Introduction
Despite significant progress in the past 20 years, graft-versus-host disease (GVHD) remains a significant cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HSCT). T-cell depletion (TCD) of the donor graft offers the potential for prevention of GVHD without the morbidity associated with immunosuppressive drugs such as methotrexate and cyclosporine. Most early trials documented that TCD could substantially limit acute and chronic GVHD. However, this reduction in GVHD did not translate into improved overall survival because of unexpected high rates of graft failure, Epstein-Barr virus–associated lymphoproliferative disorders, and disease recurrence after TCD bone marrow transplantation. Despite the problems associated with TCD, great interest remains in developing and improving this technology, particularly for recipients of HLA-mismatched grafts. If advances in graft engineering can accomplish the goal of GVHD prevention without adversely affecting engraftment, immunocompetence, and antileukemic activity, then substantial improvements in overall transplant outcome can be realized.
Graft-versus-host disease
GVHD can be classified as acute or chronic based on timing of onset and clinical features. Acute GVHD usually develops within the first 2 months of bone marrow transplantation (BMT) and affects mainly the skin, gastrointestinal tract, and liver. When pharmacologic immunosuppression is used as GVHD prophylaxis after myeloablative transplantation, moderate to severe acute GVHD (grades II-IV) occurs in 25% to 60% of matched related donor transplant recipients, and up to 45% to 70% in unrelated donor recipients.1-5Acute GVHD is also emerging as a major complication after allogeneic nonmyeloablative stem cell transplantation.6 Development of grade II to IV acute GVHD is associated with decreased survival in patients after allogeneic BMT.7 8
Chronic GVHD has a later onset than acute GVHD and is often clinically distinct. Patients can manifest sclerodermatous skin changes, keratoconjunctivitis, sicca syndrome, lichenoid oral mucosal lesions, esophageal and vaginal strictures, liver disease, and pulmonary insufficiency.9 Despite immunosuppressive agents, approximately 30% to 50% of patients will develop chronic GVHD after myeloablative HLA-identical sibling BMT. The incidence of chronic GVHD may be even higher after allogeneic transplantation using unmanipulated peripheral blood stem cells (PBSCs).10,11 Although limited chronic GVHD often resolves spontaneously with minimal intervention, extensive chronic GVHD requires prolonged immunosuppressive treatment and is associated with significant morbidity and mortality. More than 50% of patients with extensive chronic GVHD will die, mostly secondary to infections resulting from severe immune dysfunction.12
Acute GVHD is believed to occur in 3 phases: (1) tissue damage from conditioning, (2) donor T-cell activation, and (3) inflammatory effectors. In the earliest phase, inflammatory cytokines are released from host tissue in response to damage by the pretransplantation conditioning regimen.13 These cytokines, including interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α), up-regulate the expression of adhesion molecules and host major histocompatibility complex (MHC) antigens, and enhance recognition of the host tissue by mature donor T lymphocytes. During the second phase, donor T-helper 1 (Th1) cells are activated on recognition of alloantigens and secrete IL-2 and interferon-γ (IFN-γ), which recruit other T cells, cytotoxic T lymphocytes (CTLs), natural killer (NK) cells, monocytes, and macrophages14 In the last phase, mononuclear cells primed by Th1 cytokines secrete more TNF-α and IL-1, which induce cellular damage or apoptosis, and restart the cycle of inflammation.
Several clinical studies have attempted to interrupt the cytokine cascade as a strategy to prevent GVHD. Unfortunately, a blinded randomized trial of IL-1 receptor antagonist versus placebo did not demonstrate any benefit for IL-1 blockade in acute GVHD prevention.15 Another trial of recombinant human IL-11 to reduce Th1 cytokine production was halted due to unacceptable toxicity.16 Thus, although interruption of the “cytokine storm” may prevent GVHD in murine models, it appears to be insufficient in humans, and T cells remain the prime target for current therapeutic strategies in GVHD prophylaxis.
Current approaches for the prevention and treatment of GVHD involve direct blockade of T-cell function. These include the down-regulation of T lymphocytes by inhibiting cellular proliferation (methotrexate), inhibition of de novo purine synthesis (mycophenolate mofetil), suppression of IL-2 secretion by blocking calcineurin activity (cyclosporine, FK-506), interfering with downstream growth signaling pathways (sirolimus), and reduction of T-cell responsiveness by blocking the IL-2 receptor (daclizumab). However, the most effective means of GVHD prophylaxis is depletion of T cells from the donor inoculum. In many instances, TCD can dramatically reduce the incidence of GVHD even in the absence of posttransplantation immunosuppressive therapy.
Preclinical models of TCD
In 1968, Dicke and colleagues reported that irradiated mice receiving transplants with spleen cell fractions depleted of small lymphocytes enjoyed 80% to 100% survival without evidence of GVHD, whereas mice given fractions containing increasing numbers of lymphocytes all died from severe GVHD.17 Subsequently, Reisner and coworkers demonstrated similar results with mouse bone marrow and spleen cell suspensions depleted of T cells by soybean or peanut agglutinination.18 In the 1970s, antithymocyte globulin (ATG) was tried as a method of TCD, but with limited success because early ATG preparations were also toxic to hematopoietic cells. This problem was later circumvented through the absorption of ATG with spleen or liver suspensions to remove antibodies against stem cells.19 Using absorbed ATG, animals successfully received transplantations with minimal to no GVHD, across both major and minor histocompatibility barriers.20-25 In the late 1970s, it became clear from murine models that even minor alloantigen differences could stimulate GVHD, and that contamination of the marrow with as few as 0.3% T lymphocytes (3 × 104 cells) could be sufficient to cause lethal GVHD.23 In 1981, Vallera and colleagues reported on the first use of a monoclonal antibody, anti–Thy-1.2, for ex vivo TCD in a murine transplant model.26 In that study, however, many animals still died from complications of GVHD after a few months. This led to the eventual realization that antibody alone is insufficient to abrogate GVHD and that addition of rabbit complement would confer superior protection. The importance of complement would be confirmed in subsequent human studies using monoclonal antibodies for TCD.
