Abstract
This study investigated the influence of expression of proteins of the INK4 family, particularly p16, on the growth and self-renewal kinetics of hematopoietic cells. First, retrovirus-mediated gene transfer (RMGT) was used to restore p16INK4aexpression in the p16INK4a-deficient lymphoid and myeloid cell lines BV173 and K562, and it was confirmed that this inhibited their growth. Second, to sequester p16INK4a and related INK4 proteins, cyclin-dependent kinase 4 (CDK4) was retrovirally transduced into normal human CD34+ bone marrow cells and then cultured in myeloid colony-forming cell (CFC) assays. The growth of CDK4-transduced colonies was more rapid; the cell-doubling time was reduced; and, upon replating, the colonies produced greater yields of secondary colonies than mock-untransduced controls. Third, colony formation was compared by marrow cells fromp16INK4a−/− mice and wild-type mice. The results from p16INK4a−/−marrow were similar to those from CDK4-transduced human CFCs, in terms of growth rate and replating ability, and were partially reversed by RMGT ofp16INK4a. Lines of immature granulocytic cells were raised from 15 individual colonies grown from the marrow ofp16INK4a−/−mice. These had a high colony-forming ability (15%) and replating efficiency (96.7%). The p16INK4a−/−cell lines readily became growth factor–independent upon cytokine deprivation. Taken together, these results demonstrate that loss of INK4 proteins, in particular p16INK4a, increases the growth rate of myeloid colonies in vitro and, more importantly, confers an increased ability for clonal expansion on hematopoietic progenitor cells.
Introduction
Deletion of the tumor-suppressor genep16INK4a has been implicated in tumorigenesis in general1 and in leukemogenesis in particular.2-5 It has been found in 25% to 60% of cases of acute leukemia and 20% to 50% of cases of lymphoma.6Although deletion of the p16INK4a gene is uncommon in acute myeloid leukemia, messenger RNA expression is frequently undetectable, possibly as a result of DNA hypermethylation or mutations in the p16INK4a promotor region.7 These observations raise the possibility that p16INK4a expression plays an important role in the regulation of normal hematopoiesis. There is ample evidence that restoration of p16INK4a into p16INK4a-deficient leukemic cell lines reduces their proliferation rate in liquid culture and some evidence that it suppresses their ability to form colonies in semisolid culture,8,9 but the effects of p16INK4a expression on primary normal hematopoietic cell proliferation have not been examined. In particular, it is not known whether p16INK4a has any effects on the kinetics of progenitor cell renewal and differentiation. This is important because transformation of leukemic target cells must be associated with an increase in self-renewal probability above the steady-state value of 0.5 before the leukemic clone can expand, become established, and eventually predominate over normal hematopoiesis.10-12
The p16INK4a protein is the prototypic member of the INK4 family (p15INK4b, p16INK4a, p18INK4c, and p19INK4d) of cyclin-dependent kinase inhibitors (CKIs) and is part of a regulatory pathway consisting of p16INK4a, cyclin D, cyclin-dependent kinase (CDK) 4/6, retinoblastoma protein (pRB), and E2F.13 This pathway (the pRB pathway) regulates the transition from G1 to S phase of the cell cycle. The formation of complexes between cyclin D and CDK4 is inhibited by p16INK4a through binding to CDK4/6. The cyclin D–CDK4/6 complex phosphorylates pRB, resulting in the release of transcription factors so that the genes necessary for cell cycle progression can be transcribed.14
The pRB family of pocket proteins consists of pRB itself and the structurally related proteins p107 and p130. They are negative regulators of cell cycle progression, and overexpression of individual pocket proteins can cause growth arrest in G1 phase of the cell cycle. The cyclin D/CDK4 or 6 kinase complexes have been shown to be able to phosphorylate all 3 pocket proteins and overcome the cell cycle arrest imposed by the enforced expression of individual pocket proteins. The ability of the pRB family of proteins to induce cell cycle arrest is largely related to their interactions with the transcription factor E2F.14-17 Phosphorylation plays an important part in controlling the interaction of pRB-related pocket proteins with E2F as well as their ability to repress transcription directly. Hypophosphorylated forms of pocket proteins bind to E2F and repress E2F-dependent trancriptional activity, thus repressing the transcription of genes essential for DNA synthesis and/or cell cycle progression, including E2F-1, p107, pRB, cyclin A, and cyclin E.14-17 Therefore, overexpression of p16INK4a can down-regulate CDK4/6 activity, culminating in hypophosphorylation of pRB-related proteins, repression of E2F activity, and cell cycle arrest.
We have investigated the effect of p16INK4a on cell proliferation and renewal by transducing p16INK4a into the p16INK4a-deficient lymphoid and myeloid leukemia cell lines BV173 and K562. We have also studied the role of p16INK4ain normal hematopoiesis using CDK4-transduced normal human bone marrow CD34+ cells and bone marrow fromp16INK4a knockout mice. The results are consistent with the action of INK4 proteins as growth suppressors in normal hematopoiesis since INK4 deletion and abrogation increase the growth rate of hematopoietic cell colonies and the multiplication of hematopoietic progenitor cells in vitro, and facilitate the emergence of cell lines from primary hematopoietic progenitor cells.
