Abstract
Strains of human immunodeficiency virus (HIV) transmitted between individuals use the CCR5 coreceptor, but no preferential depletion of particular Th-lymphocyte subpopulations has been reported during primary HIV infection (PHI). In contrast, gut-associated Th lymphocytes are preferentially depleted in macaques recently infected by simian immunodeficiency virus. The expression of CCR5 and the intestinal homing receptor integrin α4β7 on subpopulations of Th lymphocytes was studied in 12 patients with PHI. There was a profound decrease of circulating α4β7+ Th lymphocytes and CCR5+ memory Th lymphocytes with nonlymphoid homing potential (CD62L−CD45RO+). Unlike other Th lymphocytes, this cell population remained depleted despite early control of viral replication under antiretroviral treatment. Therefore, HIV preferentially targets a specific CCR5+ subpopulation of Th lymphocytes early during infection, inducing its persistent depletion despite treatment. Protective immunity in vivo depends on Th lymphocytes carrying homing capacity to nonlymphoid tissue, and therefore these data may explain the persistent abnormalities of immune functions in patients infected with HIV.
Introduction
Strains of human immunodeficiency virus (HIV) transmitted between individuals use the CCR5 coreceptor. However, the decline in the circulating T-helper (Th) lymphocyte count during primary HIV infection (PHI) involves CCR5− cells, but not CCR5+ cells.1 The rapid recovery of CCR5− Th lymphocyte counts under highly active antiretroviral treatment (HAART) and the limited depletion of Th lymphocytes in lymph nodes of patients with PHI suggest that sequestration of CCR5− Th lymphocytes to lymphoid organs makes a large contribution to their early loss from the blood.1-3 CCR5 is only expressed by memory Th lymphocytes.4 Memory Th lymphocytes can be divided into 2 subpopulations depending on their expression of the CD62L lymph node homing receptor: CD62L+ cells with the lymphoid tissue homing potential (LHP) and CD62L− cells with the nonlymphoid tissue homing potential (NLHP).5 Intestinal mucosa–associated Th lymphocytes belong to the NLHP subpopulation and express the intestinal homing receptor integrin α4β7. Most Th lymphocytes in the gut lamina propria also express CCR5.6 Because the gastrointestinal tract is the major site of viral replication and loss of Th lymphocytes during primary infection with simian immunodeficiency virus (SIV), HIV may similarly target CCR5+ NLHP Th lymphocytes during PHI.7-10 Therefore, analysis of this particular fraction of Th lymphocytes may better reflect HIV-induced depletion of Th lymphocytes than analysis of the entire CD4+ T lymphocyte population.
We show that the counts of circulating α4β7+ and CCR5+NLHP Th lymphocytes are substantially decreased during PHI. Moreover, the abnormally low counts of CCR5+ NLHP Th lymphocytes persist unchanged for 48 weeks despite HAART, indicating that their early loss is due to early depletion rather than redistribution.
Study design
Study patients
After informed consent, 12 patients with clinical symptoms of PHI were enrolled in the ANRS 086 Primoferon A study.11The following criteria were used for inclusion: anti-HIV antibodies detectable by enzyme-linked immunosorbent assay or detectable plasma HIV RNA, and 3 bands or fewer in a Western blot test for anti-HIV antibodies. Median plasma HIV RNA concentration at inclusion was 5.8 log10 copies/mL. Antiretroviral treatment was initiated 1 ± 0.5 day (mean ± SEM) after inclusion, combining stavudine (60-80 mg/d), didanosine (300-400 mg/d), and nelfinavir (2500 mg/d) until the end of the follow-up, and pegylated-interferon (IFN)–α2b (PEG-Intron, Schering-Plough, Research Institute, Kenilworth, NJ) 1 μg/kg per week for 14 consecutive weeks.
Immunofluorescent staining and FACS analysis
Peripheral blood mononuclear cells (PBMCs) were isolated at inclusion and after 5, 13, 24, and 48 weeks. They were stained with the following monoclonal antibodies: allophycocyanin (APC)-conjugated anti-CD4 (Becton Dickinson, San Jose, CA), fluorescein isothiocyanate (FITC)–conjugated anti-CCR5, and phycoerythrin (PE)-conjugated anti β7 (both from Pharmingen, San Diego, CA) and PE-conjugated anti-CD45RO, PC5-conjugated anti-CD62L, FITC-conjugated anti-CD49d/α4 (all from Immunotech, Marseilles, France) or control immunoglobulins. Multiparametric FACS analysis of lymphocyte subpopulations was performed on 10 000 cells after gating for lymphocytes using dual-laser FACScan and CellQuest software (Becton Dickinson).
Statistical analysis
Results are median and interquartile range (IQR) values unless otherwise indicated. The unpaired Student t test was used to examine differences between PHI patients and age-matched healthy controls. P values less than .05 were considered as statistically significant.