Human trials of TCD
Because animal studies had shown that pharmacologic immunosuppression was not necessary after TCD, it was anticipated that TCD transplants in humans without cyclosporine and methotrexate would be associated with less mucositis, renal failure, infections, and other complications of conventional BMT. Furthermore, it was hoped that the lower incidence of GVHD would translate into improved long-term survival. From 1981 to 1986, hundreds of TCD transplantations were performed worldwide using various methods of TCD. Although these trials confirmed a low incidence of acute and chronic GVHD, they also revealed new and important limitations associated with TCD BMT. These included an increased incidence of graft rejection, delayed immune reconstitution, increased posttransplantation lymphoproliferative disorders (LPDs), and increased rate of disease relapse.
Methods of TCD
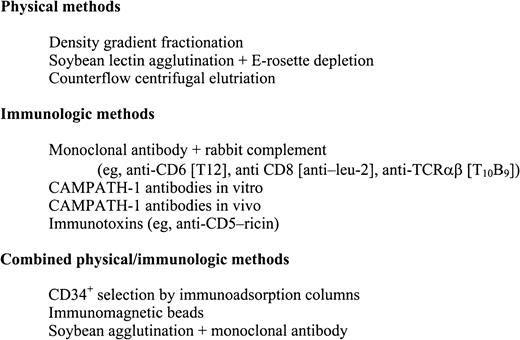
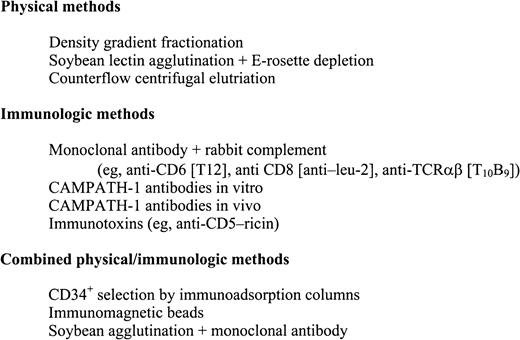
Methods of TCD in human transplantation have primarily depended on negative selection techniques by physical separation or antibody-based purging (Figure 1). Examples of physical separation techniques include differential agglutination with lectins followed by rosetting with sheep red blood cells,27,28 counterflow centrifugal elutriation,29-31 and fractionation on density gradients.32 The earliest human trials using antibody as T depletion relied on absorbed ATG.33 However, this was soon replaced by monoclonal antibodies directed against specific targets on T cells. Monoclonal antibodies have been used alone,34-36and in conjunction with homologous, heterologous, or rabbit complements,37-49 Many are directed against T-cell antigens with restricted expression, whereas others have broader targets. Some of the most widely used monoclonals are a set of antibodies called CAMPATH-1,40 which is directed against a heterogeneous 23- to 30-kd glycoprotein (CDw52) expressed on lymphocytes and monocytes. CAMPATH-1 antibodies are classified by their immunoglobulin subtype and origin. CAMPATH-1G and CAMPATH-1M are derived from the rat and are IgG2b and IgM, respectively. CAMPATH-1H, in contrast, is a recombinant DNA–derived human IgG1. CAMPATH-1 antibodies have an advantage over other anti–T-cell monoclonal antibodies in that they can fix human as well as rabbit complements. As a result, they can lyse lymphocytes in human donor serum and do not depend on rabbit complement lots that are variable in efficacy and toxicity. This property renders CAMPATH-1 antibodies useful for both in vitro or in vivo TCD. Other antibody-based methods for TCD that do not depend on complement include immunotoxins50-54 and immunomagnetic beads.55 In the 1990s, with the advent of allogeneic PBSC transplantation, CD34+ immunoadsorption columns have been developed that positively select hematopoietic progenitors and permit nonadherent cells to be washed out, effectively reducing the lymphocyte content in the infused PBSC graft.56
T-cell dose in TCD BMT
An average patient receiving a transplant with unmodified marrow receives a T-cell dose on the order of 1 to 5 × 107cells/kg recipient body weight. Initially, it was not known what degree of depletion would be necessary to prevent GVHD, because sufficiently sensitive assays for quantifying T cells were not available. Early efforts at TCD were thus intended to maximally purge lymphocytes without compromising hematopoietic precursors. It was not until the mid-1980s that limiting dilution analysis (LDA) was developed to measure functional T lymphocytes and assess cell dose.57,58 Using counterflow centrifugation elutriation (CCE), Wagner and coworkers demonstrated a 45% incidence of acute GVHD in matched related transplant recipients when the T-cell dose was 1 × 106 cells/kg, but only 22% when the dose was reduced to 0.5 × 106 cells/kg.31,59Recently, Aversa and colleagues reported that reliable engraftment without GVHD could be achieved in full haplotype mismatched transplants using high stem cell numbers plus extensive TCD to a CD3+cell dose of 3 × 104 cells/kg.60 Although these data give a clue as to the relationship between T-cell dose and GVHD, there is likely to be significant variability between donor-recipient pairs depending on minor antigen differences.
Advantages of TCD
The advantages and disadvantages of TCD are listed in Table1 and discussed in more detail below.
GVHD prevention
HLA-identical sibling transplantation.