Materials and methods
Cell lines and cell culture
The human lymphoid and myeloid cell lines BV173 and K562 have been confirmed to have theirp16INK4a genes deleted.18-20 These cells were maintained in RPMI 1640 medium (Gibco BRL, Paisley, United Kingdom) supplemented with 10% fetal calf serum (FCS) (Mycoplex) (PAA Laboratories, Linz, Austria), 2000 μM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated in flasks at 37°C in humidified 5% CO2 in air and passaged twice a week. The retroviral packaging cell lines GP+E-86 (ecotropic) and GP+envAM12 (amphotropic) were maintained in Dulbecco modified Eagle medium (Gibco BRL) supplemented with 10% FCS, 2000 μM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin, incubated in flasks at 37°C in humidified 5% CO2 in air and passaged twice a week. The WEHI-3B cell line was maintained in RPMI 1640 with 10% FCS.
Primary cell isolation and culture
Normal human bone marrow was obtained from donors of marrow for transplantation. Informed consent and Hammersmith Hospital Research Ethics Committee approval were obtained in all cases. Mononuclear cells were separated from the marrow by density gradient centrifugation by means of Ficoll Hypaque (Lymphoprep, Nycomed, Oslo, Norway). CD34+ cells were then separated from the mononuclear fraction by means of the Minimacs system (Miltenyi Biotech, Samberley, United Kingdom) according to the manufacturer's recommendations.
Wild-type mice and mice with homozygously deletedp16INK4a gene exon 2 were maintained in the Imperial College animal facility.21 At 6 weeks of age, mice were killed by cervical dislocation; the femurs were dissected out; and the marrow was flushed from the femoral cavity with 5 mL MEM alpha medium (Gibco BRL) into a sterile container.
Retrovirus-mediated gene transfer
Wild-type p16INK4a complementary DNA (cDNA) was cloned into a retroviral shuttle vector, pBN, and transfected into GP+E-86 by calcium phosphate precipitation. Supernatant from the GP+E-86 was used to infect GP+envAM12 with retroviral particles. Wild-type CDK4 cDNA22 was cloned into pBN and then transfected into GP+E-86 by calcium phosphate precipitation, and the supernatant was used to infect GP+envAM12.23
A Transwell system (Costar, High Wycombe, United Kingdom) was used for retrovirus-mediated transfer into K562 cells and primary cells.23 This system allows target cells to be incubated with producer cells without cell-to-cell contact and contamination. Amphotropic producer cells were plated in 6-well plates (Costar) at 1 day before the target cells were placed in the Transwell insert. K562 target cells (1 × 105 cells per Transwell) were suspended in medium containing a final concentration of 4 μg/mL polybrene, exposed to the producer cells for two 3-day cycles of infection, and then selected in G418-containing medium for 2 weeks prior to further study. Human CD34+ target cells were placed in the Transwell insert in medium containing 50 ng/mL interleukin 3 (IL-3), 100 ng/mL stem cell factor, 10 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (all from First Link, Brierley Hill, United Kingdom), and 4 μg/mL protamine sulfate. The CD34+ cells were harvested from the Transwell inserts after 2-day exposure to the producer cells. The selection of the CD34+ cells is described under “Colony assays and evaluation” below.
Growth rate of BV173 and K562 cells
BV173 and K562 cells were cultured at an initial concentration of 2 × 104 cells per milliliter. The p16INK4a-transduced and empty vector–transduced cells, but not the parent cells, were cultured in the presence of G418. Cell concentrations were measured by hemocytometry at daily intervals.
Western blotting
After 107 cells were pelleted by centrifugation in Eppendorf tubes, the supernatant was removed, and the cells were lysed in 120 μL RIPA buffer (1% NP40, 0.5% sodium deoxycholate, 0.3M NaCl, and Complete protease inhibitor cocktail [Boehringer Mannheim, Germany]). The protein concentration of the lysate was determined by means of a MicroProtein Determination kit (Sigma, Poole, United Kingdom). We electrophoresed 80 μg protein in 5%, 10%, and 15% (vol/vol) polyacrylamide gels (29:1 acrylamide to bisacrylamide) (Bio-Rad Laboratories, Hemel Hempstead, United Kingdom) and then blotted it onto a nitrocellulose membrane (Hybond-N) (Amersham, Little Chalfont, United Kingdom). The membrane was stained with antibodies to p16INK4a (Becton Dickinson, United Kingdom), pRB, E2F-1, p107, p130, p15, and p18 (Santa Cruz Biotechnology, CA), and the protein signals were visualized by electrochemiluminescence (ECL kit) (Amersham).
