Abstract
Signaling motifs located within the cytoplasmic domain of certain receptors contribute to lysosome fusion. Most studies have described lysosome fusion with respect to endocytic receptors. Phagolysosome fusion has not been extensively studied. To test the hypothesis that the tail of FcγRIIA participates in phagolysosomal fusion, a “reverse” genetic complementation system was used. It was previously shown that complement receptor type 3 (CR3) can rescue the phagocytic activity of a mutant FcγRIIA lacking its cytoplasmic domain (tail-minus form). This system has allowed us to study Fcγ receptor–dependent phagocytosis and phagolysosome fusion in the presence and absence of the cytoplasmic domain of FcγRIIA. Fluorescent dextran was used to label lysosomes. After target internalization, wild-type FcγRIIA–mediated phagolysosome formation was observed as indicated by colocalization of fluorescent dextran and the phagosome. In addition, when studying mutants of FcγRIIA containing a full-length cytoplasmic tail with the 2 ITAM tyrosines mutated to phenylalanine, (1) phagocytosis was abolished, (2) CR3 restored phagocytosis, and (3) lysosomal fusion was similar to that observed with the wild-type receptor. In contrast, in the presence of CR3 and the tail-minus form of FcγRIIA, internalized particles did not colocalize with dextran. Electron microscopy revealed that the lysosomal enzyme acid phosphatase colocalized with immunoglobulin G–coated targets internalized by wild-type FcγRIIA but not by tail-minus FcγRIIA and CR3. Thus, the tail of FcγRIIA contributes to phagolysosome fusion by a mechanism that does not require a functional ITAM sequence.
Introduction
Phagocytosis is a crucial step toward the eventual destruction of foreign particles by the immune system. After separation from the plasma membrane, a phagosome must traffic to and fuse with lysosomes. Lysosomes contain a battery of hydrolytic enzymes within a low pH environment.1 Endosome-to-lysosome recognition is mediated by signaling motifs located within the cytoplasmic domains of certain receptors.2,3 Some investigators have suggested that the tyrosine-containing ITAM motif found within the cytoplasmic domain of receptors such as the γ chain of Fcγ receptor I (FcγRI) and FcγRIII and the cytoplasmic domain of FcγRIIA is responsible for phagocytic signaling in antibody-dependent phagocytosis.4,5 It is possible that signal sequences located in the cytoplasmic tails of these receptors are responsible for mediating trafficking to lysosomes and the eventual fusion of the 2 organelles. However, when the cytoplasmic domain of FcγRIIA or its crucial tyrosine residues are removed, phagocytosis is abolished.4 5 After the phagocytic activity of the receptor is lost, there is no mechanism to study downstream properties of the receptors such as lysosome fusion.
FcγRs are known to cooperate with complement receptors during immunoglobulin G (IgG)–dependent phagocytosis and oxidant production.6-9 One mechanism of Fcγ-to-complement receptor cooperation involves physical association of these receptors.10-13 These receptor interactions and cooperation phenomena have been extended to urokinase-type plasminogen activator receptors and CD14.8,14 It has previously been observed that complement receptor type 3 (CR3) (αM β2, CD11b/CD18) rescues the phagocytic activity of the disabled tail-minus form of FcγRIIA.11 The ability of CR3 to complement the phagocytic function of tail-minus FcγRIIA has allowed us to study the role of the cytoplasmic domain of FcγRIIA in phagolysosomal fusion.
In the current study we observed that CR3 reconstitutes FcγR-dependent phagocytosis in a FcγRIIA mutant where tyrosine residues are mutated to phenylalanine (FcγRIIA-ITAM mutant). By employing a reverse genetic complementation strategy and 2 mutant forms of FcγRIIA, we have studied the cytoplasmic domain's role in phagolysosome formation.
We examined the hypothesis that the cytoplasmic domain of FcγRIIA contributes to phagolysosome formation and observed that the cytoplasmic domain of FcγRIIA participates in phagolysosome fusion. This was shown by colocalization of IgG-coated cells with either fluorescent dextran or acid phosphatase, 2 independent experimental strategies to label lysosomes. Although wild-type FcγRIIA supported phagolysosome formation, the tail-minus form of FcγRIIA did not. However, FcγRIIA (ITAM mutant), complemented with CR3 to restore phagocytosis, retained their intrinsic lysosome signaling capacity. Thus, the cytoplasmic tail of FcγRIIA contributes to fusion of phagosomes with lysosomes.
