Introduction
The development of cell and organ transplantation is one of the great success stories of modern medicine. Transplantation of various cells, tissues, and solid organs has entered the realm of the clinically routine.1-4 Milestones in cell therapy include the development of transfusion medicine, hemopoietic stem cell transplantation, and adoptive immunotherapy. Progress in cell therapy outside hematology has been slower to develop, but recent progress has been made in Parkinson disease5,6 and diabetes mellitus.7 Most recently, advances in stem cell biology may presage an impending revolution in cell therapy. Several studies have demonstrated that the stem cells of healthy adults retain enormous plasticity. For example, hemopoietic stem cells can differentiate into muscle,8,9 liver cells,10and neurons11,12; neural stem cells can differentiate into blood,13 liver, kidney, or muscle cells14; and muscle stem cells can differentiate into blood.15Human embryonic stem cells, which are capable of contributing to all human tissues, have been generated for the first time.16These discoveries make imaginable the prospect of developing a new generation of cell therapeutics applicable to a vast array of diseases. However, before this vision can be realized many obstacles must be overcome, with one of the most important obstacles resting in the present inability to control what happens to a cell after it has been transplanted.
Currently available tools for controlling the survival, growth, and differentiation of transplanted cell populations are blunt instruments. In most cases, graft-versus-host disease (GVHD) is only poorly controlled with immunosuppressive drugs, which in turn cause a punishing array of side effects. Lineage-restricted growth factors such as erythropoietin and granulocyte colony-stimulating factor (G-CSF) have had a major effect on clinical hematology, but growth factors are powerless in situations in which it is desirable to preferentially expand a subpopulation of cells with a particular lineage. These situations may arise in the setting of mixed chimerism, in which donor and host hemopoietic elements coexist; or in gene therapy, in which only a fraction of cells contain the therapeutic gene. Under these circumstances, it would be useful to have a means of specifically influencing the in vivo behavior of genetically modified cells in terms of proliferation, death, or differentiation. Toward this end, conditional systems have been developed that rely on the principle that protein interactions control virtually all cellular processes; in particular, protein dimerization (or oligomerization) is at the heart of many signaling pathways controlling cell death, proliferation, and differentiation.
These conditional systems share 2 main components: a fusion protein and a drug. The fusion protein contains an intracellular signaling molecule linked to a protein that provides a high-affinity binding site for a drug called a chemical inducer of dimerization (CID). Individually the signaling domains are functionally inert. Activation occurs upon bringing 2 signaling domains into close proximity with one another, a process termed dimerization. Proximity is controlled through addition of the CID, which in turn activates signaling. The signaling fusion thus serves as a switch that is turned on in the presence of the CID and off following withdrawal of the CID. This method has the potential to control the behavior of genetically modified cells both in vitro and in vivo. The generation of pharmacologically regulated alleles has important implications for analyzing the effect of signaling molecules such as growth factor receptors, Janus kinases (JAK), signal transducers and activators of transcription, and others. This review focuses primarily on potential clinical applications of dimerizer systems using intracellular growth factor–receptor sequences.
Systems for achieving controlled proximity
Systems for achieving controlled proximity should meet several criteria before being considered for clinical use. The first set of criteria pertains to the CID. Obviously, the CID should not exist naturally in humans, since constitutive activation would occur, and it should be nontoxic. For testing in experimental systems, a monomeric counterpart of the CID provides a valuable control for competitive-inhibition assays. The second set of criteria pertains to the fusion protein. The fusion should be entirely human in origin because expression of nonhuman sequences can induce clinically relevant immunologic responses.17 The fusion should be relatively small because of the limited packaging capacity of many gene-delivery systems and the anticipated need to incorporate a second therapeutic gene into the vector in many cases. Finally, the fusion should not undergo constitutive dimerization and must be strictly dependent on the presence of the CID for spatial proximity and therefore activation.