Colony assays and evaluation
Colony assay
Myeloid (granulocyte-macrophage) colony formation by primary cells was assayed by plating 1 × 105 mouse bone marrow cells per milliliter, or 1 × 103 CD34+ human cells per milliliter in methylcellulose (Methocult H423, Metachem Diagnostics, Northampton, United Kingdom). The murine cells were stimulated by 20% (vol/vol) WEHI-3B cell–conditioned medium, and the human cells were stimulated by recombinant cytokines (50 ng/mL stem cell factor, 1 ng/mL GM-CSF, 10 ng/mL IL-3, and 100 ng/mL granulocyte CSF, all from First Link). In the case of retrovirally transduced cells, 1.5 mg/mL G418 was added. This concentration reduces the survival of cells that do not express neomycin resistance (neor) to 1.55%.23 Triplicate cultures in 35-mm petri dishes were incubated at 37°C in humidified 5% CO2 in air.
Transgene expression
Following the transduction procedure, the number of colonies that grew in the presence of G418 was half the number that grew in the absence of G418. This implies a transduction efficiency of approximately 50%. Nested polymerase chain reaction (PCR) was used to confirm the presence of neor in colonies grown for 14 days in the presence of G418. Individual colonies were plucked, and PCR was performed by means of specifically designed primers (first-step primers: CAAGCGAAACATCGCA-TCGAGCGA and GAAGAACTCGTCAAGAAGGCGATA; second-step primers: ATGGAAGCCGGTCTTGTCGAT and GATACCGTAAAGCACGAGGAA). Of 125 colonies analyzed by PCR, 95% expressed neor.
Video recording to measure colony growth rate
Colony-culture dishes were removed from the incubator at intervals of 1 to 2 days and placed on a grid that was designed so that the dish could be located in precisely the same position and orientation on each occasion. Whole-culture plates were filmed by moving the cultures past a video camera mounted on an inverted microscope and attached to a domestic video recorder. The entire plate was recorded, irrespective of whether a colony was present. The recordings were reviewed retrospectively, and the positions of the colonies and the numbers of cells they contained transferred to a paper replica of the grid.24
Colony replating for secondary colony formation
On the seventh day of culture, 120 colonies of more than 50 cells were individually plucked and replated into 100 μL methylcellulose plus serum and cytokines in the wells of flat-based 96-well microtiter plates. G418 was added to secondary cultures of colonies grown from retrovirally transduced cells that had been exposed to G418 during the primary culture. The numbers of colonies in each well were scored 7 days after replating, and the percentage of positive wells and the total number of secondary colonies were calculated. In addition, the data were plotted as the cumulative distribution of numbers of secondary colonies per primary colony, and the area under the curve (AUC) was calculated by means of the trapezium rule.25
The main reason for calculating the AUC is that the distribution of secondary colonies per primary colony is highly skewed so that median rather than mean values should be used. However, when fewer than 50% of the primary colonies form secondary colonies, the median will be zero and the result will be uninformative. Thus, the AUC is used to provide an overall measure of amplification of the colony-forming cells regardless of the replating efficiency of the particular population under investigation.26
Generation of cell lines from p16INK4a−/−mouse marrow
Fifty individual colonies plucked from the methylcellulose cultures of p16INK4a−/− mouse marrow and 50 from cultures of wild-type mouse marrow. Each colony was transferred to 100 μL of medium plus serum and WEHI-3B cell–conditioned medium in separate wells of a round-bottomed 96-well microtiter plate. Of thep16INK4a−/− colonies, 15 colonies (30%), but none of the wild-type colonies, expanded in the liquid culture and were individually transferred to 48-well plates, 24-well plates, and finally to 75-cm2 tissue-culture flasks. We recloned the 15 cell lines by plating them at 104 cells per milliliter in the granulocyte-macrophage colony-forming unit (CFU-GM) assay and then plucking 10 individual colonies from each for expansion. At random, 2 expanded colonies were selected from each cell line and maintained in medium with serum and WEHI-3B cell–conditioned medium. The remainder were discarded.
Growth-factor independence
We selected 4 of the cell lines at random and tested them for growth-factor independence either by immediately transferring them to medium lacking WEHI-3B cell–conditioned medium or by reducing the concentration stepwise (20%, 10%, 7.5%, 5%, 2.5%, 0%) at 4-day intervals. Trypan blue–excluding cells were counted at 2-day intervals with a hemocytometer.
Statistical analysis
Nonparametric statistical analysis (Mann-Whitney Utest) was performed with StatView SE+Graphics software for the Macintosh computer (Abacus Concepts, Berkeley, CA). AUCs were calculated with an Excel5 (Microsoft, Seattle, WA) spreadsheet on a Macintosh computer.
Results
Restoration of p16INK4a expression inhibits proliferation by BV173 and K562 cells
The CKI p16INK4a was reintroduced into the p16INK4a-deficient BV173 and K562 cell lines by infection with p16INK4a -expressing retrovirus. Following the 2-week selection period, transduced cells were maintained in selection medium, and the parent cell lines were cultured without selection. BV173 and K562 cells transduced with the empty vector proliferated with the same kinetics as the respective parent cell lines. Successful transduction of the empty vector into BV173 cells and K562 cells was confirmed by RT-PCR for the Neorgene, which was positive in all cases. Neorexpression was not detected in untransduced K562 and BV173 cells. These results show that the transduction procedure per se had no effect on cell proliferation.