In 1981, Rodt and coworkers reported on the use of absorbed human ATG as TCD in HLA-compatible sibling transplants and demonstrated that only 2 of 12 patients developed mild skin GVHD.33 In contrast, concurrent TCD studies using murine monoclonal antibodies alone reported GVHD rates of approximately 50%.35,36 These results prompted investigators to add complement in hopes of enhancing lymphocytolysis and improving GVHD outcome. Studies in the mid-1980s confirmed that TCD using monoclonal antibodies plus rabbit complement, which removed 2 to 3 logs of T lymphocytes, uniformly decreased the incidence of clinically significant GVHD to 10% to 20% after matched sibling transplantation.37,39,41-43 In the late 1980s and early 1990s, similar results were reported in larger phase II trials of TCD using anti-CD5, -CD6, and -CD8 antibodies plus complement, CAMPATH-1, or immunotoxins.49,54,61-64 In some of these studies, the low incidence of GVHD was achieved even in the absence of immunosuppression after transplantation.49 54
Studies of HLA-identical sibling PBSC transplantation using CD34+ selection have reported great variation in acute GVHD incidence from 8% to 100%.65-69 This variability was likely influenced by factors such as the number of CD3+cells infused, immunosuppressive agents given after transplantation, and type of CD34+ selection device used. Other TCD methods are also being investigated with PBSC transplantation. Hale and associates have recently reported that HLA-matched sibling PBSC transplantation purged with CAMPATH-1H or CAMPATH-1G is associated with only 4% and 11% incidence of grade II to IV acute GVHD, respectively.70
Alternative donor transplantation.
Retrospective data from the National Marrow Donor Registry (NMDR) have demonstrated that TCD is a significant negative predictor for the development of grade III to IV acute GVHD after unrelated BMT.71 The International Bone Marrow Transplant Registry (IBMTR) recently reported on 1868 leukemia patients who received allogeneic marrow transplants from donors other than HLA-identical siblings. The incidence of grade II to IV GVHD was between 34% and 38% in the TCD group, as compared to 57% in the non-TCD group (P < .0001).72 HLA-matched unrelated marrow transplants depleted of T cells with anti-CD6 monoclonal antibody plus complement as the sole form of GVHD prophylaxis is associated with an approximately 40% incidence of grade II to IV GVHD.73 A similar result has been reported for TCD using the αβ T-cell receptor antibody T10B9 in unrelated marrow transplants for patients with chronic myelogenous leukemia (CML).74 In another study, the incidence of grade II to IV acute GVHD was only 24% among patients who received unrelated marrow grafts T-cell depleted with CAMPATH-1.75
T-cell depletion has also been used in HLA-mismatched transplant settings. In single institution studies, grade II to IV GVHD incidence has ranged from 18% to 40% in recipients of HLA-mismatched BMT after TCD using anti-CD6 or T10B9 monoclonal antibody.76-78 In leukemia patients receiving HLA haploidentical marrow from related donors, a low occurrence of GVHD has been observed when the graft was augmented with PBSCs and T-cell depleted using lectin agglutination and E-rosetting.60 79
Organ dysfunction after TCD BMT
T-cell depletion appears to be associated with less organ toxicity early after transplantation compared to conventional BMT. The incidence of hepatic veno-occlusive disease has been reported to be only 3.1% after TCD BMT, and only 1.2% among patients receiving total body irradiation (TBI) as part of their conditioning.80 This reduction in hepatotoxicity may be due to the fact that the TCD patients are spared from injurious effects of methotrexate or cyclosporine/tacrolimus. Alternatively, decreases in alloreactivity may lead to lower levels of circulating cytokines, which could damage hepatic endothelium. Finally, because TCD patients may have earlier neutrophil recovery in the absence of methotrexate, they are also less likely to require antibiotics such as amphotericin that could have deleterious effects on hepatorenal function.
T-cell depletion has also been associated with decreased pulmonary complications after BMT. Breuer and coworkers have reported that fatal interstitial pneumonitis occurred in only 6 of 80 (7.5%) consecutive allogeneic BMT where TCD was used as GVHD prophylaxis.81In another series, the incidence of life-threatening pulmonary complications within the first 60 days of BMT was 8% among those who received TCD as the sole form of GVHD prophylaxis, but 33% among those who received cyclosporine and methotrexate (P < .0001).82 In this study, the protective effect of TCD against pulmonary complications was independent of acute GVHD in multivariate analysis, perhaps implicating deleterious effects of GVHD prophylactic medications.
Transplant-related mortality
Because TCD protects against GVHD and reduces organ dysfunction after BMT, one would expect these benefits to translate into lower transplant-related mortality (TRM). In a number of series, TRM after TCD BMT has indeed been quite low, ranging from 2% to 15%.49,83-86 However, other TCD studies have reported TRM rates from 20% to 32% even after matched sibling transplantations, with many deaths being secondary to graft failure, infections, and Epstein-Barr virus (EBV)–associated LPDs.54 87-89 This variability highlights the fact that elements other than GVHD, such as the intensity of conditioning, posttransplantation immunosuppression, graft failure, and immune reconstitution, also contribute significantly to TRM. It further reinforces the point that for a TCD regimen to be successful, it must do more than just protect against GVHD. It must also preserve graft-versus-leukemia (GVL) activity and engraftment potential and spare patients from excessive transplant-related toxicity.