Materials and methods
Cell culture and transfections
Chinese hamster ovary (CHO) cells were transfected by electroporation with a mixture of 1.5 μg pSVneo, 5 μg pBACD11b (generated by replacing the CD11a complementary DNA in pBACD11a15 with the CD11b complementary DNA,16 a gift from D. Hickstein (University of Washington, Seattle, WA), 5 μg pCMVBACD18, and 5 μg of either pRcCMVCD32 or a variant of this CD32 plasmid containing a tail-minus mutation, as described.4 Expansion and selection were performed as previously described.11 Seven different clones were generated: 161-24, which was not transfected but exposed to the transfection protocol; 161-84, which expressed only CR3; 131-3, which expressed wild-type FcγRIIA; 135-12, expressing FcγRIIA tailless alone; 169-8 and 169-24, which both express the FcγRIIA tailless and CR3; and 173-46, expressing both the wild-type FcγRIIA and CR3.
In addition, one crucial experiment is to compare wild-type FcγRIIA with both FcγRIIA tailless and an ITAM mutant of FcγRIIA that expresses a full-length FcγRIIA cytoplasmic domain with Tyr→Phe mutations in both of the ITAM motifs (FcγRIIA ITAM mutant). Therefore, we transiently transfected wild-type FcγRIIA, tailless FcγRIIA, and the ITAM mutant FcγRIIA into an untransfected CHO cell line or a CR3-expressing CHO cell line using FuGene6 transfection reagent. For experiments, cells were seeded onto 25-mm2coverslips and allowed to adhere overnight at 37°C in 5% CO2. Cells were tested for expression using both indirect immunofluorescence flow cytometry and fluorescence microscopy as previously described.11 Transient expression of FcγRIIA and FcγRIIA (ITAM mutant) was equivalent as detected by fluorescence-activated cell sorting. Mean fluorescence intensities of the transiently transfected receptors are shown in the figure legends (Figure 3).
Lysosome labeling
Transfectants were grown on glass coverslips (Corning, Corning, NY) overnight at 37°C. A total of 5 μg rhodamine-conjugated dextran (10 000 molecular weight; Molecular Probes, Eugene, OR) was added to each coverslip for 90 minutes at 37°C. Cells were washed with phosphate-buffered saline followed by addition of fresh media to the coverslips as described by Oh and Swanson.17Imaging of lysosomes was performed using an Axiovert 135 fluorescence microscope (Carl Zeiss, Thornwood, NY) using mercury illumination. Optical filters for rhodamine excitation and emission were 530DF22 and 590DF30, respectively (Omega, Brattlesboro, VT). Images were observed using an intensified charge-coupled device (ICCD) (Hamamatsu, Hamamatsu City, Japan) coupled to a Scion LG-3 (Scion, Frederick, MD) image capture board on a Dell Precision 410 Workstation (Round Rock, TX). Images were processed using Scion Image software.
Phagocytosis of erythrocytes
Sheep red blood cells (SRBCs) (Alsever; Rockland Scientific, Gilbertsville, PA) were opsonized with the highest subagglutinating concentration of IgG rabbit antisheep erythrocyte antibody (ICN, Costa Mesa, CA). Subsequently, antibody-coated cells (EAs) were added at a target-to-effector ratio of 10:1 (EA/transfectant). The EAs were incubated with transfectants for 45 minutes at 37°C in culture media. Coverslips were then placed on ice to stop phagocytosis. Bound external EAs were either removed by hypotonic lysis in 0.25 × phosphate-buffered saline or labeled with a secondary fluorescent anti-IgG. Therefore, external EAs become fluorescent, and internal EAs are not susceptible to the secondary labeling.