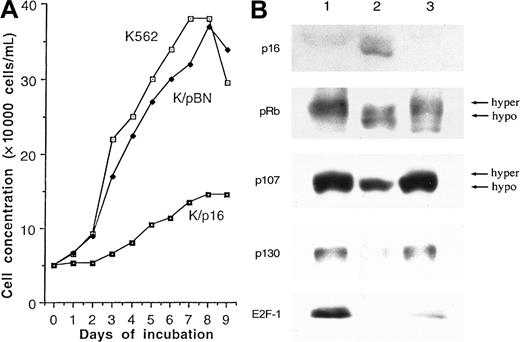
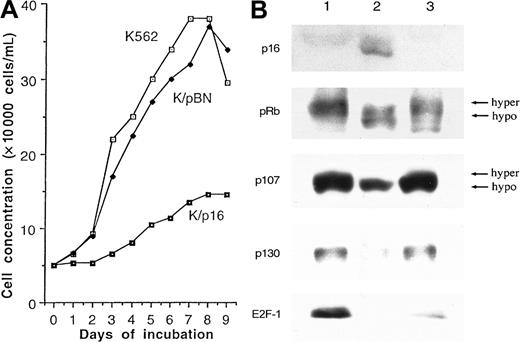
BV173 cells transduced with p16INK4a failed to grow at all in any of 4 separate experiments (data not shown). Similarly, in 3 of 4 experiments on K562 cells, p16INK4a-expressing cells failed to grow. In the fourth experiment, the p16INK4a-transduced cells exhibited a gradual increase in number but showed a considerably reduced growth rate compared with the parent cells and empty vector–transduced controls (Figure 1A). These results confirmed that p16INK4a expression inhibits or prevents cell growth. This slow growth provided the opportunity to accumulate sufficient cells for further investigations. The expression of p16INK4a was confirmed by Western blotting after 16 passages after selection (Figure 1B).
K562 cells.
(A) Growth curves for p16INK4a-transduced (K/p16), empty vector–transduced (K/pBN), and untransduced K562 cells. The p16INK4a-transduced and empty vector–transduced cells, but not the parental cells, were cultured in the presence of G418. The results are from the 1 experiment (out of 4) in which the p16INK4a-transduced cells showed any significant growth. (B) Western blot analysis of untransduced K562 cells (lane 1), K562 cells transduced with p16 (lane 2), and cells transduced with empty vector (lane 3) after 16 passages. The results show expression of p16INK4a by the p16INK4a-transduced cells. Expression of p16INK4a (lane 2) results in loss of the hyperphosphorylated forms of pRB and p107 and in reductions in the expression levels of p107, p130, and E2F-1.
K562 cells.
(A) Growth curves for p16INK4a-transduced (K/p16), empty vector–transduced (K/pBN), and untransduced K562 cells. The p16INK4a-transduced and empty vector–transduced cells, but not the parental cells, were cultured in the presence of G418. The results are from the 1 experiment (out of 4) in which the p16INK4a-transduced cells showed any significant growth. (B) Western blot analysis of untransduced K562 cells (lane 1), K562 cells transduced with p16 (lane 2), and cells transduced with empty vector (lane 3) after 16 passages. The results show expression of p16INK4a by the p16INK4a-transduced cells. Expression of p16INK4a (lane 2) results in loss of the hyperphosphorylated forms of pRB and p107 and in reductions in the expression levels of p107, p130, and E2F-1.
Previous studies have demonstrated that p16INK4a requires a functional pRB pathway to induce cell cycle arrest.27-30Also, it has been shown that p16INK4a prevents CDK4/6 from forming complexes with cyclin D, thereby suppressing phosphorylation of the pRB family of proteins.31 32 To demonstrate that the p16INK4a/pRB pathway is intact in K562 cells and that it responds to expression of p16, we studied the expression of downstream targets of p16INK4a by Western blotting. The results (Figure 1B) show that p16INK4a expression in K562 cells reduced phosphorylation of pRB and p107 and reduced expression levels of the E2F-regulated genes E2F-1, p107, and p130. Although there is an unexplained decrease in expression of E2F-1 by the vector-only controls, there was a complete disappearance of E2F-1 from the p16-transduced cells. These findings are consistent with previous results showing that the growth-suppressive properties of p16INK4a are accomplished by maintaining the pRB-related pocket proteins in their hypophosphorylated states and repressing the transcription of E2F target genes.