Steroid-based systems
The creation of steroid-regulated signaling molecules was first described in studies using the N-terminal glucocorticoid-binding domain (GBD) of the glucocorticoid receptor.18 Fusion proteins containing the GBD are complexed with heat shock protein 90 (hsp90), which dissociates on hormone binding.19 This in turn has various functional consequences, depending on the protein to which the GBD is fused. When the GBD is fused to a transcription factor, glucocorticoid binding relieves hsp90-mediated repression of transactivation. Additionally, hsp90 dissociation allows nuclear translocation of the fusion protein and self-association of GBD domains, leading to dimerization or multimerization.20Similar mechanisms appear to underlie other steroid-responsive systems, the best characterized of which is the estrogen-binding domain (EBD). Estrogen binding to a fusion protein incorporating the EBD also allows dimerization.21 Of note, the self-association domains of the GBD or EBD do not rely on the drug to enforce spatial proximity directly. Rather, the steroid binding relieves hsp90-mediated repression of dimerization. This contrasts with the coumermycin-gyrase B (GyrB)–based system and the FK1012-FK506 binding protein (FKBP)–based system described below. In these systems, a symmetric small molecule forms a bridge between 2 dimerization domains (Figure1). The steroid-based system was described in detail by Picard.22
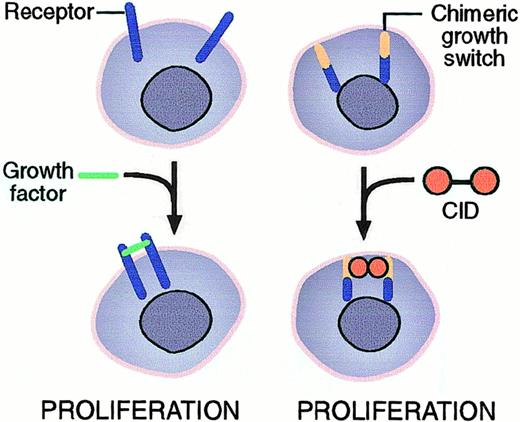
Induction of dimerization by a CID.
A chimeric growth switch consisting of receptor sequences and a dimerization domain is activated on addition of a CID. The CID enforces dimerization by binding 2 dimerization domains on 2 neighboring molecules with a 1:2 stoichiometry. Dimerization causes signaling from the receptor sequences. The principle is the same for the coumermycin-GyrB system and the FK1012-FKBP system. The molecule shown also carries a myristylation domain derived from c-src for targeting to the inner cell membrane.
Induction of dimerization by a CID.
A chimeric growth switch consisting of receptor sequences and a dimerization domain is activated on addition of a CID. The CID enforces dimerization by binding 2 dimerization domains on 2 neighboring molecules with a 1:2 stoichiometry. Dimerization causes signaling from the receptor sequences. The principle is the same for the coumermycin-GyrB system and the FK1012-FKBP system. The molecule shown also carries a myristylation domain derived from c-src for targeting to the inner cell membrane.
In response to the anticipated concern that binding by endogenous estrogen would produce constitutive activation in vivo, a point mutation in the hormone-binding domain that decreases the affinity for estrogen by 3 orders of magnitude was introduced.23 This modified domain has an unaltered affinity for tamoxifen, and a system for generating conditionally active signaling proteins based on the mutated EBD has been described.24,25 The clinical utility of this system must be established in vivo, since the antagonism of endogenous estrogen receptors by tamoxifen may be undesirable in some settings. An advantage of the steroid-based system is that it allows creation of conditional alleles by mechanisms other than dimerization. For instance, Heyworth et al26 demonstrated that a conditionally active GATA-2 allele induces cell-cycle exit and differentiation in hemopoietic cell lines and primary murine bone marrow cells.