Fluorescence microscopy
Goat antirabbit IgG F(ab′)2 fragments conjugated with fluorescein isothiocyanate (ICN) were added to the coverslips for 30 minutes on ice to detect the external EAs. The coverslips were observed using bright-field microscopy or by fluorescence microscopy using the system described above. Narrow band-pass discriminating filters were used with excitation at 482 nm and emission at 530 nm for fluorescein isothiocyanate fluorescence (not shown).11
Electron microscopy
Transfectants expressing either wild-type FcγRIIA (131-3) or tailless FcγRIIA with CR3 (169-123) were incubated with opsonized sheep erythrocytes for 45 minutes at 37°C in culture media. The cells were washed and then fixed with glutaraldehyde overnight at 4°C. To detect the lysosomal compartment, we stained for acid phosphatase using modified Gormori media consisting of 13.9 mM β-glycerophosphate, 1 mM Pb(NO3)2, 0.05 M acetate buffer, 0.08% CaCl2, and 5% sucrose. Cells were treated with the acid phosphatase stain for 1 hour at 37°C with gentle agitation. The cells were washed extensively with cacodylate buffer and then postfixed with osmium tetroxide for 1 hour at room temperature. The cells were dehydrated and embedded in Spurr resin as described previously.18 Thin sections were viewed with a Joel 35e (Tokyo, Japan) electron microscope. Micrographs were taken using an in-column digital camera system coupled to a Macintosh G3 computer and processed with Adobe Photoshop 5.0. Quantitative data are combined with fluorescence data. Individual quantitative data are shown in the figure legends (Figures 2 and 4).
Results
Receptor expression and phagocytosis
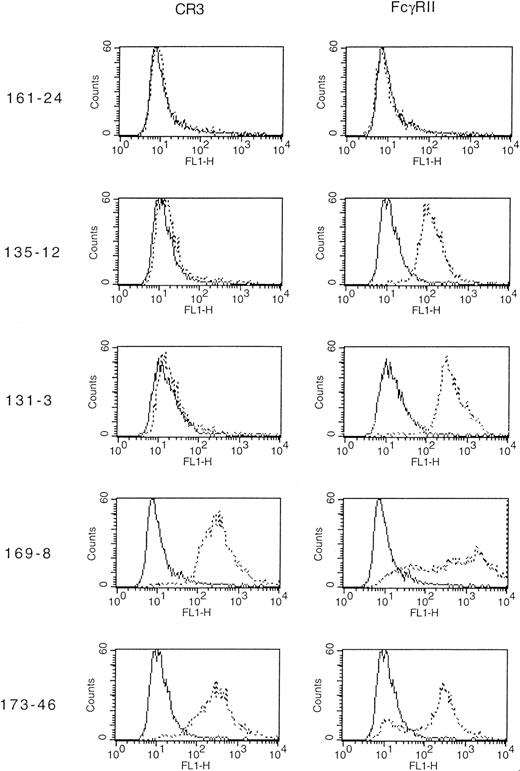
Transfected CHO cells were studied for expression of FcγRIIA and CR3 using flow cytometry (Figure 1). Several cell lines were produced. Clone 131-3 expressed wild-type FcγRIIA; 135-12 expressed the tail-minus mutant of FcγRIIA; and 161-24 expressed neither of the receptors but was exposed to the transfection protocol. Clones 169-8 and 169-23 both expressed the tailless mutant FcγRIIA in combination with CR3. We also constructed a wild-type FcγRIIA and CR3 clone (173-46). As shown in Figure 1, indirect immunofluorescence analysis confirmed the phenotypes of the cell lines. In addition, we used a phagocytosis-defective FcγRIIA that had a full-length cytoplasmic domain with only the tyrosine residues in each of the ITAM motifs mutated to phenylalanine (FcγRIIA ITAM mutant). This mutation has previously been shown to abolish IgG-dependent phagocytosis via FcγRIIA.5 We also transfected wild-type FcγRIIA, FcγRIIA tailless, and FcγRIIA (ITAM mutant) transiently into untransfected CHO cells or a CR3-expressing cell line. Expression was determined via indirect immunofluorescence quantitated by flow cytometry. Expression of wild-type FcγRIIA (MFI 97/89), tailless FcγRIIA (MFI 87/96), and this FcγRIIA (ITAM mutant) (MFI 93/91) were equivalent in CHO/CR3-transfected cells.
Indirect immunofluorescence flow cytometric analysis of cell lines expressing FcγRIIA (CD32) or CR3 (CD11b/CD18).