Transduction of CDK4 into human CD34+ cells increases colony growth rate and replating ability
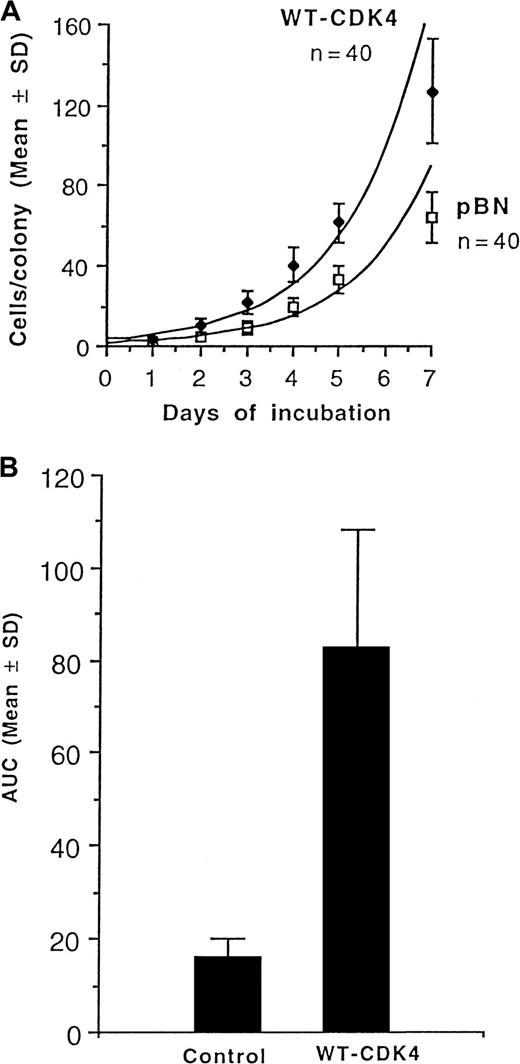
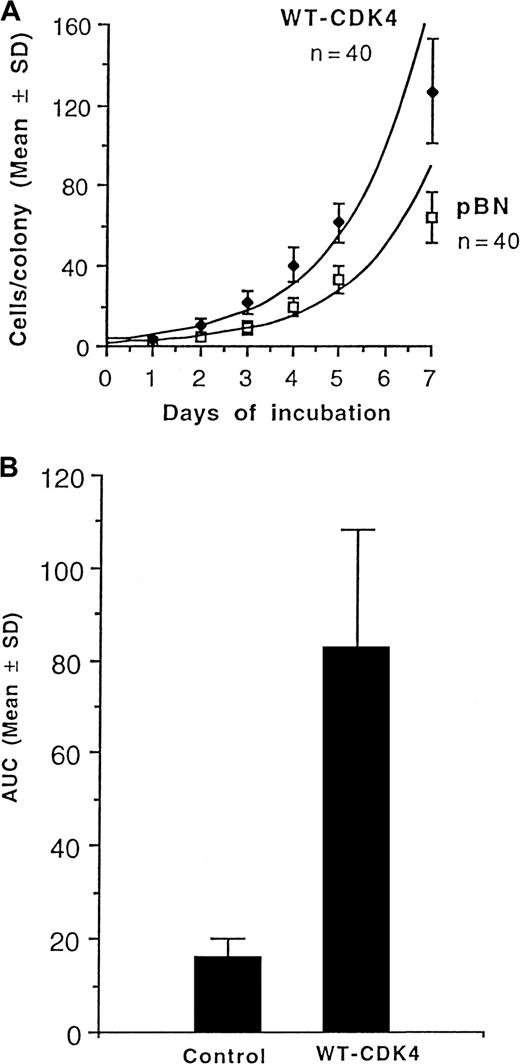
Figure 2A shows that expression of CDK4 in human CD34+ cells increases the growth rate of colonies in clonogenic assays. Moreover, their ability to form secondary colonies upon replating was also significantly enhanced (Figure 2B, Table 1) in terms of the percentage of colonies forming secondary colonies upon replating (2.4-fold), the total number of secondary colonies (8.2-fold), and the AUC (5.2-fold).
Growth and replication of CFU-GM.
(A) Growth rates of CFU-GM colonies grown from normal human bone marrow CD34+ cells transduced with wild-type CDK4 (WT-CDK4) or empty vector (pBN). n = number of colonies analyzed in each group. (B) Replicative capacity of CFU-GM, as reflected by the AUC, grown from WT-CDK4–transduced or control-transduced CD34+ normal human marrow cells. Samples from 3 donors were analyzed.
Growth and replication of CFU-GM.
(A) Growth rates of CFU-GM colonies grown from normal human bone marrow CD34+ cells transduced with wild-type CDK4 (WT-CDK4) or empty vector (pBN). n = number of colonies analyzed in each group. (B) Replicative capacity of CFU-GM, as reflected by the AUC, grown from WT-CDK4–transduced or control-transduced CD34+ normal human marrow cells. Samples from 3 donors were analyzed.
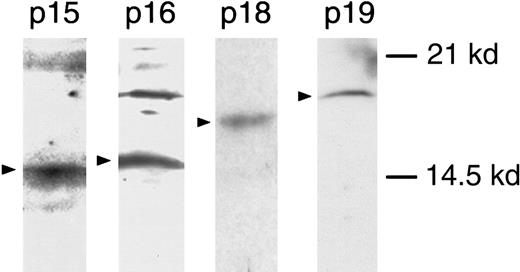
These results indicate that expression of p16INK4amay exert growth-suppressive effects on normal human myeloid progenitor cell growth. However, CDK4 sequesters p15INK4b, p18INK4c, and p19INK4d, as well as p16INK4a, all of which can potentially function as tumor-suppressor genes. The Western blot analysis of CD34+cell lysates in Figure 3 shows expression of p15INK4b, p18INK4c, and p19INK4d, as well as p16INK4a. Thus, although the effects of CDK4 transduction can be attributed to sequestration of INK4-family proteins, they cannot be specifically attributed to the abrogation of p16INK4a.