Coumermycin-GyrB–based systems
A dimerizer system that uses the antibiotic coumermycin has also been developed.27 This system relies on the binding of coumermycin to 2 copies of an N-terminal 24-kd fragment derived fromEscherichia coli DNA GyrB. Novobiocin, a competitive monomer of coumermycin, enhances the usefulness of this system in experimental models. However, the anticipated immunogenicity of the bacterial sequences will likely hinder transition of this system into clinical use. Additionally, coumermycin has not been developed for clinical use, mainly because of poor bioavailability, adverse gastrointestinal effects of its oral form, and local irritation on parenteral administration.28
FKBP12-based systems
A dimerizer system that has advantages with respect to clinical development is based on the immunophilin FKBP12, a rotamase that binds the lipid-soluble immunosuppressive drug FK506 and its dimeric counterpart, FK1012.29 Although the FK506-FKBP12 complex inhibits calcineurin and thus suppresses signaling by means of the T-cell receptor, FKBP12 in complex with FK1012 (or any of an increasing number of chemically designed FK1012 analogues) does not inhibit calcineurin and is therefore not immunosuppressive.
Binding of FK1012 to endogenous FKBP12 sequences is undesirable for 2 reasons. First, endogenous FKBP12 may act as a sink, sequestering the drug so that it cannot perform its task of binding FKBP12 motifs in the fusion protein. Second and more important, binding to endogenous protein might interfere with the physiologic involvement of FKBP12 in processes such as calcium-channel function30 and sperm motility.31 FKBP12 knockout mice were found to have severe congenital cardiomyopathy, indicating that FKBP12 has a role in the development and perhaps the maintenance of the myocardium.32 To prevent binding to endogenous FKBP12, a new generation of dimerizers has been developed in which a chemical modification or “bump” has been incorporated into the CID, thereby precluding binding to FKBP12. To accommodate binding to bumped CIDs, a hole was engineered into FKBP12 by introducing a single phenylalanine-to-valine substitution at amino acid position 36 (now termed F36V), thereby providing lock-and-key specificity at the FKB12-CID interface.33 34 This approach circumvents toxicity that might arise from binding to endogenous FKBP12.
A bumped CID, AP1903, has been tested in a phase I trial in healthy human subjects. A single intravenous dose achieved serum levels predicted to be effective, without producing apparent toxic effects.66 A possible disadvantage of the F36V substitution is generation of a T-cell epitope that might lead to immune-mediated rejection of the transduced cells. Although this has not been observed in murine transplantation models, immune responses must be carefully evaluated in studies using large animals and in clinical trials.
Pharmacologic regulation of cell growth
A method for pharmacologically regulating the growth of genetically modified cells would have several possible applications. For example, inefficient gene transfer into stem cells is a major obstacle in gene therapy. To circumvent this obstacle, a gene encoding a CID-responsive protein that regulates cell growth might be linked to a second gene that encodes a therapeutic protein. After gene transfer, the transduced population could be selectively expanded by using CIDs either in vitro or in vivo (Figure 2). In vivo selection using CIDs would have particular advantages in gene therapy. In contrast to selection methods that rely on use of drug-resistance genes, CIDs offer the possibility of achieving selection without the toxic effects associated with administration of cytotoxic drugs. Additionally, selection could bypass the need for myeloablative conditioning, thereby further improving safety of the treatment. Finally, CID-mediated selection would probably circumvent the variable transgene expression that is attributable to position effects,35-37 since CID-responsiveness requires expression of the transgene.
In vivo selection of hemopoietic cells by using CID-selectable genes.
The vector encodes 2 genes, a therapeutic gene and a CID-selectable gene. After ex vivo transduction and reinfusion of hemopoietic stem cells, administering a CID in vivo signals a proliferative stimulus targeted exclusively to the transduced cell population, which then selectively expands.
In vivo selection of hemopoietic cells by using CID-selectable genes.
The vector encodes 2 genes, a therapeutic gene and a CID-selectable gene. After ex vivo transduction and reinfusion of hemopoietic stem cells, administering a CID in vivo signals a proliferative stimulus targeted exclusively to the transduced cell population, which then selectively expands.
