We used postgrafting immunosuppression with mycophenolate mofetil (MMF) and cyclosporine (CSP) to control both graft-versus-host and host-versus-graft reactions in a canine model of dog leukocyte antigen (DLA)–identical littermate marrow transplants. This way, the single pretransplantation dose of total body irradiation (TBI) otherwise needed for sustained allografts could be lowered from 920 cGy to the sublethal level of 200 cGy.1 We next substituted 450 cGy irradiation targeted to the cervical, thoracic, and upper abdominal lymph node chain for 200 cGy TBI in this model.2Prompt and sustained engraftment was seen in unirradiated marrow and lymph nodes as early as week 6. This indicated that creation of marrow space by cytotoxic agents was not required for homing of transplanted stem cells. Rather, some degree of host immunosuppression was sufficient to set the stage for successful allografts. The concept was validated in a 30-year-old patient with common variable immunodeficiency disease who received no conditioning therapy and in whom durable allogeneic marrow engraftment was achieved solely with postgrafting MMF/CSP.3
The field of lymph node chain irradiation in these studies included the thymus. Reports by others on nonmyeloablative regimens for major histocompatibility complex (MHC)–matched and mismatched murine and porcine transplantations have emphasized the pivotal importance of thymus irradiation in the success of grafts.4 5 It is not clear whether the engraftment of the transplanted cells was facilitated through creation of thymic space or elimination of thymic alloreactivity by thymic irradiation. Here, we evaluated in the canine model whether the success of central lymph node chain radiation could largely be attributed to inclusion of the thymus in the radiation field. We asked whether sustained grafts of DLA-identical littermate marrow could be achieved using 450 cGy thymic irradiation before and MMF/CSP after transplantation.
Litters of beagles weighing from 7.5 to 11.4 kg (median, 9.0 kg) and 6 to 9 months old (median, 7 months) were used. Research was conducted per the principles outlined in the Guide for Laboratory Animal Facilities and Care (National Academy of Sciences, National Research Council). The Institutional Animal Care and Use Committee of the Fred Hutchinson Cancer Research Center approved the research protocol. Kennels are certified with the American Association for Accreditation of Laboratory Animal Care. A high-energy linear accelerator (Varian CLINAC 6, Palo Alto, CA) delivered 450 cGy thymic irradiation at 200 cGy/min in a single setting with a tightly collimated 6 million electron volt beam using 2 isocentric parallel-opposed anterior and posterior ports, 2.5 cm wide and 3.5 cm long, to include the thymus with margins. A radiograph verified the portal placement. Selection of DLA-matched pairs, transplants of 1.5 to 6.2 × 108 (median, 2.7 × 108) nucleated marrow cells/kg (day 0), posttransplantation care, administration and dose schedules of MMF and CSP, assessment of engraftment, and histopathologic examinations were done as previously described.1 2