Western blot analysis of cell lysates.
Western blot analysis of INK4 proteins in CD34+ cell lysates using antibodies against p15, p16, p18, and p19.
Western blot analysis of cell lysates.
Western blot analysis of INK4 proteins in CD34+ cell lysates using antibodies against p15, p16, p18, and p19.
Deletion of p16INK4a increases myeloid progenitor cell proliferation in gene knockout mice
There was an obvious increase in colony growth rate and size in cultures of p16INK4a knockout marrow compared with cultures of wild-type marrow. The data in Figure4A show that the numbers of cells per colony increase more rapidly in cultures ofp16INK4a knockout marrow compared with wild-type cultures and indicate a reduction in cell-doubling time from 15 hours to 12 hours (P = .003). Cytospin preparations of 50 p16INK4a knockout marrow colonies and 50 wild-type marrow colonies were examined to evaluate cell morphology. All colonies consisted of cells of the myeloid and/or monocyte lineage. There were no major differences in the lineage representation or stage of cell differentiation in colonies grown from p16INK4a knockout marrow compared with wild-type colonies.
Growth and replication of CFU-GM.
(A) Growth rates of CFU-GM colonies grown from marrow ofp16INK4a knockout (−/−) and intact (+/+) mice. n = number of colonies analyzed in each group. (B) Replicative capacity of CFU-GM, as reflected by the AUC, grown from marrow ofp16INK4a knockout (−/−) and intact (+/+) mice. n = 3 for each group.
Growth and replication of CFU-GM.
(A) Growth rates of CFU-GM colonies grown from marrow ofp16INK4a knockout (−/−) and intact (+/+) mice. n = number of colonies analyzed in each group. (B) Replicative capacity of CFU-GM, as reflected by the AUC, grown from marrow ofp16INK4a knockout (−/−) and intact (+/+) mice. n = 3 for each group.
The results of replating the colonies into secondary cultures showed that the enhanced colony growth rate was associated with increased multiplication of clonogenic cells within the developing colonies (Table 1, Figure 4B). Deletion of p16INK4aresulted in significant increases in the percentage of colonies that formed secondary colonies upon replating (1.6-fold), in the total number of secondary colonies (5.4-fold), and in the magnitude of the AUC (3.5-fold). Retrovirus-mediated gene transfer (RMGT)–mediated resoration of p16INK4a into bone marrow cells of 2 of p16INK4a−/− mice resulted in 45% and 46% reductions in the magnitude of the AUC. This shows thatp16INK4a gene deletion played a significant part in the increased AUC exhibited by p16INK4a−/−bone marrow cells.
P16INK4agene deletion facilitates cell line propagation in vitro
None of the wild-type colonies expanded sufficiently to produce cell lines, and all cells were dead within 2 weeks of plucking the colonies. After recloning, the 15p16INK4a−/− cell lines showed a higher colony-forming efficiency than thep16INK4a−/− primary bone marrow cells. Almost all of the colonies (96.7%) produced secondary colonies upon replating, and there was also an increase in the level of the AUC (Table 1).
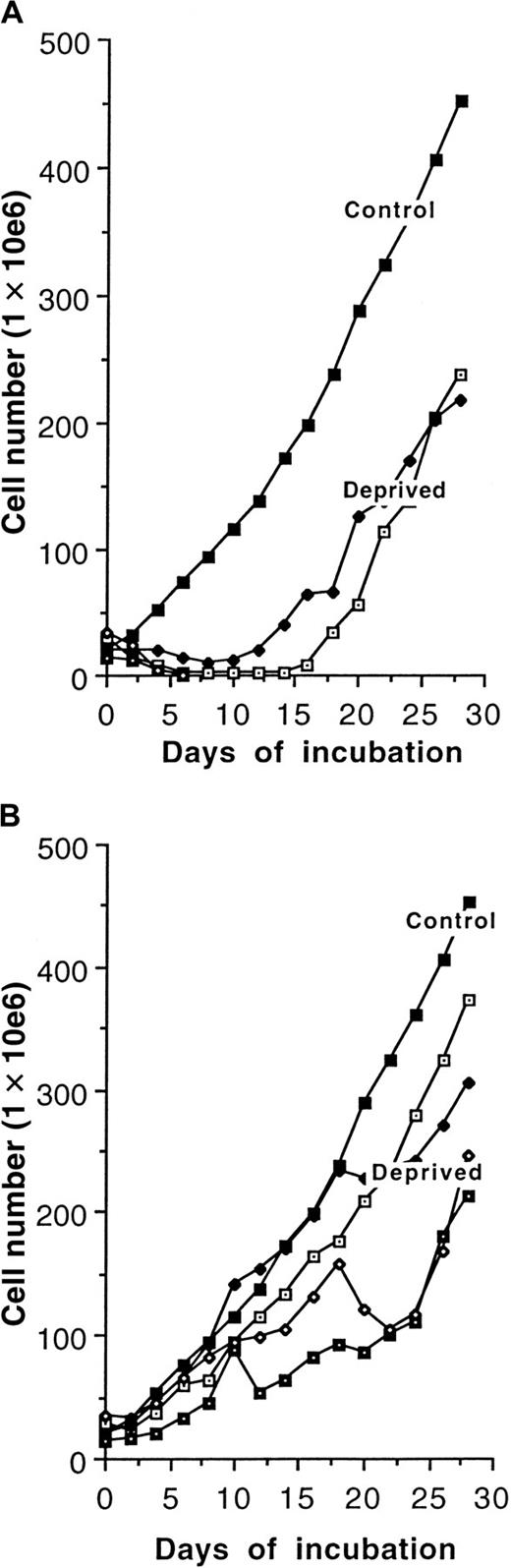
Figure 5A-B shows the results of immediate and stepwise growth-factor deprivation. When growth factor was immediately withdrawn from 4 cell lines, 2 of them died out rapidly, but the other 2 survived for 2 weeks and then grew at the same rate as the controls in WEHI-3B cell–conditioned medium (Figure 5A). When growth factor was withdrawn from the same cell lines over a period of 2 weeks, all of the cell lines survived and were able to grow without WEHI-3B cell–conditioned medium at the same rate as the controls (Figure 5B).