Because growth factors provide a paradigm for controlling cell proliferation, most efforts to regulate the growth of genetically modified cells use the signaling domains of growth factor receptors. In theory, the simplest approach would involve use of genes that encode naturally occurring full-length receptors, such as the receptor for erythropoietin.38 However, this approach could not be used clinically because the presence of endogenous ligand in vivo induces sustained growth of cells bearing the transgene.39 Efforts to bring cell proliferation under pharmacologic control have the potential for clinical applicability in that growth factor receptors can be stripped of their extracellular domains, thereby precluding activation by endogenous ligands. Instead, the residual receptor signaling domain is linked to motifs that render the fusion dependent on CID. The feasibility of this approach was demonstrated by using the interleukin 3 (IL-3)–dependent murine pro-B-cell line Ba/F3. Ba/F3 cells have an especially useful property: when forced to express a heterologous receptor, they can be converted to dependency on the corresponding ligand. For example, expression of the erythropoietin receptor allows Ba/F3 cell growth in erythropoietin despite the absence of IL-3.40 Similarly, Ba/F3 cells expressing an FKBP12-erythropoietin receptor fusion were shown to be dependent on FK1012, thereby demonstrating the feasibility of pharmacologic control over cell proliferation.41
Similar results have been obtained with each of the dimerizer systems described above, including signaling domains taken from the G-CSF receptor,42,43 c-kit,44 mpl,45flt-3, and gp130 (unpublished data). Dimerization of JAK was also shown to suffice for Ba/F3 cell growth in the absence of IL-3.46,47 Recent data suggests that dimerization is necessary but not sufficient for activation of receptor signaling48 and that preformed receptor complexes on the cell surface are activated by a conformational change on ligand binding.49 50 The extent to which these natural mechanisms are mimicked by CIDs is unknown.
Applications in the hemopoietic system
In applying pharmacologically regulated cell growth to the hemopoietic system, stable expression of the transgene is essential. Stable expression requires (1) vectors (such as retroviral vectors) that can stably integrate into DNA, (2) gene transfer into a long-lived cell such as the hemopoietic stem cell, and (3) incorporation ofcis regulatory sequences that guarantee expression in the desired cell type. In this regard, sequences in the long-terminal repeats of murine stem cell virus–based vectors were shown to direct expression in very early human hemopoietic cells (ie, severe combined immunodeficiency–nonobese diabetic repopulating cells).51
In vitro studies.
CID-mediated expansion of primary hemopoietic cells has been described in a few reports.34,42,45,52 After retroviral transduction, cells are cultured in the absence of added growth factors and in either the presence or absence of a CID. Only a few receptors have been evaluated by using this approach, with the most extensive studies involving the thrombopoietin receptor mpl. Murine bone marrow cells transduced by using a vector encoding a FKBP12-Mpl fusion failed to grow in the absence of drug or added growth factors, with all cells dying in 7 to 14 days. In contrast, FK1012 dramatically stimulated cell proliferation for up to nearly a year of culture, with an initial brief phase of multilineage differentiation followed by a longer phase of preferential megakaryocytic differentiation.45 Although mpl is capable of inducing expansion of multipotential cells, including day-12 colony-forming units–spleen, mpl-expanded cells are incapable of long-term repopulation and show no potential for B or T lymphoid differentiation (unpublished data). These findings are consistent with an ability of mpl to induce self-renewal of multipotential hemopoietic progenitor cells, which may be the functional equivalents of certain common myeloid progenitors.53 Studies using the estrogen system found that CID-dependent dimerization of a G-CSF receptor–EBD fusion supports the terminal differentiation of committed murine progenitor cells.42
CID-dependent expansion of primary cells has also been extended to CD34-selected human cord blood cells.52 After transduction, cells cultured in the presence of AP1903, a bumped CID, expanded 13.8- to 186-fold relative to cells cultured in the absence of AP1903, and the cell type that emerged in suspension culture was erythroid. Contrary to results in the murine system, cell expansion was transient. Possible reasons for the differences between the murine and human systems are currently under investigation.
In vivo studies.