The indicated 5 clones were subjected to indirect immunofluorescence using primary murine monoclonal antibodies specific for FcγRIIA (CD32), CR3 (CD11b/CD18), or a negative control reagent. In each panel, the solid line represents cells stained with negative control reagent, whereas the dotted line indicates staining with the appropriate anti-CD antibody.
Indirect immunofluorescence flow cytometric analysis of cell lines expressing FcγRIIA (CD32) or CR3 (CD11b/CD18).
The indicated 5 clones were subjected to indirect immunofluorescence using primary murine monoclonal antibodies specific for FcγRIIA (CD32), CR3 (CD11b/CD18), or a negative control reagent. In each panel, the solid line represents cells stained with negative control reagent, whereas the dotted line indicates staining with the appropriate anti-CD antibody.
To confirm that the receptors were functional, we examined phagocytosis using IgG-coated sheep erythrocytes (EAs). After incubation of EAs with the transfectants for 30 minutes at 37°C, we observed that the wild-type FcγRIIA (clone 131-3) was capable of internalizing IgG-coated erythrocytes. However, the FcγRIIA tailless (clone 135-12) and the FcγRIIA (ITAM mutant) were not able to phagocytose EAs, as previously reported.4,5 11 We observed that the coexpression of CR3 with either of the mutant FcγRIIAs restored FcγR-dependent phagocytosis (Table 1).
Fluorescence detection of phagosome-lysosome fusion
We next studied whether the cytoplasmic tail of FcγRIIA participates in phagolysosomal fusion. Fluorescently labeled dextran was used to label lysosomes.17 Fluorescent dextran is taken up by pinocytosis and then delivered to lysosomes. This allows the fluorescent dextran to spill from the preloaded lysosomes into the phagosome. After incubation with dextran, the transfectants exhibited dextran located in small punctate vesicles when viewed with fluorescence microscopy (data not shown).
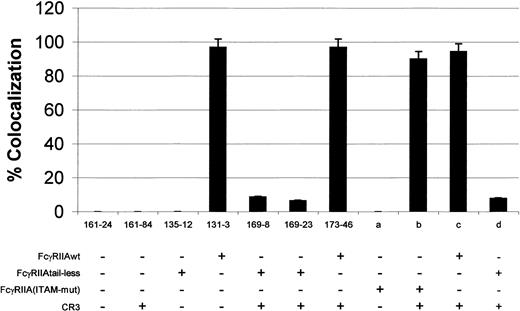
Previous work has shown that coexpression of CR3 and a phagocytosis-defective tailless FcγRIIA restored IgG-dependent phagocytosis.11 Similarily, studies have suggested that CR3 does not mediate phagolysosome fusion by itself (R.G.W., L.M.-B., R.F.T., H.R.P., unpublished observations, September 1999).19 Therefore, we used this approach, cotransfection of FcγRIIA and CR3, to examine postphagocytic events in the presence and absence of the cytoplasmic tail of FcγRIIA or in an ITAM mutant of FcγRIIA. As shown in Figure2, wild-type FcγRIIA (clone 131-3) transfectants exhibited colocalization of fluorescent dextran with the internalized IgG-coated particle. This effect was seen as soon as 15 minutes after addition of targets and did not change significantly up to 60 minutes after phagocytosis. In addition, more than 95% (101 of 104 dextran studies) of the internalized targets were positive for lysosome fusion as determined by rhodamine dextran colocalization (Figure 3 and Table 1). However, when the cell lines containing the mutant tailless form of FcγRIIA in the presence of CR3 were studied (clones 169-8 and 169-23), very little colocalization of IgG-coated cells with the dextran was observed (Figures 2, 3, and Table 1). Little or no colocalization of dextran with EAs was observed from 15 minutes to 60 minutes after phagocytosis. Internalized targets displayed fusion with lysosomes in 6.4% and 8.7% of the cells for clones 169-8 and 169-23, respectively. Table 1 shows pooled data from both fluorescent dextran and acid phosphatase studies. Individually, clone 169-8 showed colocalization in 2 of 35 targets studied. Similarily, clone 169-23 contained 3 of 41 targets colocalized with dextran. In addition, tailless FcγRIIA was transiently transfected into a CR3-expressing stable cell line (Figure 3, column d). Similar phagolysosomal fusion data were observed as with cells stably expressing both CR3 and tailless FcγRIIA. The FcγRIIA ITAM mutant without CR3 is unable to induce phagocytosis of IgG-coated cells and, therefore, no lysosomal fusion can occur (Figure 3, column a). However, in the presence of CR3 and FcγRIIA ITAM mutant, phagocytosis was restored, and near wild-type levels of lysosome fusion was detected (Figure 3, column b). We also studied clone 173-46, which expressed wild-type FcγRIIA and CR3 to determine if CR3 might affect phagolysosome formation. As seen in Figure 3, expression of CR3 did not affect the ability of wild-type FcγRIIA to participate in phagolysosome fusion (173-46 and Figure 3, column c).
