
Syk and Src-like tyrosine kinases are respectively involved in the s-FKN–induced ERKs and p38 activations.
MonoMac6 cells, untreated or preincubated 2 hours with 10 μM PP2 or 5 μg/mL piceatannol, were exposed following different time courses to 60 nM s-FKN. The activity and the amount of each MAPK were evaluated as described in “Materials and methods.” The results are representative of 3 experiments.
The inhibitory profile of the 2 tyrosine kinases inhibitors on p38 activation (Figure 2C) mirrored that observed with ERKs, because only PP2 was partially efficient, revealing a specific involvement of Src-like kinases, but not Syk, in the control of s-FKN-induced p38 activation.
s-FKN induces Syk phosphorylation and activates c-Src in MonoMac6 cells
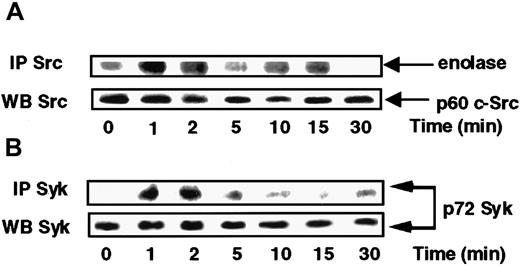
On the basis of the above data suggesting a possible involvement of Src and Syk in the respective control of p38 and ERKs, we sought to verify whether Src and Syk activities were indeed activated in response to s-FKN. To this end, Src activity present in anti-c-Src immunoprecipitates from lysates of cells exposed to s-FKN was tested. Figure 3A shows that c-Src kinase was sharply activated, peaking at 1 to 2 minutes before declining to the basal level by 5 minutes and rebonding in a biphasic manner by 15 minutes. Syk was immunoprecipitated from s-FKN–treated MonoMac6 cells and pellets were examined for their phosphotyrosine profile. Stimulation of MonoMac6 cells by s-FKN also induced a rapid increase in the level of Syk tyrosine phosphorylation appearing within 1 to 2 minutes (Figure 3B).
s-FKN activates Syk and Src tyrosine kinases in MonoMac6 cells.
MonoMac6 cells were incubated with 60 nM s-FKN for the indicated times. Src (A) and Syk (B) kinases were immunoprecipitated (IP) with specific antibodies from cell lysates, resolved by SDS-PAGE, and electrophoretically transferred to Immobilon-P membrane. Src activity was assessed by in vitro kinase assay using enolase as substrate, and Syk activity by immunoblotting using antiphosphotyrosine 4G10 monoclonal antibodies. The amounts of Syk and c-Src immunoprecipitated were evaluated by Western blotting (WB) analysis with the appropriate antibodies. The results are representative of 2 experiments.
s-FKN activates Syk and Src tyrosine kinases in MonoMac6 cells.
MonoMac6 cells were incubated with 60 nM s-FKN for the indicated times. Src (A) and Syk (B) kinases were immunoprecipitated (IP) with specific antibodies from cell lysates, resolved by SDS-PAGE, and electrophoretically transferred to Immobilon-P membrane. Src activity was assessed by in vitro kinase assay using enolase as substrate, and Syk activity by immunoblotting using antiphosphotyrosine 4G10 monoclonal antibodies. The amounts of Syk and c-Src immunoprecipitated were evaluated by Western blotting (WB) analysis with the appropriate antibodies. The results are representative of 2 experiments.
s-FKN–induced ERKs, JNK1, and p38 pathways display distinctive sensitivities to cholera and pertussis toxins
Chemokine receptors have long been known to be coupled with G proteins sensitive to bacterial toxins.27 To better delineate the involvement of Go/i and Gs proteins in the differential control of ERKs, JNK1, and p38, MonoMac6 cells were pretreated for 4 hours, with pertussis toxin, an inhibitor of Go/i proteins, or cholera toxin, an activator of Gs proteins.