Growth factor independence.
(A) Effects of immediate total WEHI-3B CM withdrawal from 4 cell lines derived from p16INK4a−/− mouse bone marrow. (B) Effects of stepwise WEHI-3B CM withdrawal from 4 cell lines derived from p16INK4a−/− mouse bone marrow.
Growth factor independence.
(A) Effects of immediate total WEHI-3B CM withdrawal from 4 cell lines derived from p16INK4a−/− mouse bone marrow. (B) Effects of stepwise WEHI-3B CM withdrawal from 4 cell lines derived from p16INK4a−/− mouse bone marrow.
Discussion
We have used 3 in vitro model systems to investigate the influence of p16INK4a on hematopoietic progenitor cell proliferation. There were consistent increases in the growth rates and sizes of colonies grown from p16INK4a knockout mouse bone marrow and from CDK4-transduced normal human CD34+ cells. These observations are in line with the reduced growth rate of K562 cells in which p16INK4a expression had been restored. The assays of colonies for their content of progenitor cells, by replating them into secondary cultures, revealed that colonies of normal cells from which p16INK4a/INK4 proteins had been deleted or abrogated contained increased numbers of clonogenic cells and that this increase was counteracted by RMGT ofp16INK4a intop16INK4a−/− mouse bone marrow cells.
We found that, in most cases, restoration of p16INK4aexpression in p16INK4-deleted lymphoid (BV173) and myeloid (K562) cell lines completely repress cell proliferation, indicating that p16INK4a has a role in controlling proliferation in these hematopoietic cells. Interestingly, we found in one experiment that expression of p16INK4a in K562 cells did not completely suppress cell growth in spite of the fact that most if not all of the cells stained with anti–p16 fluorescein isothiocyanate (data not shown). This result indicates that the effects of p16INK4a may vary, possibly according to the levels of p16INK4a protein in the cells.
It has been reported that p16INK4a can induce cell cycle arrest only in cells with an intact and functional pRB pathway.27-30 Consequently, it was important to confirm that changes in the expression of p16INK4a can induce hypophosphorylation of pRB-related pocket proteins and repression of E2F activity. The p16INK4a-transduced K562 cells had increased levels of the hypophosphorylated forms of pRB and p107 compared with untransduced cells and cells transduced with empty expression vector. This observation is consistent with previous reports showing that p16INK4a represses the activity of cyclin D–dependent kinases, which are responsible for the phosphorylation of all 3 of these pocket proteins.33-36 We also found that restoration of p16INK4a was associated with down-regulation of pRB, p107, and a component of the E2F transcription factor family (E2F-1). This is attributable to the fact that pRB, p107, and E2F-1 are E2F-regulated genes33-35 and are repressed by the accumulation of dephosphorylated forms of the pocket proteins. Overall, these results imply that the pRB pathway in K562 cells is intact and that it responds to overexpression of p16INK4a. However, these cell line models cannot be used to evaluate changes in progenitor cell renewal and differentiation and do not provide information about the function of p16INK4a in untransformed primary cells. To characterize further the functional role of p16INK4a or related INK4 family proteins, we investigated the effects of sequestering p16INK4a/INK4-family proteins in primary human CD34+ cells.