Differential interference contrast and fluorescence micrographs displaying colocalization of internalized targets and fluorescent dextran.
(A,C) Differential interference contrast (DIC) images of transfectants. (B,D) Fluorescent micrographs indicating the location of the preloaded fluorescent dextran. Panels A and B show clone 131-3 (n = 3) expressing wild-type FcγRIIA. Panels C and D show clone 169-23 (n = 5), which expresses mutant tail-minus FcγRIIA and CR3. Colocalization of the fluorescent dextran (arrowheads) can be observed with the wild-type FcγRIIA but not in the clone expressing mutant tailless FcγRIIA and CR3 (169-23). Of 104 internal targets, 101 were colocalized with fluorescent dextran in wild-type FcγRIIA-transfected cells. However, tailless FcγRIIA only showed target colocalization with dextran in 3 of 41 cases (original magnification × 1100). TRITC indicates tetramethylrhodamine isothiocyanate.
Differential interference contrast and fluorescence micrographs displaying colocalization of internalized targets and fluorescent dextran.
(A,C) Differential interference contrast (DIC) images of transfectants. (B,D) Fluorescent micrographs indicating the location of the preloaded fluorescent dextran. Panels A and B show clone 131-3 (n = 3) expressing wild-type FcγRIIA. Panels C and D show clone 169-23 (n = 5), which expresses mutant tail-minus FcγRIIA and CR3. Colocalization of the fluorescent dextran (arrowheads) can be observed with the wild-type FcγRIIA but not in the clone expressing mutant tailless FcγRIIA and CR3 (169-23). Of 104 internal targets, 101 were colocalized with fluorescent dextran in wild-type FcγRIIA-transfected cells. However, tailless FcγRIIA only showed target colocalization with dextran in 3 of 41 cases (original magnification × 1100). TRITC indicates tetramethylrhodamine isothiocyanate.
Percent lysosome marker colocalization with internalized target.
Cells were preloaded with rhodamine dextran and then allowed to internalize opsonized erythrocytes or were stained for acid phosphatase after phagocytosis. Lysosome fusion is determined by the colocalization of the internalized target and the lysosomal marker. As shown, clones 131-3 and 173-46 expressing wild-type FcγRIIA show more than 97% of the internalized targets colocalized with one of the markers. However, internalization via tailless FcγRIIA, using CR3 to mediate phagocytosis (clones 169-8 and 169-23, n = 5 for both lines), exhibited very little colocalization of the targets with either fluorescent dextran or acid phosphatase (P < .001 comparing wild-type FcγRIIA with tailless FcγRIIA). Columns a-d represent experiments with transient transfections of the FcγRIIA constructs. FcγRIIA immunoreceptor tyrosine-based activation motif (ITAM) mutants (MFI 93) displayed no colocalization of targets and marker due to the absence of phagocytosis (column a). However, in the presence of CR3 to restore phagocytosis, FcγRIIA ITAM mutants (column b) (MFI 91) displayed near wild-type FcγRIIA (column c) (MFI 89) levels of target/marker colocalization. Tailless FcγRIIA (column d) transiently transfected (MFI 96) displayed very little colocalization of targets with dextran.
Percent lysosome marker colocalization with internalized target.