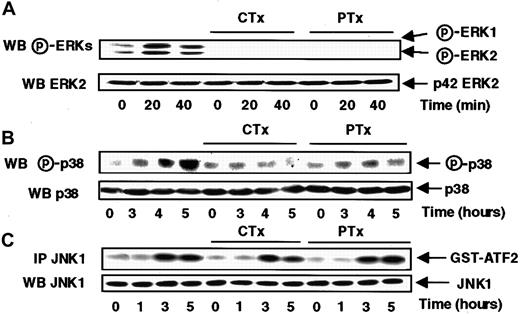
Figure 4, panels A and B, show that s-FKN–mediated ERK1, ERK2, and p38 activation was abrogated by a previous treatment of MonoMac6 cells with either cholera or pertussis toxin, suggesting that those pathways did require an upstream coordinated activation of both Gs and Go/i proteins.
Cholera and pertussis toxins abrogate the s-FKN–induced ERKs and p38 activations, but do not affect the JNK1 activation.
MonoMac6 cells, untreated or preincubated 4 hours with 250 ng/mL pertussis toxin (PTX) or 10 μg/mL cholera toxin (CTX), were exposed to 60 nM s-FKN following different time courses. The activity and the amount of each MAPK were evaluated as previously described in Figure 1and in “Materials and methods.” The results are representative of 3 experiments.
Cholera and pertussis toxins abrogate the s-FKN–induced ERKs and p38 activations, but do not affect the JNK1 activation.
MonoMac6 cells, untreated or preincubated 4 hours with 250 ng/mL pertussis toxin (PTX) or 10 μg/mL cholera toxin (CTX), were exposed to 60 nM s-FKN following different time courses. The activity and the amount of each MAPK were evaluated as previously described in Figure 1and in “Materials and methods.” The results are representative of 3 experiments.
Conversely, JNK1 activity remained unaffected when the cells were treated either for 16 hours by 100 ng/mL pertussis toxin (data not shown) or for 4 hours by 250 ng/mL pertussis toxin or 10 μg/mL cholera toxin (Figure 4C), indicating that neither Gs nor Go/i proteins are involved in the control of JNK1 activation in response to s-FKN.
s-FKN stimulates PI kinase activity in MonoMac6 cells
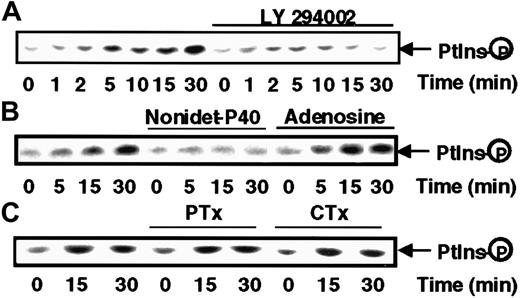
Because of the importance of phosphorylation of inositide phospholipids in the activation of several kinases and cytoskeleton remodeling, we sought to determine whether PI kinase was activated in response to s-FNK. Antiphosphotyrosine immunoprecipitates from lysates of s-FKN–treated MonoMac6 cells were tested for their ability to phosphorylate PI. As shown in Figure 5, s-FKN rapidly stimulates a lipid kinase activity detectable within 5 minutes (upper panel), which was virtually abrogated by a pretreatment of the cells with LY294002, a specific inhibitor of the PI3-kinase.28 To define more precisely the nature of the PI kinase activity, we took advantage of the fact that PI3-kinase has been shown to be strongly inhibited by NP-40 detergent and insensitive to the presence of adenosine, a pattern that was symmetrical to that exhibited by PI4K.29 The data presented in Figure 5, lower panel, indicate that the s-FKN–induced lipid-kinase displays all the features of a PI3K. We verified that in our system, PI3K inhibition was without effect on any of the 3 MAPK cascades (data not shown).
s-FKN stimulates PI3K activity.
MonoMac6 cells, untreated or, as indicated in the figure, preincubated 2 hours with 10 μM LY294002 or 16 hours with 100 ng/mL pertussis toxin (PTX) or 4 hours with 10 μg/mL cholera toxin (CTX), were exposed to 60 nM s-FKN following different time courses. Cell lysates were immunoprecipitated with antiphosphotyrosine monoclonal antibody and immune complexes were analyzed for PI kinase activity in the absence (A,C) or presence of 0.2% Nonidet-P40 or 200 μM adenosine (B). The results are representative of 2 experiments.
s-FKN stimulates PI3K activity.