Limitations associated with TCD
Graft failure
Prior to the advent of TCD, graft failure after allogeneic BMT was uncommon, occurring in 1% to 5% of patients having transplantations for leukemia.90,91 In contrast, most TCD BMT series in the 1980s and early 1990s reported higher rates of graft failure.31,32,40,41,43-46,51,52,61,87,92-99 In some of these studies, the incidence of graft failure was as high as 50% to 70%.92,99 In an analysis from the IBMTR of more than 3000 patients who received TCD or non-TCD BMT for leukemia, TCD was associated with a 9-fold increased risk for graft failure compared to unmanipulated marrow transplantation (P < .0001).100
A number of studies have retrospectively analyzed risk factors associated with graft failure after TCD. Aside from HLA disparity, one important factor appears to be the dose of TBI administered before transplantation. Patients who are conditioned with higher doses of TBI consistently have lower incidence of graft failure compared to those who receive lower TBI doses.92,99,101,102 In addition, donor-recipient sex disparity, male donor gender, donor age over 25 years, and absence of posttransplantation immunosuppression, have also been reported to be significant risk factors for graft failure.95,99 100
Graft failure after TCD BMT can occur as 3 distinct patterns.92 These include (1) failure of initial engraftment, (2) partial or full initial engraftment followed by graft rejection within 2 weeks of BMT, and (3) delayed graft failure that can occur months after BMT. The pathophysiologic mechanisms behind these different patterns are not well understood, although it is widely accepted that early graft failure after TCD transplantation results primarily from immunologic rejection of donor hematopoietic elements by host lymphoid elements that have survived the conditioning process. Direct evidence for the role of the host immune system have come from the identification of host T lymphocytes from patients at the time of graft rejection, which exhibit donor-specific cytotoxic activity,103-112 and which suppress donor proliferation of granulocyte-macrophage colony-forming units and erythroid colony forming units in vitro.93,104 Clinical evidence further supporting this model includes observations that genetic disparity between donor and recipient correlates with graft rejection and that increased immunosuppression, either through the conditioning regimen or posttransplantation immunosuppressive agents, appears to protect against graft failure.92,95,99-102 113
It does not appear that failure of initial engraftment is caused by injury to hematopoietic progenitors or auxiliary cells during marrow manipulation, because autologous marrows purged with monoclonal antibodies and complement engraft without difficulty.61,99,114-116 Viral infections, such as cytomegalovirus (CMV) or human herpes virus 6, may contribute to graft failure after BMT.117-121 Although TCD BMT patients may have a higher risk of CMV reactivation after transplantation,122-125 direct clinical evidence implicating this and other viruses to graft failure after TCD transplantation is limited.
Other clinical observations also offer insight into the pathophysiology of graft failure. Many studies have reported that mixed lymphoid and myeloid chimerism is more common after TCD BMT and that such chimerism may be associated with graft failure.99,102,126-130 Viable host-derived hematopoietic cells can often be recovered from patients after TCD marrow transplantation,131 132 and their coexistence with the donor graft implies a state of immune tolerance between the graft and host. This may be especially true for patients who have persistent stable mixed chimerism with fully functional hematopoietic grafts. For patients whose mixed chimerism is transient, however, it is possible that graft failure could result when host lymphoid tolerance of the graft is broken. At the current time, it is unknown whether the mixed chimeric state is a cause of graft rejection or an effect of the graft rejection process itself.
The higher incidence of graft failure associated with donor-patient gender disparity has led researchers to investigate gender-specific antigens as potential targets for graft rejection. Studies suggest that female patients receiving grafts from male donors may be at higher risk for graft rejection because female recipient T lymphocytes can recognize male-specific minor antigens in the donor graft. The H-Y antigens encoded on the Y chromosome have been implicated as targets for this process. During rejection of male stem cell grafts, H-Y–specific CTL clones have been isolated from the peripheral blood of female recipients.110 Multiple H-Y antigens are believed to be involved in graft rejection. Known H-Y epitopes recognized by HLA-A2–restricted CTL clones include peptides derived from the SMCY and DFFRYgenes.133-135 More recently, a third H-Y epitope that is recognized by HLA-B60–restricted CTL clones has been localized to theUTY gene on the Y chromosome.136 The identification of these H-Y antigens could prove useful in the selection of donor recipient pairs for stem cell transplantation and could be especially relevant in female recipients who may have been sensitized to male antigens through prior blood transfusions.
Because graft rejection is felt to be mediated by residual host T cells that have escaped myeloablation, early preventive strategies were focused on intensifying the myeloablative regimen. Some investigators also believed that intensive conditioning regimens would empty out the host marrow and increase “hematopoietic space” for the incoming donor graft. High doses of cytarabine, thiotepa, and anthracyclines have been incorporated into standard ablative regimens and have reduced the rate of graft failure after TCD BMT.89,137-141However, these were also more likely to be associated with increased regimen-related toxicity. Other researchers have investigated the addition of total lymphoid irradiation (TLI) as a strategy against graft failure. Although TLI has been shown to reduce graft failure after conventional BMT for aplastic anemia,142-144 its benefits are less clear after TCD BMT. Although single-arm studies of TCD BMT have reported a low incidence of graft failure when TLI was included in the conditioning regimen,87,145,146 an older prospective randomized trial of TCD transplantation failed to show any benefit.147
An alternative method of immunosuppression commonly used to reduce graft failure after TCD BMT is the simultaneous depletion of host immune cells using ATG or CAMPATH-1 at the time of transplantation. Studies in animals from the late 1980s showed that in vivo depletion of recipient lymphocytes using anti–T-cell monoclonal antibodies could prevent rejection of the TCD marrow even across major histocompatibility barriers.148,149 The presumed mechanism behind this observation was that the treatment eliminated host lymphocytes that were active in the graft rejection process. In 1994, Hale and his associates adapted this approach to humans and reported that simultaneous donor and host TCD with CAMPATH-1 reduced the incidence of graft failure to less than 10%, without compromising GVHD prophylaxis.87 In their subsequent series of 70 AML patients receiving transplants from HLA-identical sibling donors using this combined TCD approach, the incidence of graft failure was only 6%, significantly lower than that with donor marrow TCD alone.86
Another widely applied strategy for reducing graft failure involves limited or selective TCD. The premise of this approach arose from the belief that most early methods of TCD had attempted to deplete as many T lymphocytes from the marrow as possible and that the exhaustive depletion nonspecifically removed ancillary marrow elements that were necessary to sustain engraftment. It was thus postulated that by selectively purging only certain subsets of T cells, such as mature T lymphocytes, but sparing NK cells, immature thymocytes, monocytes, and other hematopoietic elements, one could maintain the protective effects against GVHD without increasing the risk for graft failure. Several selective antibody-purging methods have been successfully used. Studies using anti-CD5 immunotoxins, anti-CD6, and anti-TCRαβ (T10B9) antibodies have all demonstrated low graft failure rates without compromising GVHD prophylaxis.49,52-54,83,84 150-152
T-cell add-back to the marrow inoculum has been studied as a means of reducing graft rejection after TCD BMT. Using centrifugal elutriation technique for T-cell separation, Wagner and coworkers have shown the reintroduction of T lymphocytes to a dose of 0.5 × 106cells/kg protects against GVHD while maintaining engraftment at over 95%.59 Researchers have also added back donor T cells to marrow that had been T depleted using CAMPATH-1. However, these studies are problematic because of an increased risk of severe GVHD as the number of T cells added back is increased.153 154
Dose escalation of CD34+ stem cells may be an effective strategy for overcoming graft failure after TCD BMT. Preclinical models have shown that mice given “megadoses” of TCD marrow could engraft despite sublethal doses of conditioning irradiation.155 In human studies, the addition of CD34+ cells to TCD marrow to augment stem cell dose has permitted reliable engraftment in leukemia patients despite full HLA haplotype mismatches.60 79
Delayed immune reconstitution
Regeneration of the immune system after allogeneic BMT is a slow process that is often protracted by the presence of GVHD and its treatment. Peripheral blood T- lymphocyte counts do not normalize until 3 to 12 months after BMT,156 and their functional recovery frequently takes even longer.157-159 In vitro studies of T cells after BMT have demonstrated reduced proliferative response to mitogenic stimuli, abnormal cytotoxic T-cell effector function, and impaired ability to collaborate with B cells in immunoglobulin synthesis.157 160-166 Because of this delayed immune reconstitution, patients after allogeneic BMT remain susceptible to opportunistic infections for months to years.