Cells were preloaded with rhodamine dextran and then allowed to internalize opsonized erythrocytes or were stained for acid phosphatase after phagocytosis. Lysosome fusion is determined by the colocalization of the internalized target and the lysosomal marker. As shown, clones 131-3 and 173-46 expressing wild-type FcγRIIA show more than 97% of the internalized targets colocalized with one of the markers. However, internalization via tailless FcγRIIA, using CR3 to mediate phagocytosis (clones 169-8 and 169-23, n = 5 for both lines), exhibited very little colocalization of the targets with either fluorescent dextran or acid phosphatase (P < .001 comparing wild-type FcγRIIA with tailless FcγRIIA). Columns a-d represent experiments with transient transfections of the FcγRIIA constructs. FcγRIIA immunoreceptor tyrosine-based activation motif (ITAM) mutants (MFI 93) displayed no colocalization of targets and marker due to the absence of phagocytosis (column a). However, in the presence of CR3 to restore phagocytosis, FcγRIIA ITAM mutants (column b) (MFI 91) displayed near wild-type FcγRIIA (column c) (MFI 89) levels of target/marker colocalization. Tailless FcγRIIA (column d) transiently transfected (MFI 96) displayed very little colocalization of targets with dextran.
Electron microscopy of phagosome-lysosome fusion
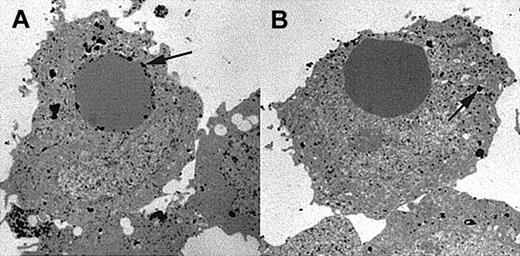
As a second independent means of detecting phagosome-lysosome fusion following phagocytosis, we employed electron microscopy using a specific lysosomal stain. Acid phosphatase is an enzyme specific for lysosomes and has been used extensively to stain CHO cells.20 Therefore, we used this enzyme to detect the localization of lysosomal enzymes inside cells. After incubation of transfectants expressing either wild-type FcγRIIA or tailless FcγRIIA in the presence of CR3 with IgG-coated sheep erythrocytes, the cells were fixed and stained for acid phosphatase. After embedding, thin sections were viewed with an electron microscope. Acid phosphatase appeared as dark electron dense patches, revealing the location of lysosomal enzyme activity. Figure 4 shows representative micrographs of experiments repeated on 4 independent occasions. As shown, in the presence of the wild-type FcγRIIA (clone 131-3) we observed acid phosphatase staining near the internalized target, indicating phagolysosomal fusion in 61 of 63 cases studied with acid phasphatase (Figure 4A). However, cells expressing the tail-minus form of FcγRIIA (clone 169-23) did not support phagolysosome formation (only 9 of 97 targets colocalized with acid phosphatase). Thus, the acid phosphatase staining was found throughout the entire cytoplasm as punctate granules and was not localized near internalized targets (Figure 4B). These results suggest that the cytoplasmic domain of FcγRIIA targets the internalized particle for fusion with lysosomes.
Electron micrographs showing location of acid phosphatase, a lysosomal enzyme.
Micrographs are representative examples of experiments repeated 4 times. Transfectant CHO cells were allowed to internalize IgG-coated EAs and then fixed and stained for acid phosphatase. (A) Internalization via wild-type FcγRIIA (clone 131-3) exhibits strong acid phosphatase activity near the internalized target in 61 of 63 internal targets counted (n = 4 for both lines). (B) However, internalization via tail-minus FcγRIIA (clone 169-23), using CR3 to mediate the phagocytic signal, does not show colocalization of the target with acid phosphatase activity (arrows). When counted, only 9 of 97 internal targets show colocalization with acid phasphatase (original magnification × 6000).
Electron micrographs showing location of acid phosphatase, a lysosomal enzyme.
Micrographs are representative examples of experiments repeated 4 times. Transfectant CHO cells were allowed to internalize IgG-coated EAs and then fixed and stained for acid phosphatase. (A) Internalization via wild-type FcγRIIA (clone 131-3) exhibits strong acid phosphatase activity near the internalized target in 61 of 63 internal targets counted (n = 4 for both lines). (B) However, internalization via tail-minus FcγRIIA (clone 169-23), using CR3 to mediate the phagocytic signal, does not show colocalization of the target with acid phosphatase activity (arrows). When counted, only 9 of 97 internal targets show colocalization with acid phasphatase (original magnification × 6000).