Results and discussion
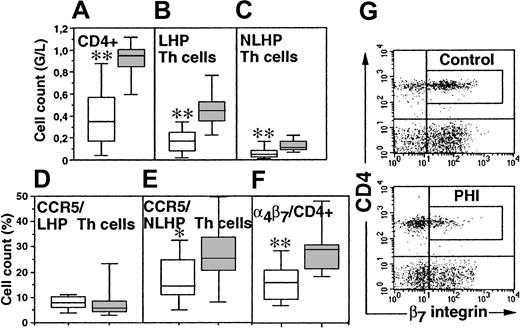
At inclusion, CD4+ cell counts of PHI patients were significantly lower than those of controls (P = .0001; Figure 1A). Both naive (CD62L+CD45RO−) and memory (CD45RO+) Th lymphocyte counts were lower in patients (P = .0018 and P = .0001, respectively).12-14 Among memory Th lymphocytes, the counts of both the CD62L+ and CD62L−subpopulations were decreased (Figure 1B,C).
Th lymphocyte subpopulations in PHI patients.
Absolute cell counts of CD4+ (A), LHP (CD62L+CD45RO+) Th cells (B), and NLHP (CD62L−CD45RO+) Th cells (C) and the percentage of CCR5-expressing LHP Th cells (D), CCR5-expressing NLHP Th cells (E), and α4β7-expressing Th cells (F) were determined at inclusion in 12 PHI patients (hollow boxes) and 12 age-matched healthy controls (solid boxes). Box plots depict median (horizontal line within box), 75% to 25% IQR (upper and lower limits of the box, respectively), and range (upper and lower horizontal bars outside the box). (G) Dot plots of β7-integrin staining of CD4+ T cells of a representative control individual and a selected PHI patient. *P < .05; **P < .005 by 2-tailed Student ttest.
Th lymphocyte subpopulations in PHI patients.
Absolute cell counts of CD4+ (A), LHP (CD62L+CD45RO+) Th cells (B), and NLHP (CD62L−CD45RO+) Th cells (C) and the percentage of CCR5-expressing LHP Th cells (D), CCR5-expressing NLHP Th cells (E), and α4β7-expressing Th cells (F) were determined at inclusion in 12 PHI patients (hollow boxes) and 12 age-matched healthy controls (solid boxes). Box plots depict median (horizontal line within box), 75% to 25% IQR (upper and lower limits of the box, respectively), and range (upper and lower horizontal bars outside the box). (G) Dot plots of β7-integrin staining of CD4+ T cells of a representative control individual and a selected PHI patient. *P < .05; **P < .005 by 2-tailed Student ttest.
The proportion of CD62L+CD45RO+ Th lymphocytes expressing CCR5 was in the same range in patients and controls (Figure1D). Therefore, CD62L+CD45RO+ Th lymphocyte counts are decreased during PHI independent of their expression of CCR5. In contrast, the percentage of CD62L−CD45RO+ Th cells expressing CCR5 was lower in patients than in controls (14.5% versus 25.3%, respectively;P = .036; Figure 1E).
The preferential decrease of CD62L−CD45RO+ Th lymphocytes expressing CCR5 was associated with a decrease of Th lymphocytes expressing α4β7+. The α4β7+ cells made up 27.3% of Th cells in controls, but only 15.8% in patients (P = .0009; Figure 1F). The absolute number of circulating α4β7+ Th lymphocytes was only 48 × 106/L in patients and 240 × 106/L in healthy controls (P = .0001). In controls, circulating Th lymphocytes expressing high levels of the β7 integrin were enriched in CCR5+ cells as compared to β7− Th lymphocytes (median percentage: 20.8 versus 6.2, respectively; P = .02). There were almost no Th lymphocytes expressing high levels of β7 in patients (P = .0078, as compared to controls; Figure 1G).
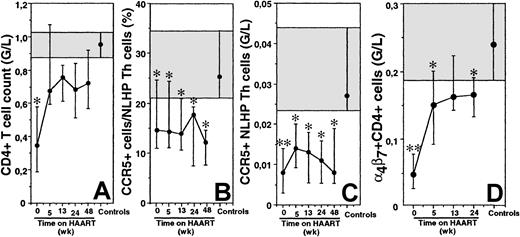
The HAART combination administered in this study led to an early control of HIV replication and a rapid recovery of peripheral Th cells (Figure 2A).11 We determined whether this restoration involved all subpopulations of Th lymphocytes. The recovery was rapid for naive and CD62L+CD45RO+ Th lymphocytes (not shown), as already reported in patients treated at the chronic phase of the infection.15,16 These findings are consistent with a mechanism of sequestration in lymphoid organs causing the early decrease of most Th lymphocytes.1 17 This recovery was also rapid for CD62L−CD45RO+ Th lymphocytes. The proportion of CCR5-expressing cells among CD62L+CD45RO+ Th cells remained within the normal range for 48 weeks (not shown). In contrast, the proportion of CCR5-expressing cells among CD62L−CD45RO+ Th lymphocytes remained low, with no tendency for recovery. On week 48, the fraction of CCR5+ cells among CD62L−CD45RO+ Th lymphocytes remained significantly lower than that in healthy individuals (P = .012, Figure 2B), and the absolute number of circulating CCR5+CD62L−CD45RO+ Th cells was only 30% that of controls (P = .013; Figure2C). The α4β7+ Th lymphocyte counts increased during HAART, but their absolute number did not fully recover (P = .01 on week 24 as compared to controls; Figure 2D).