MonoMac6 cells, untreated or, as indicated in the figure, preincubated 2 hours with 10 μM LY294002 or 16 hours with 100 ng/mL pertussis toxin (PTX) or 4 hours with 10 μg/mL cholera toxin (CTX), were exposed to 60 nM s-FKN following different time courses. Cell lysates were immunoprecipitated with antiphosphotyrosine monoclonal antibody and immune complexes were analyzed for PI kinase activity in the absence (A,C) or presence of 0.2% Nonidet-P40 or 200 μM adenosine (B). The results are representative of 2 experiments.
s-FKN–induced MonoMac6 cells adhesion to fibronectin is dependent on ERKs, SAPKs, and PI3K pathway activation
Chemokine receptors identified to date on leukocytes, including CX3CR1, all exhibit a 7-transmembrane G protein-linked structure and have been shown to transduce signals leading to cytoskeletal reorganization, integrin activation, and other functions leading to cell migration.10,11 We examined the potential involvement of each of s-FKN–triggered signaling pathways in the s-FKN–enhanced MonoMac6 cells adhesion to fibronectin. Cells were pretreated either with PD98059, a specific MEK inhibitor,30 or with SB202190, a SAPKs inhibitor,31 or with LY294002, a PI3K inhibitor,28 before being tested for their ability to interact with the fibronectin-coated inserts. Our results indicate that s-FKN markedly enhanced adhesion of MonoMac6 cells to fibronectin and that cells remained immobilized even after a lag period of 3 hours, which is compatible with the signaling time course observed for the SAPKs pathways. As shown in Figure 6, enhancement of MonoMac6 cells binding to fibronectin induced by s-FKN was diminished by 58% in the presence of SB202190 (column 2) and by almost 30% with either PD98059 (column 3), LY294002 (column 4), or pertussis toxin (column 5). Interestingly, ablation of the totality of the MAPK activities by the use of SB202190 plus PD98059 led to 85% inhibition of s-FKN–induced adhesion (column 6). Along the same line, consistent with a PI3K-dependent pathway distinct from MAPK cascades, exposure of MonoMac6 cells to a mixture of SB202190 and LY294002 resulted in an 80% inhibition of the chemokine-induced adhesion (column 7). We verified in our migration system that all of these inhibitors have any significant effect on basal MonoMac6 adhesion to fibronectin (data not shown).
s-FKN–induced MonoMac6 cells adhesion to fibronectin is partially dependent on ERKs, SAPKs, PI3K, and Go/Gi proteins-mediated signaling pathways.
[3H]-Methyl thymidine-labeled MonoMac6 cells were washed and pretreated for 16 hours with either 100 ng/mL pertussis toxin (PTX) or for 2 hours with 30 μM SB202190, or PD98059, or 10 μM LY294002, or with combined inhibitors. Cells were then placed in the upper well of migration chambers in presence of 60 nM s-FKN and tested for their ability to adhere to fibronectin-coated filters. After a 3-hour period, filters were collected and washed twice in phosphate buffer saline. The amount of adherent cells to fibronectin was evaluated by liquid scintillation β counts as described in “Materials and methods.” Data are mean ± SD from triplicates of a single experiment representative of 3 others.
s-FKN–induced MonoMac6 cells adhesion to fibronectin is partially dependent on ERKs, SAPKs, PI3K, and Go/Gi proteins-mediated signaling pathways.
[3H]-Methyl thymidine-labeled MonoMac6 cells were washed and pretreated for 16 hours with either 100 ng/mL pertussis toxin (PTX) or for 2 hours with 30 μM SB202190, or PD98059, or 10 μM LY294002, or with combined inhibitors. Cells were then placed in the upper well of migration chambers in presence of 60 nM s-FKN and tested for their ability to adhere to fibronectin-coated filters. After a 3-hour period, filters were collected and washed twice in phosphate buffer saline. The amount of adherent cells to fibronectin was evaluated by liquid scintillation β counts as described in “Materials and methods.” Data are mean ± SD from triplicates of a single experiment representative of 3 others.