After allogeneic BMT, regeneration of T-cell populations occurs by both thymic-dependent and thymic-independent pathways. The former pathway involves the positive or negative selection of thymocytes in the thymus and produces a T-cell population with a diverse T-cell receptor (TCR) repertoire. In contrast, the thymicindependent pathway, which reconstitutes the T-cell compartment by expansion of T cells infused along with the graft, produces a T-cell population with limited TCR diversity. Although the thymic- dependent pathway appears to be important in the regeneration of T cells after chemotherapy in children, it may be less active in adults whose thymuses are involuted.167,168 However, even in adults, the host thymus is required after BMT for the reconstitution of CD4+ and CD8+ naı̈ve subsets, and maintenance of TCR diversity.169
Immune recovery after TCD BMT has been studied in a number of transplant centers using various methods of TCD.163,165,170-175 After allogeneic BMT, NK cells appear to be the first lymphoid subset to emerge, usually within 2 to 3 weeks after transplantation, followed by B cells (3-6 months) and T cells (3-12 months). Phenotypic analyses reveal that total lymphocyte numbers are usually higher early after BMT in recipients of conventional marrow compared to TCD grafts. Furthermore, the reconstituted T-cell compartment is predominantly of the CD8+ subset, and most TCD BMT patients will have a deficit in CD4+ cells, with an inverted CD4+ to CD8+ ratio for up to 2 years.165 The number of CD4+ cells normalizes at 7 to 9 months after conventional BMT, but this process is delayed after TCD BMT.170
Functional recovery of T cells appears to be impaired after TCD BMT as well. Welte and colleagues reported that in recipients of TCD marrow, the proliferative response of peripheral blood mononuclear cells to exogenous IL-2 stimulation remained abnormal for up to 6 months, compared to only 1 month for recipients of conventional BMT.163 Similarly, the proliferative response of T cells to mitogenic stimulation can be impaired for over 18 months after TCD BMT.165 As another reflection of their decreased function, T lymphocytes from recipients of TCD BMT have significantly restricted variability in their TCR repertoires.173,175 This may be explained in part by the fact that the T-cell compartment after BMT is expanded mainly from lymphocytes cotransfused with the marrow, and therefore recipients of TCD transplants would have many fewer precursors with which to reconstitute their repertoire than recipients of conventional BMT. In support of this hypothesis, TCR analyses have shown the peripheral T-lymphocyte pool after TCD BMT to be the progeny of a limited number of precursors.173
The delayed reconstitution of CD4+ cells and impaired recovery of T-cell repertoire diversity have led many to speculate that TCD BMT recipients may be at higher risk for opportunistic infections. Although there is little reported evidence to suggest an increased risk of bacterial or fungal infections after TCD transplantation, a number of studies have demonstrated a higher probability of reactivation for viruses such as CMV,122,124,125 and EBV, leading to EBV-associated B-cell lymphoproliferative disorders (EBV-LPDs).54,176 177
EBV-LPDs
Although EBV-LPDs are well known in immunosuppressed patients after solid organ transplantation, it is surprisingly uncommon after BMT except in the TCD setting, where its incidence has been reported to be between 5% to 30%.54,176,177 Recipients of TCD transplants using HLA-mismatched or unrelated donor marrow appear to be at particularly high risk,138,176,178 as are patients with severe GVHD and those treated with certain anti–T-cell monoclonal antibodies.54,176 179 EBV-LPDs are felt to arise mainly from infected donor B cells that have been cotransplanted with the allograft. However, there have been cases of EBV lymphoma in B cells from EBV-seronegative donors, suggesting that de novo infection in transplant recipients or reactivation of EBV in recipient cells can occur.
Although combinations of IFN-α, intravenous γ-globulin, and high-dose acyclovir have some activity against EBV-LPDs, their response rates have been disappointing.177,180,181 In the past few years, donor lymphocyte infusion (DLI) has emerged as a potential effective treatment of EBV-LPDs.182 The therapeutic effect from DLI stems from the development of EBV-specific immunity mediated by CTLs present in the infusion. As an extension of this strategy, researchers have now demonstrated that administration of EBV-specific CTLs cultivated in vitro from donor lymphocytes is effective against EBV-LPDs.183-186 For patients who are not candidates for DLI or EBV-specific CTL therapy, recent small case series have reported promising responses with the use of the anti-CD20 monoclonal antibody rituximab.187,188 Rituximab is relatively safe and may prove to be as effective as DLI in the treatment of EBV-LPD. Polymerase chain reaction methods to detect EBV DNA may be an effective means of diagnosing patients with EBV-LPDs prior to the onset of clinically evident disease.189 As these and other methods of early detection improve, clinicians may now intervene with DLI or anti-CD20 antibody at a stage of low disease burden, and perhaps alter the poor outcome often associated with this condition.