In the primary human CFU-GM model, we used CDK4 to sequester p16INK4a and mimic the inactivation of p16INK4a.30 This interaction between CDK4 and p16INK4a occurs pathologically in glioma.22CDK4 amplification has been shown to be an alternative top16INK4a homozygous gene deletion in glioma cell lines and may be experimentally mimicked in vitro by retrovirus-mediated transduction of CDK4.30 However, overexpression of CDK4 eliminates all INK4 family members, and p15INK4b, p18INK4c, and p16INK4dcan potentially function as tumor-suppressor genes. As confirmation in other studies,37-39 we found that normal human CD34+ cells express p15INK4b, p18INK4c, and p19INK4d, in addition to p16INK4a. Thus, on this evidence alone, we cannot attribute the effects found in primary hematopoietic progenitor cells specifically to abrogation of p16INK4a. To investigate the role of p16INK4a itself, we also set up CFU-GM assays from the bone marrow of p16INK4a−/− mice. Consistent with the data from human CD34+ cells, we found that CFU-GM from p16INK4a−/− mice have a greatly increased growth rate and replating ability. These results from the knockout-mouse model cannot be explained by deletion ofp15INK4b, p18INK4c, orp19INK4d since these genes remain intact in the animals. Consequently, it is likely that p16INK4a has a similar role in human and murine hematopoietic progenitor cells.
The INK4a locus encodes 2 potential tumor suppressors with different reading frames. P16INK4a is encoded by exons 1α, 2, and 3, and the alternative reading frame (ARF) consists of exons 1β, 2, and 3 and encodes p19ARF in mice and p14ARF in humans.40-43 It is therefore noteworthy that the p16INK4a−/− mice used in our studies have p16INK4a inactivated through deletion of p16INK4a exon 2,21 and it is thus possible that this deletion could also inactivate p19ARF. However, recent mutation studies have shown that only exon 1β is necessary for the activity of p19ARF, and it is conceivable that the cells from thep16INK4aexon2−/− mice produce a truncated protein that is still active. Indeed, cells from these mice have been shown to up-regulate p53 expression in response to oncogenic Ras, suggesting that the ARF activity is functional.44Deletion of p16INK4a will cause pRB to be hyperphosphorylated and thereby increase E2F-1 activity. Moreover, expression of CDK4 in normal human CFU-GM will cause pRB hyperphosphorylation, induction of E2F-1 activity, and an increase in transcription of the ARF gene because the ARFgene has been shown to be positively regulated by E2F-1. In this case, overexpression of ARF should have a negative effect on proliferation by inducing cell cycle arrest and apoptosis. However, transduction of human CFU-GM with CDK4 increased the growth rate and replating efficiency, consistent with a role for p16INK4a in progenitor cell proliferation. It is also relevant that the ARF proteins target p53 by binding to MDM2, rather than CDK4, and do not exert their effects through the Rb pathway,45 46 so that the results obtained in the human CFU-GM model cannot be explained by an action on ARF. Finally, the results of restoringp16INK4 expression to K562 cells, in which the human p14ARF is also deleted, and top16INK4a−/− mouse bone marrow cells show that p16INK4a can have important effects irrespective of the presence or absence of p14/19ARF.
Hematopoietic progenitor cells fromp16INK4a−/− mice were able to survive in the absence of stromal cell support for at least 6 months, suggesting that lack of p16INK4a function considerably increases the longevity of hematopoietic cell clones and may even render them immortal. The increased clonogenic activity and replating activity are consistent with the kinetic requirements of increased self-renewal for clonal expansion.10,11 The growth factor–deprivation experiments indicate that the majority of the cell lines initially were growth factor–dependent but that they readily became growth factor–independent upon withdrawal of WEHI-3B cell–conditioned medium. This result is consistent with the idea that cyclin D/CDK4–dependent kinase has a critical role in integrating mitogenic signals from growth factors with the cell cycle machinery.31,32 Furthermore, our finding is supported by a recent report that both p16INK4a and p19ARFhave roles in cellular immortalization and that they act in overlapping pathways.47 At this stage, we cannot rule out a role for secondary genetic abnormalities, possibly as a result of the increased cell proliferation and/or increased genetic instability in cells lacking the p16INK4a protein, in transformation to growth-factor independence. We also do not exclude the possibility that the tumor-suppressor gene ARF may have a role in immortalization of these cells.
Overall, our results strongly implicate p16INK4a in the regulation of hematopoietic-progenitor cell kinetics although yet-to-be-defined roles for other members of the INK4 family cannot be ruled out entirely. They suggest a role for the INK4/CDK4/pRB pathway in regulating the kinetics of progenitor cell renewal and differentiation, and highlight their importance for clonal survival.
We thank Dr M. Serrano (Centro Nacional de Biotecnologia, Madrid, Spain) for the generous donation of the p16INK4acDNA and the INK4a knockout mice, and Dr J. V. Melo for designing the PCR primers.
J.L.L. S.B.M., N.C.P.C., E.W.-F.L., J.G., and M.Y.G. are supported by the Leukaemia Research Fund of Great Britain; W.C. was the recipient of a PhD Studentship from the Siriraj Hospital, Government of Thailand; B.Z. was supported by the China Scholarship Council; and N.S.B.T. is supported by the Charles Wolfson Charitable Trust.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
M. Y. Gordon, LRF Centre for Adult Leukaemia, Imperial College School of Medicine, Hammersmith Campus, DuCane Rd, London W12 0NN, UK; e-mail: myrtle.gordon@ic.ac.uk.