Discussion
s-FKN has been recently reported to exert, in vitro, a chemotactic effect on monocytes, NK cells, and T lymphocytes3,4,8 and to induce the firm adhesion of monocytes and CD8 T lymphocytes.5,14 Although some activation signals triggered in response to other chemokines have been reported,19 32-34 the molecular mechanisms responsible for cell activation by s-FKN remain largely unknown. On the basis of this observation, we became interested in elucidating, in MonoMac6 cells, the effect of s-FKN on the signal transduction pathways induced from its receptor identified as CX3CR1.
s-FKN not only stimulated ERK1 and ERK2 in MonoMac6 cell line but also promoted an important increase in SAPKs activities such as JNK1 and p38. Interestingly, the activation kinetic profiles of the different members of the MAPK family fall into 2 categories with a rapid and transient activation of the 2 ERKs activities (within 1 hour), whereas JNK1 and p38 SAPKs exhibited a delayed and sustained time course that persisted for up to 5 hours. These results suggest that complex mechanisms could be triggered in response to s-FKN interaction with its receptor, leading to independent controls of the different MAPKs cascades.
s-FKN receptor belongs to a large family of 7 transmembrane-spanning, heterotrimeric GPCRs.10,35 Ligands interacting GPCR have been demonstrated to activate different tyrosine kinases that bridge the G proteins to the ERK pathway.24,36-38 Various nonreceptor tyrosine kinases have recently been shown to participate in myeloid cell signaling responses to growth factors and to IgG binding to Fc receptors, including Lyn, Yes, Hck, Fgr, c-Src,39-43as well as Syk.44-47 Syk has also been reported to be activated in monocytes stimulated by the engagement of integrins.48-50 Furthermore, this tyrosine kinase has been shown to control JNK activation on CD28-mediated T-cell stimulation.31
To examine the possible involvement of Syk- and Src-like protein tyrosine kinases in s-FKN–induced MAPKs and SAPKs activation, MonoMac6 cells were preincubated in presence of either PP2, a potent inhibitor of the Src kinase family25 or piceatannol, a specific inhibitor of the Syk tyrosine kinase family26 before being exposed to the chemokine. We could demonstrate, in our model, that tyrosine kinases exerted a complex control on the different congeners of the MAPK family, where s-FKN–induced ERKs and p38 activities were respectively abrogated by piceatannol and dramatically attenuated by PP2. This implies that, on s-FKN stimulation, Syk positively controlled the ERKs pathway, whereas c-Src potentiated p38 activation. It is noteworthy that under the same conditions JNK1 activation was insensitive to any of the tyrosine kinase inhibitors. We verified that s-FKN effectively activated both Src and Syk activities following a rapid and sharp profile of stimulation, occurring within minutes, compatible with a subsequent activation of ERKs and p38.
Taken collectively, our data lend support to a model where s-FKN activates independently Syk and Src that in turn stimulate, respectively, the ERKs and the p38 pathways, whereas the JNK1 pathway seems to be totally independent of these nonreceptor tyrosine kinases.
Chemokines receptors have long been known to be coupled with G proteins sensitive to bacterial toxins.27 Analysis of s-FKN effects on leukocytes has revealed that cell migration responses and calcium mobilization are mediated in a pertussis toxin-dependent manner,8 suggesting a coupling of CX3CR1 to Go/Gi class of G proteins. However, it has recently been demonstrated that the association of chemokines receptors to different G proteins depends on both the receptor and the cell line studied.12 51 To further evaluate the coupling mechanism(s) of the s-FKN receptor and the nature of possible G proteins involved in the signaling events activated in response to this chemokine, MonoMac6 cells were pretreated with pertussis toxin, an inhibitor of Go/i proteins, or cholera toxin, an activator of Gs proteins.
Interestingly, we found that ERKs and SAPKs exhibited a differential sensitivity to these toxins. The s-FKN–mediated ERK1, ERK2, and p38 activation was abrogated by the treatment of MonoMac6 cells with both cholera and pertussis toxins, suggesting those pathways are under the coordinated control of Gs and Go/i proteins. JNK1 differed from the other MAPK in the sense that it was insensitive to the action of both kinds of toxin, consistent with the idea that s-FKN up-regulates this cascade through a Gs or Go/i protein-independent pathway.