Spurred by the advances in the understanding and treatment of EBV-LPDs, researchers have in recent years concentrated their efforts on prevention of this disease. B-cell depletion of the donor graft has been investigated as a strategy to reduce EBV-LPDs after TCD BMT. Cavazzana-Calvo and colleagues have shown that combined ex vivo depletion of B and T cells from the graft using monoclonal antibodies effectively prevented EBV-LPDs and improved survival compared to control patients who received TCD grafts without B-cell purging.190 Similarly, Hale and Waldman have reported that TCD BMT using CAMPATH-1 was associated with a minimal risk for EBV-LPDs.191 They attributed this risk reduction to the fact that CAMPATH-1 depletes both T and B cells, thereby removing donor B cells that are potentially infected with EBV from the donor marrow.191 Finally, the prophylactic administration of EBV-specific CTLs has been shown to reduced the incidence of EBV-LPDs after TCD BMT.185 186
Leukemia relapse
The higher incidence of leukemia relapse associated with TCD BMT was first suggested in a prospective randomized trial which included 40 patients with acute and chronic leukemia.44 Of the 20 patients randomized to the TCD arm, 7 had clinically apparent relapse, compared to only 2 in the control (non-TCD) arm.44Although not statistically significant, this difference prompted researchers to investigate the possibility of higher disease relapse associated with TCD. Multiple retrospective studies have subsequently demonstrated that leukemia relapse is indeed more frequent after TCD BMT, especially for CML.8,100,126 192-196
The increased rate of leukemia relapse after TCD BMT has been linked, as least in part, to the reduction in GVHD and concomitant loss of the GVL activity. In recipients of T-cell–replete marrow, patients who develop clinically significant GVHD have a lower incidence of leukemia relapse compared to those who do not develop GVHD.197-199Additional evidence linking T cells and GVL activity comes from studies using donor lymphocyte infusions in patients with CML who have relapsed after BMT, where complete response rates of 70% to 80% have been attained.200 201
TCD BMT for CML.
An increased rate of relapse has been observed in virtually all studies using TCD of HLA-identical sibling bone marrow on patients with CML.100,126,192,193 The risk of relapse after matched related TCD BMT for CML chronic phase has been estimated to be 5- to 6-fold higher than that after conventional BMT.100,192 This relative risk has been reported to be as high as 18 for TCD transplantations done in accelerated phase CML.193 However, the increase in CML relapse is not as apparent after TCD transplantation using matched unrelated marrow.199,202-204 In one series, the 3-year probability of relapse for CML chronic phase was only 8% for recipients of unrelated TCD allografts, compared to 47% for those who received TCD marrow from HLA identical siblings.204
TCD BMT for acute leukemia.
Unlike CML, TCD appears to have only a modest effect on the relapse rate of patients receiving transplants for acute leukemia. Reported rates of relapse after TCD BMT for acute myelogenous leukemia (AML) in first remission have ranged from 0% to 31% in different centers and are at least partly influenced by factors such as intensity of conditioning, extent and method of TCD, patient selection, and posttransplantation immunosuppression.83,89,205-207Retrospective data from the IBMTR, which included 731 TCD and 2480 non-TCD BMTs for leukemia, have shown that TCD is associated with a 1.7- to 2.0-fold increased risk for recurrence in patients with acute lymphoblastic leukemia (ALL) in any phase and in patients with AML undergoing transplantation in relapse or first remission.100 However, in a small randomized trial comparing TCD with methotrexate and cyclosporine as GVHD prophylaxis for leukemia patients undergoing HLA-matched related BMT, a higher relapse rate was observed after TCD BMT only in patients with CML, but not in patients with acute leukemia.208
Leukemic relapse after alternative donor TCD BMT.
There have been conflicting reports on whether TCD adversely affects leukemia-free survival after alternative donor transplantation. In an analysis of 462 patients from the NMDP, TCD was associated with a significant increased risk for leukemia relapse at 2 years.71 More recently, Champlin and colleagues reported on the IBMTR experience from 870 patients who underwent T-depleted unrelated or mismatched donor BMT for leukemia.72 They discovered that the leukemia-free survival for patients whose grafts were depleted with “narrow specificity” antibodies (eg, anti-CD5, CD6, anti-TCRαβ) were significantly higher than those whose grafts had been depleted with “broad specificity” antibodies (eg, anti-CD2, ATG, CAMPATH-1, elutriation, or lectin/sheep red blood cell agglutination).72 Furthermore, the 5-year leukemia-free survival among recipients of narrow specificity TCD BMT was similar to that in recipients of non-TCD BMT (29% versus 31%,P = NS). These important results suggest that, at least in the setting of unrelated or mismatched BMT, TCD using narrow specificity antibodies is not associated with loss of GVL effect, but retains the advantage of decreased GVHD compared to conventional BMT.
TCD BMT for lymphoma/myeloma.
Although studies have shown lower lymphoma and myeloma relapse after allogeneic BMT than autologous transplantation,209-214 use of allogeneic BMT has been restricted in these diseases primarily because of disproportionately high rates of transplant-related mortality. Because TCD is associated with less toxicity, it has been suggested that TCD transplants may be particularly beneficial in these conditions. Small studies of matched sibling TCD BMT for non-Hodgkin lymphoma (NHL) and multiple myeloma (MM) have indeed demonstrated low rates of treatment-related toxicity and mortality.151 215However, whether TCD will protect against relapse and improve overall survival remains unknown.