Use of the F36V-Mpl fusion for in vivo selection of transduced murine hemopoietic cells has been reported.34 After transplantation of F36V-Mpl–transduced marrow cells into lethally irradiated mice, administration of another bumped CID, AP20187, produced a dramatic in vivo expansion of transduced red cells, platelets, and to a lesser extent, granulocytes. Although the nature of the in vivo expanded cell (committed progenitor or transplantable stem cell) was not conclusively determined, stem cells containing the transgene were not exhausted by CID treatment, as indicated by their persistence in recipients of secondary transplants. Future work must determine the ideal stage of hemopoiesis at which selection should be applied.
Clinical applicability of CID-mediated in vivo cell expansion will ultimately depend on achieving similar results in a large animal. In addition to demonstrating efficacy, it will be particularly important to show that CID-induced proliferation in this model remains strictly drug dependent in vivo. It is noteworthy that a single amino acid substitution in growth factor receptors,54 as well as in FKBP-containing fusion proteins, can dramatically influence the propensity for constitutive dimerization.55 Because a myeloproliferative syndrome or even frank leukemia might result from constitutive activation of receptor sequences, the behavior of the growth switch must be rigorously tested in mice and large animals before a clinical trial can be contemplated. Progress has been made in the development of an expression cassette for the β-globin gene.56 Thalassemia may be an especially attractive target for this approach because, as noted above, CID-mediated activation of the mpl signaling domain induces a preferential in vivo expansion of transduced erythroid cells. In view of the relatively few therapeutic options available to many patients with end-stage thalassemia, a clinical trial in this patient group may eventually be warranted.
Applications outside the hemopoietic system
The low efficiency of gene transfer is also an important obstacle to successful gene therapy for diseases originating outside the hemopoietic system. For example, CID-mediated expansion of transduced cells might be applied to gene therapy for liver diseases. The feasibility of using CIDs for liver cell expansion has been demonstrated both in vitro and in vivo57 (A. Lieber, personal communication, January 2001). Other investigations attempted to use CIDs to expand transduced muscle cells. In one study, a murine myoblast cell line that is normally dependent on fibroblast growth factor 1 (FGF1) was converted to AP20187 dependency on expression of an F36V-FGF1 receptor fusion (C. Murry and M. Whitney, personal communication, February 2001).
Pharmacologic regulation of cell death
Complementing the use of CIDs to induce growth of genetically modified cells are situations in which CIDs might be useful for conditionally eliminating genetically modified cells. Transfer of a death switch or suicide gene may have applications in the treatment of malignant and other acquired diseases. The most notable application is in adoptive immunotherapy for relapse after allogeneic marrow transplantation. For example, relapse after allogeneic transplantation for chronic myelogenous leukemia (CML) can be treated by adoptive transfer of donor lymphocytes, in which the graft-versus-leukemia effect produces complete remissions in 60% to 70% of patients.58 However, the development of life-threatening GVHD is a major impediment to this approach. Introduction of a suicide gene into donor lymphocytes provides a potential method for retaining specific control over survival of those lymphocytes after adoptive transfer.59 This strategy was shown to be feasible in a clinical trial using a gene encoding herpes simplex virus thymidine kinase (HSV-TK).60 HSV-TK converts the antiviral drug ganciclovir into a toxic metabolite, thereby eliminating the transduced cell population while sparing nontransduced cells. A major limitation to using HSV-TK clinically is that the viral protein induces immunologic responses, as was observed in a clinical trial.17 An additional limitation to use of HSV-TK in patients who have undergone marrow transplantation is that a need for ganciclovir therapy may arise because of cytomegalovirus infection and the HSV-TK–transduced T cells would then become victims of collateral damage.
In addition to applications in adoptive immunotherapy, suicide-gene therapy might be useful in treating disorders in which a locally confined cell mass must be reduced—for example, benign prostate hyperplasia.61 Malignant cells are an obvious target for the suicide-gene approach, and many investigators have experimentally assessed this strategy for cancer gene therapy.62 This method faces serious difficulty, however, in that complete and selective transduction of all tumor cells in vivo currently appears to be impossible, notwithstanding the “bystander effect” that has been claimed for some suicide systems.