Persistent depletion of CCR5-expressing NLHP Th lymphocytes in PHI patients undergoing HAART treatment.
The median (IQR) CD4+ T cell count (A), the median (IQR) percentage of CCR5-expressing NLHP Th cells (B), the median (IQR) CCR5-expressing NLHP Th cell count (C), and the median (IQR) α4β7+ Th cell count (D) were followed in 12 PHI patients treated with HAART. Shaded areas represent the 75% to 25% IQR values for healthy controls. The solid symbol in the shaded area represents median for the controls. *P < .05; **P < .005 by 2-tailed Student t test.
Persistent depletion of CCR5-expressing NLHP Th lymphocytes in PHI patients undergoing HAART treatment.
The median (IQR) CD4+ T cell count (A), the median (IQR) percentage of CCR5-expressing NLHP Th cells (B), the median (IQR) CCR5-expressing NLHP Th cell count (C), and the median (IQR) α4β7+ Th cell count (D) were followed in 12 PHI patients treated with HAART. Shaded areas represent the 75% to 25% IQR values for healthy controls. The solid symbol in the shaded area represents median for the controls. *P < .05; **P < .005 by 2-tailed Student t test.
These results show that despite CCR5 coreceptor usage by primary HIV isolates, HIV does not lead to a homogenous decrease of CCR5-expressing Th lymphocytes during PHI. Rather, it preferentially targets Th lymphocytes expressing CCR5 in the NLHP subpopulation of memory Th lymphocytes. The absence of recovery of this CCR5+ cell population under treatment indicates that, in contrast to other populations of Th lymphocytes, sequestration and redistribution cannot account for its early changes. Rather, CCR5+ NLHP Th cells are lost during PHI, and they are not replaced during the following year despite control of viral replication. Because the vast majority of lamina propria Th lymphocytes express CCR5, depletion of CCR5+ NLHP Th lymphocytes may reflect a preferential compartmentalization of the Th lymphocyte depletion in the gastrointestinal tract at the early stage of HIV infection, as reported in SIV primary infection of macaques.8 9 This would be consistent with the substantial loss of α4β7+ Th lymphocytes during PHI. The partial recovery of α4β7+ Th cells under treatment may reflect expansion of these cells or conversion from α4β7− Th lymphocytes. Such processes do not allow recovery of CCR5+ NLHP Th lymphocytes.
The selective and persistent depletion of CCR5+ NLHP Th lymphocytes after PHI is of particular interest in view of recent data demonstrating redistribution of antigen-experienced Th cells from lymphoid organs to nonlymphoid tissues during primary immune responses.18 Moreover, the nonlymphoid compartment seems to promote survival of memory Th lymphocytes. It remains to be determined whether the loss of CCR5+ NLHP memory Th lymphocytes accounts for the loss of anti-HIV–specific memory Th lymphocytes during PHI. NLHP memory Th lymphocytes are richer than LHP in Th1 lymphocytes.19-21 In addition, CCR5 is a marker of the Th1 subpopulation. The depletion of CCR5+ NLHP may thus contribute to the impairment of Th1 responses in HIV-infected patients.22 Our data also suggest the need to reconsider the relevance of markers currently used to evaluate immune reconstitution in HIV-infected patients. It may be valuable to include analysis of CCR5 expression on particular subpopulations of Th lymphocytes, such as NLHP memory Th lymphocytes.
R.K. and A.R. contributed equally to this work. Ghislaine Lubart and Anne-Marie Delavalle are acknowledged for their technical assistance. The Primoferon A Study Group is composed of the authors and Catherine Lemonnier (Schering-Plough France); F. Duchenne and B. Hoen (Centre Hospitalier-Universitaire [CHU] Besançon); P. Goubin, R. Verdon, and C. Bazin (CHU Caen); J. L. Touraine and J. M. Livrozet (Hôpital E Herriot, Lyon); V. Baillat and J. Reynes (CHU Montpellier); F. Grihon (Hôpital de Noyon); F. Cartier and F. Souala (CHU Rennes); D. Sissoko and Y. Mouton (CHU Tourcoing); C. Yung, P. Lesprita, and Y. Levy (CHU Henri Mondor, Créteil); J. M. Molina, S. Balkan, D. Ponscarme, and C. Lascoux-Combe (CHU Saint-Louis, Paris); C. Rouzioux and M. Burgard (CHU Necker-Enfants-Malades, Paris); C. Pignon (Hôpital A Béclère, Clamart); and G. Chene (Bordeaux), all in France; and M. Laughlin, Schering-Plough Research Institute, Kenilworth, NJ.
Supported by the Agence Nationale de Recherches sur le SIDA (ANRS).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dominique Emilie, Institut Paris-Sud sur les Cytokines, 32, rue des Carnets, 92 140 Clamart, France; e-mail:emilie@ipsc.u-psud.fr.