Strategies for reducing relapse after TCD BMT
Selective TCD
Although both GVL activity and GVHD are initiated by T cells in the donor graft, evidence suggests that different subsets of T lymphocytes may be involved in these processes and that GVL activity could exist in the absence of GVHD.216,217 In animal models, both donor CD4+ and CD8+ cells play a significant role in GVHD, but donor CD4+ cells in the absence of CD8+ cells can still mediate GVL activity.218 Based in part on these observations, Champlin and colleagues have shown that CD8+ depletion followed by posttransplantation cyclosporine can reduce the incidence and severity of GVHD without compromising GVL activity. In their initial study of 36 leukemia patients given CD8+-depleted matched sibling grafts, 2 (8%) developed grade III to IV GVHD, and only 3 (11%) had leukemia relapse at 2 years.62 In a follow-up double-blind randomized trial, the cyclosporine plus CD8+depletion arm experienced significantly less grade II to IV GVHD compared to the control arm receiving cyclosporine alone (20% versus 80%,P < .004), and the leukemia relapse rates were similar between the 2 groups.219
Additional evidence to suggest that GVL activity can be separated from GVHD has emerged from studies of DLI given for relapsed disease after BMT. Although many DLI series have demonstrated an association between development of GVHD and antileukemic response, it is clear that in a number of patients, remission can be attained in the absence of GVHD. The GVHD/GVL dichotomy is further evidenced in DLI studies using CD8+ depletion. CD8+-depleted DLI has been shown to significantly reduce the incidence of GVHD, but retain GVL activity and clinical responses in patients with relapsed CML.220 221 Although studies of CD8+ depletion have been encouraging, the pathophysiologic differences between GVHD and GVL are likely more complicated than just CD4 and CD8 cells. Definitive dissection of the anatomic subsets underlying GVHD and GVL remains a crucial but elusive goal for investigators hoping to reduce disease relapse after TCD BMT.
Cytokine stimulation
Another strategy aimed at decreasing disease relapse rates after TCD BMT has been the administration of IL-2 after transplantation to stimulate activity of NK cells. Evidence has emerged to suggest that NK cells may be important mediators of antileukemic activity. Baker and coworkers have recently reported in murine models that CD8+NK/T cells expanded in culture with IL-2, IFN-γ, and anti-CD3 monoclonal antibody exhibit potent cytotoxic activity against syngeneic and allogeneic tumor targets both in vitro and in vivo.222When allogeneic mice across major histocompatibility barriers received these expanded CD8+ NK/T cell transplants, little GVHD was observed despite doses up to 20 × 106 cells. In contrast, when the same donor-recipient pairs were given transplants with 2.5 × 106 splenocytes rich in T cells, all developed acute GVHD and died within 20 days. Evidence for NK cell alloreactivity in GVL has also been suggested in human studies. Addition of IL-2 to peripheral blood lymphocytes collected from CML patients after allogeneic BMT has been shown to induce an NK cell cytolytic response against cryopreserved leukemic cell targets.223,224 In an analysis of 20 patients who received allografts grafts mismatched at the HLA-C or Bw4allele in the direction of GVHD, Ruggeri and coworkers were able to isolate donor versus recipient alloreactive NK cell clones at a high frequency early after transplantation, even though none of these patients had clinical evidence of GVHD.225 They further demonstrated that these alloreactive NK cell clones could lyse the patients' pretransplantation cryopreserved leukemia cells in vitro, suggesting that GVL activity mediated by NK cells exists in these patients without GVHD.
Interleukin-2 is known to stimulate both NK cells and activated T cells at high concentrations. However, IL-2 at low doses appears to stimulate NK cells preferentially. As such, it has been postulated that administration of low doses of IL-2 may enhance GVL activity through NK cell stimulation without inducing GVHD. Prolonged infusion of low-dose recombinant IL-2 following TCD allogeneic BMT has been shown to be well tolerated and results in a marked increase in NK cell numbers.226 Mononuclear cells taken from patients after low-dose IL-2 treatment have also been shown to possess substantially enhanced in vitro cytolytic activity against tumor cell lines.226 In a pilot clinical trial, the prolonged infusion of IL-2 of at least 4 weeks after TCD BMT appeared to lower the incidence of disease relapse relative to historical controls.227 Although promising, these preliminary results have yet to be tested in randomized studies.
DLI
Adoptive immunotherapy using DLI has also emerged as a potential approach to compensate for the higher rate of leukemia relapse after TCD BMT. Sehn and colleagues have retrospectively analyzed the outcomes of 46 CML patients who underwent TCD allogeneic BMT, and compared them to 40 similar patients who received non-TCD allogeneic transplants using cyclosporine and methotrexate.84 They found that, as expected, the TCD group had a lower incidence of GVHD and treatment-related mortality, but a higher probability of hematologic or molecular relapse compared to the non-TCD group. However, most of the patients suffering relapse were successfully salvaged with DLI, compensating for the initial higher relapse rate associated with TCD. Similarly, Drobyski and coworkers reported that despite a high cumulative incidence of CML relapse (49%) at 3 years after TCD BMT, the 5-year overall survival rate in their cohort was 80% because most patients having relapse achieved durable remissions with DLI therapy.85 Taken together, these results suggest that TCD BMT followed by posttransplantation DLI at disease relapse could be a reasonable option for patients with CML, especially for those with concurrent comorbidity or advanced age who would otherwise be suboptimal candidates for non-TCD BMT.
Future directions in TCD BMT
Functional TCD/induction of T-cell anergy
Instead of focusing on removal of anatomic subsets, some investigators have turned their attention to TCD techniques in which only alloreactive T cells are removed from the graft. In one such system, donor T cells are coincubated with recipient mononuclear cells in vitro. After a defined interval, alloreactive cells are identified by expression of activation markers, such as CD25 or CD69, and are physically separated from the remaining cells by immunomagnetic cell sorting.228,229 Using this method, Koh and colleagues have shown that approximately 90% of the alloreactive component could be purged while preserving more than 70% residual immunity as measured by third-party alloantigen response.228 It is postulated that this method would preferentially purge alloreactive lymphocytes from the graft that are responsible for GVHD, but retain nonreactive T cells, which may improve posttransplantation immune reconstitution and enhance engraftment.