Chemokines have been demonstrated to transmit, via their GPCRs, intracellular signals resulting in the activation of PI kinases as evidenced for SDF-1α in Jurkat cells,52 RANTES in NK cells53 and in T lymphocytes,54 IL-8 in human neutrophils,55 and MCP-1 in the monocytic THP-1 cell line.18
We found that s-FKN rapidly induced a lipid kinase activity in the MonoMac6 cells. This activity was blocked by a pretreatment of the cells by LY294002 or in presence of detergent added in the kinase assay, pointing to the involvement of a PI3K. However, pretreatment of the cells with LY294002 failed to alter any of the chemokine-mediated MAPKs (ERKs, JNK1, or p38) providing evidence that PI3K activity controls a distinct pathway.
To date, scarce information is available on the mechanisms implicated during the diapedesis step in response to s-FKN. This chemokine has been reported to mediate both chemotaxis and adhesion of monocytes,3 but other reports claim that the chemokine-like domain or the entire extracellular domain of s-FKN fail to mobilize human monocytes and THP-1 cells.4 Despite this discrepancy, evidence from many groups likely supports a role of s-FKN in mediating cell adhesion.8,13,14 In this context, we wanted to define which of the s-FKN–activated pathway(s) intervene(s) in its proadhesive properties. Cell adhesion was reduced by 58% with SB202190, a SAPKs inhibitor,31 and by 30%, with either PD98059, a MEK inhibitor,30 or LY294002, a PI3K inhibitor.28 Furthermore, inhibition of all the MAPK cascades (by SB202190 and PD98059) or the concomitant abrogation of SAPK and PI3K (by SB202190 and LY294002) resulted in both cases to more than 80% inhibition of cells adherence, highlighting the concomitant requirement of of SAPKs, ERKs, and PI3K pathways for the mediation of the proadhesive activity of the s-FKN. Finally, the inhibitory effect of pertussis toxin revealed that the strong adhesive effect of s-FKN was also partially dependent on Go/Gi protein activation, in accord with a recent study from Goda et al.14
In conclusion, our data point to a complex coordinated activation of SAPKs, ERKs, and PI3K to efficiently mediate the firm adhesion process engendered by s-FKN in human monocytes.
Supported by the Institut National de la Santè et de la Recherche Mèdicale, the Ligue Nationale Contre le Cancer, ComitèDèpartemental des Alpes Maritimes (subvention 2000) and the Association pour la Recherche sur le Cancer (grant 5417). B.C. and M.P. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bernard Rossi, INSERM U364, IFR 50, Facultè de Mèdecine, Avenue de Valombrose, 06107 Nice Cedex 02, France; e-mail: rossi@taloa.unice.fr.

![Fig. 6. s-FKN–induced MonoMac6 cells adhesion to fibronectin is partially dependent on ERKs, SAPKs, PI3K, and Go/Gi proteins-mediated signaling pathways. / [3H]-Methyl thymidine-labeled MonoMac6 cells were washed and pretreated for 16 hours with either 100 ng/mL pertussis toxin (PTX) or for 2 hours with 30 μM SB202190, or PD98059, or 10 μM LY294002, or with combined inhibitors. Cells were then placed in the upper well of migration chambers in presence of 60 nM s-FKN and tested for their ability to adhere to fibronectin-coated filters. After a 3-hour period, filters were collected and washed twice in phosphate buffer saline. The amount of adherent cells to fibronectin was evaluated by liquid scintillation β counts as described in “Materials and methods.” Data are mean ± SD from triplicates of a single experiment representative of 3 others.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/7/10.1182_blood.v97.7.2031/6/m_h80710847006.jpeg?Expires=1765946009&Signature=QWMlsfDD5-zubvH-AZ-mQFBeFOjOVeEgQ7qYkf~kWJnJhBcR9dG9oZRTz-5SJy0XYR~JF9B2Mi97vr9Nr~65agG9lw2nRmbz2KH7m2RAb9oiU50z5V0kxZHe61JFeIrsY6cRcKZ8EQ0~9P0A2koyZC8X2LT2ZNi3HP3OS58UZ1quawNXGCz5Uubxl6emcS7T~fo5PikqWBKHXl8RFVnJ4ztvd0~1Nh4hPQ7XBMgJBp4~G6-8sqTDdKYlnQxh3gyRIFHn0Ec5PZtiz2RBn0~elUWKqbdBDojfbiuC0SR8dnCtylBlnSOuPGkpqWtarl8pImxI5fPoFyjMh3qeNTn-QQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