The suicide genes described above make S-phase–dependent drugs toxic and thus target only cycling cells. FKBP12-based death switches that theoretically should be independent of the S phase have been developed.61,63-65 Unlike suicide systems that rely on expression of viral proteins, CID-based death switches are entirely human in origin and therefore have a lower probability of provoking immunogenicity. The fas receptor belongs to the tumor necrosis factor receptor family and is believed to signal on trimerization. Likewise, intracellular fas-receptor sequences fused to 2 tandem-oriented dimerizer domains63,65 can mediate controlled apoptosis in cell lines and transduced human T cells on CID-mediated multimerization. Of note, a single FKBP domain allows only dimerization and therefore cannot induce efficient cell killing. One disadvantage of a fas-based system may be the highly variable susceptibility of different cell types to fas-mediated apoptosis.61AP1903-induced multimerization of caspases 1, 3, 8, and 9, as well as Fadd sequences, also yields apoptosis switches, and the efficacy of these apoptotic proteins appears to be less dependent on cell type.61 65
Phase I and phase II clinical trials are currently in preparation at the Fred Hutchinson Cancer Research Center in Seattle and in Milan, Italy, to evaluate a FKBP-fas fusion suicide gene in the setting of adoptive immunotherapy. The fusion is labeled with an extracellular low-affinity nerve growth factor receptor (NGFR) tag to allow transduced cells to be selected by using an anti-NGFR antibody. In vitro, this death switch can induce apoptosis of a considerable proportion of transduced lymphocytes after only an hour of exposure to AP1903.67 Patients who have relapse after allogeneic bone marrow transplantation for CML will receive donor leukocyte infusions with genetically altered lymphocytes containing the novel death switch. Administration of AP1903 in this situation is expected to eliminate the modified lymphocytes in vivo and thus serve as a specific treatment for severe GVHD.
Future prospects
Studies using murine bone marrow have suggested that different receptor sequences produce different outcomes (unpublished data). A question that must be resolved is whether desired cell types can be specifically elicited by introducing the appropriate signal. For instance, mpl sequences allow preferential expansion of erythroid cells and, to a lesser extent, megakaryocytes. Which signaling domains would allow controlled expansion of myeloid cells? Is it possible to equip hemopoietic or even more primitive stem cells with a receptor or other signaling molecule that preferentially induces their in vivo differentiation toward a particular cell type or hemopoietic lineage?
Because of the plasticity of stem cells derived from bone marrow, delivering the right signal at the right time to these cells may enhance their contribution to tissues such as liver10 and muscle.8,9 Delivery of the signal in vivo might allow subsequent local expansion of these cells to a therapeutically relevant proportion. In addition to liver and muscle cells, the system could be used for other nonhemopoietic cell types, such as dopaminergic cells, for treatment of Parkinson disease; or β islet cells, for the treatment of diabetes mellitus—diseases for which a shortage of available donors currently creates major limitations to cell therapy.5,6 Finally, with the development of human embryonic stem cells, it may eventually be possible to engineer virtually every tissue to replace failing organs. Many of these strategies face important technical obstacles and raise ethical and regulatory questions beyond the scope of this review. However, the feasibility of pharmacologic regulation of genetically modified cell populations in vivo has been demonstrated.34 The stage is now set for a wide range of potential applications.
We thank T. Papayannopoulou and J. Abkowitz for helpful comments and suggestions on the manuscript.
Supported by grants 5R01DK52997, 1R01DK57525, 2P01HL53750, and 2P01DK47754 from the National Institutes of Health, an American Society of Hematology Junior Faculty Scholar Award, and an award from the Fanconi Anemia Research Fund.
©2001 by The American Society of Hematology
References
Author notes
C. Anthony Blau, Mailstop 357710, Health Sciences Building, University of Washington, Seattle, WA 98195; e-mail:tblau@u.washington.edu.