As an extension of functional TCD, other investigators have attempted to induce anergy in donor T cells prior to transplantation as a means of reducing GVHD. Murine marrow transplant studies have shown that GVHD could be reduced even across major genetic barriers by treating the recipient with CTLA4-Ig, an agent that blocks the CTLA4-BB1 (also called CD28-B7) interaction between T lymphocytes and antigen-presenting cells.230,231 Blockade of this and other costimulatory pathways (LFA-1/ICAM, CD40-CD40L) have since been shown to deactivate T cells and induce a state of alloimmune tolerance after BMT.231-233 A recent study in pediatric patients has suggested that costimulatory signal blockade using CTLA-Ig may reduce GVHD after HLA-mismatched BMT.234 If differential targets for GVHD and GVL can be identified, it may be possible to expose donor marrow in vitro to GVHD targets in the presence of CTLA4-Ig to induce GVHD specific anergy, while preserving the T-cell response to tumor antigens for a full GVL effect.
Delayed T-cell add-back/prophylactic DLI
The success of DLI in salvaging CML patients after BMT has led many researchers to investigate the potential of using delayed T-cell add-back to eliminate residual disease after TCD BMT. The attraction of this approach is that it allows one to reap the benefits of TCD early after BMT (ie, decreased GVHD and transplant-related toxicity), and yet be able to restore the GVL effect at a later time with DLI. Alyea and colleagues have recently conducted a trial of delayed T-cell add-back in 14 patients with MM after TCD allogeneic BMT.235 Of the 11 patients who had persistent myeloma 6 months after transplantation, 10 responses (6 complete, 4 partial) were observed after DLI, and the 2-year progression-free survival for the 14 patients who received DLI was significantly improved compared to a comparable historical cohort who received TCD BMT without DLI. After nonmyeloablative stem cell transplantation, the GVL effect induced by DLI may be sufficient to prevent relapse in leukemic patients with minimal residual disease.236
Even though DLI is performed when the patient is further removed from conditioning therapy and the associated inflammatory cytokine milieu, GVHD remains a major complication. Existing data on DLI show a GVHD incidence between 40% and 80%, and an attendant mortality of nearly 20%. The solution to this GVHD problem may lie in the correct timing and dosing of lymphocytes with the T-cell add-back, but neither of these parameters is known at this time. In patients given DLI for relapsed disease, GVHD incidence and severity have been decreased without loss of GVL by lowering the dose of lymphocytes infused,237 or by selectively depleting CD8+cells from the lymphocyte pool.221,238 For prophylactic DLI to be viable, it must reduce relapse rates without inducing GVHD. This may be possible by using DLI with a fixed number of selected T cells. A randomized trial of prophylactic DLI 6 months after TCD BMT has attempted to address this issue. Patients were randomized to receive either unselected or CD8+-depleted lymphocytes, adjusted to a CD4+ dose of 1.0 × 107CD4+ cells/kg. None of 9 patients receiving CD8+-depleted lymphocytes developed GVHD, compared to 6 of 9 patients receiving unselected cells (P = .003). All patients experienced conversion to complete donor chimerism, and relapse rates were similarly low in both groups.244
In recent years, researchers have introduced the herpes simplex thymidine kinase (HSTK) gene into donor T cells as a novel approach for controlling GVHD after DLI. The insertion of this “suicide” gene into donor T lymphocytes renders them susceptible to destruction with ganciclovir, and therefore provides a reliable means of eliminating these cells should severe GVHD develop after the infusion.239,240 The use of suicide gene therapy may also be applicable in conjunction with TCD BMT. Tiberghien and coworkers have recently shown that HSTK gene-modified T lymphocytes infused along with TCD marrow at the time of transplantation did not interfere with engraftment.241 More significantly, they were able to demonstrate long-lasting circulation of the gene-modified cells after transplantation, and in 2 of 3 patients, GVHD response on treatment with ganciclovir.241 A case of chronic cutaneous GVHD responsive to ganciclovir has also been recently reported in a patient who had received T cells bearing the HSTK gene at the time of BMT.242
Vaccine strategies
As new leukemia antigens are identified that are potential targets for the GVL response, allogeneic tumor vaccines may be developed to stimulate specific antileukemic activity without GVHD. As an example, a recently identified peptide (PR1) from the serine protease proteinase 3, which is aberrantly expressed in myeloid leukemias, may serve as a specific target for the GVL response.243 Investigators have successfully isolated PR-1–specific CTLs from the peripheral blood of patients with CML. PR-1–specific CTLs were found in 11 of 12 patients who responded to IFN therapy, but none of the 7 patients who did not respond. Furthermore, 6 of 8 patients who responded to allogeneic BMT had PR-1–specific CTLs in their blood, but in the one patient who relapsed after BMT, no PR-1–specific CTL could be detected.243Based on these results, clinical trials of a PR-1 peptide vaccine are currently being conducted in patients with myeloid leukemia, and adoptive cellular therapy using PR-1–specific CTLs may one day replace DLI as a method for eradicating leukemia cells without GVHD after TCD transplantation.
Conclusion
It is frustrating that after 2 decades, we have not been able to establish the role of TCD in transplantation. We still do not have a clear idea of who should receive a TCD BMT, or how marrow or stem cells should be purged. It remains unclear whether additional medications are needed to promote engraftment or control GVHD, or what the nature and timing of immunomodulating manipulations to reduce the risk of relapse should be. There have been no definitive randomized trials to answer these questions to date, partly because researchers have not been able to agree on a single TCD strategy or the best way to engineer a graft. The optimal number of T cells to include in the graft remains unknown and may in fact vary among donor-recipient pairs. It would be ideal to be able to manipulate different lymphoid subgroups responsible for GVHD and GVL. Being able to do so will be critical to the future success of allogeneic stem cell transplantation.
Supported in part by NIH grant AI29530.
R.J.S. is a clinical scholar of the Leukemia Society of America.
© 2001 by The American society of Hematology
References
Author notes
Robert J. Soiffer, Dana-Farber Cancer Institute, 44 Binney St, D1B58, Boston, MA 02115; e-mail:robert_soiffer@dfci.harvard.edu.