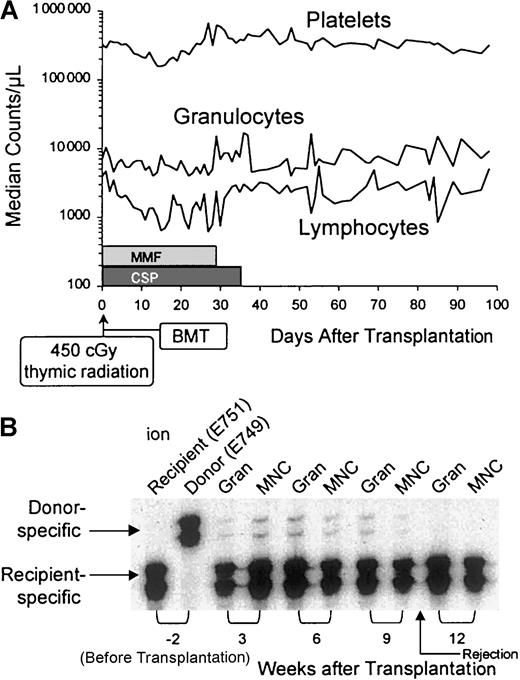
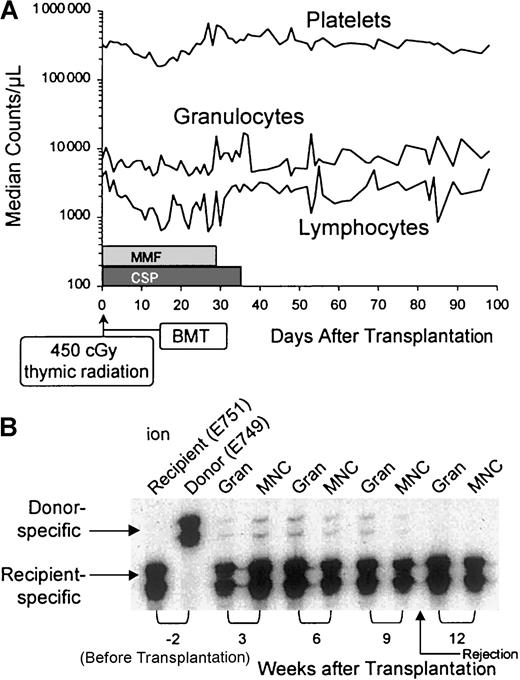
All 5 recipients showed initial evidence of mixed donor-host hematopoietic chimerism, with the donor contributions ranging from 1% to 5% (Table 1). Four dogs lost their grafts between 9 and 14 weeks after transplantation, and they survived with complete autologous recovery. One dog died on day 23 with systemic canine herpes virus infection, at a time when donor cell contribution in the marrow was 1%. We speculate that this dog would have rejected his graft over the next 6 to 10 weeks, similarly to the other 4 dogs in the study. The dogs' median platelet and granulocyte counts showed minimal transient changes after transplantation, whereas median lymphocyte counts transiently declined from 3500/μL to almost 600/μL between weeks 2 and 4, presumably due to the combined effects of thymic radiation and postgrafting MMF (Figure 1). Analysis of peripheral blood samples (dog E751) by microsatellite markers provides an example of the transient nature of donor engraftment.
Effects of thymic radiation and postgrafting MMF/CSP.
(A) Platelet, granulocyte, and lymphocyte changes in 5 dogs given 450 cGy thymus irradiation, DLA-identical littermate marrow grafts, and MMF/CSP after transplantation. (B) Results of microsatellite marker studies of donor and recipient (dog E751) cells before transplantation (lanes 1 and 2) and recipient cells after marrow transplantation (lanes 3-10). Gran indicates granulocytes; MNC, mononuclear cells.
Effects of thymic radiation and postgrafting MMF/CSP.
(A) Platelet, granulocyte, and lymphocyte changes in 5 dogs given 450 cGy thymus irradiation, DLA-identical littermate marrow grafts, and MMF/CSP after transplantation. (B) Results of microsatellite marker studies of donor and recipient (dog E751) cells before transplantation (lanes 1 and 2) and recipient cells after marrow transplantation (lanes 3-10). Gran indicates granulocytes; MNC, mononuclear cells.
We conclude that thymic irradiation in itself provided insufficient host immunosuppression for durable donor-cell engraftment. The duration of engraftment was comparable to that seen in dogs conditioned with 100 cGy of TBI.1 In comparison, mixed hematopoietic chimerism lasting at most 3 weeks was seen in dogs not given pretransplantation irradiation and only treated with postgrafting MMF/CSP.1 The current data do not support a pivotal role of thymus irradiation for durable allografts in this MHC-matched model. Rather, it seems that irradiation of a larger proportion of recipient lymphoid tissues is required, such as is afforded by the previously described irradiation of the lymph node chain.2
Supported in part by National Institutes of Health grants CA78902, CA15704, DK42716, and HL36444. R.S. also received support from the Laura Landro Salomon Endowment Fund and a prize from the Josef Steiner Krebsstiftung, Bern, Switzerland; M.T.L. received additional support from a Lady Tata Memorial Trust International research grant, London, United Kingdom.