Discussion
The goal of this study was to determine if the cytoplasmic tail of FcγRIIA participates in phagolysosomal fusion. Previous studies have shown that the cytoplasmic tail of FcγRIIA is necessary for phagocytosis.4,5 Specifically, the tail's ITAM (YXXL) sequence of the FcγRIIA cytoplasmic domain is required for phagocytosis.5 Although it is known that a dileucine motif located in the cytoplasmic domain of FcγRIIB mediates endocytosis and basolateral sorting in MDCK cells,2 the potential role of FcγRIIA's cytoplasmic domain in lysosomal delivery is unknown. Because the FcγRIIA tail does not possess a known phagolysosomal delivery sequence such as a dileucine motif, the tail's potential ability to support phagolysosome fusion is uncertain. However, because phagolysosome formation requires phagocytosis, phagocytic signaling must remain intact. To dissect the mechanisms involved in phagolysosome formation from phagocytosis (internalization) per se, we have genetically complemented, with CR3, the phagocytic function of a FcγRIIA tail-minus mutant and ITAM mutants of FcγRIIA. Thus, in this study we use CR3 as a mechanism to allow particles to be internalized in the absence of the normal FcγRIIA phagocytic machinery. We now show for the first time that the cytoplasmic tail of FcγRIIA participates in phagolysosomal fusion and that this signal is distinct from a functional ITAM.
As mentioned above, one consensus sequence for endolysosome formation is the dileucine sequence. This motif has been described in various receptors such as FcγRIIB, the cation-dependent mannose 6–phosphate receptor, and the LDL receptor.2,21,22 These motifs may be involved in the direct interaction of the receptors with lysosomes, or they may act to recruit other cytoplasmic helper proteins such as Rho, which could then direct the receptors toward lysosomes. Alternatively, the endolysosome fusion signal may not be a linear sequence of amino acids but, rather, a 3-dimensional motif such as that found on nascent lysosomal enzymes in the endoplasmic reticulum.21 22
Several motifs have been shown to be important in mediating phagocytosis, delivery to intracellular compartments, and cytoskeletal manipulation. Various ITAMs or ITAM-like motifs and their likely 3-dimensional structures have been implicated in the recruitment and binding of signaling molecules.23-25 One such heavily studied molecule is Syk kinase. Syk has been suggested to be a crucial mediator of Fcγ receptor–mediated phagocytosis and transport to lysosomes.23,24 In addition, one study has suggested that kinase activity is required for directing FcγRI to the lysosomal compartment.26 It is likely that the ability to recruit Syk leads to the further recruitment of other downstream signaling molecules.
Phagolysosome delivery motifs likely contribute to the recruitment of additional signaling components such as GTP-binding proteins (eg, Rho). Rho has also been shown to be a crucial partner in mediating Fcγ receptor phagocytosis27 and in altering the actin cytoskeleton in response to extracellular signals.28-32Other molecules such as phosphatidylinositol-3 kinase are also recruited to the sites of engulfment to mediate phagocytosis in other cell types, possibly participating in receptor-mediated phosphorylation.33,34 Additional studies have shown the importance of Rho in the regulation of endosome dynamics.35 These data show that the effects we observed in the transfectant studies, specifically where the wild-type receptor aggregated many internalized targets into one or more large phagosomes while the tailless FcγRIIA with CR3 did not mediate this aggregation of endosomes, may be due to Rho recruitment. FcγRIIA may contain a signal motif in the cytoplasmic domain that is responsible for the eventual recruitment of Rho.
In this study, using a transfectant CHO model system, we have shown that the cytoplasmic tail of FcγRIIA participates in phagolysosome fusion. The data presented in this study are relevant to studies involving phagocytosis and phagolysosome fusion of various microbes such as Mycobacterium tuberculosis and Toxoplasma gondii.36 37 The suggestion that M tuberculosis does not allow phagolysosome fusion unless opsonized with IgG supports our studies that the cytoplasmic tail of FcγRs (in this study FcγRIIA) participates in phagolysosome formation. Overall, lysosomal targeting/fusion may be a more complex phenomenon than initially hypothesized. The approach used in this study may be useful in future studies involving other receptor interactions and intracellular signaling pathways in addition to elucidating the mechanism(s) by which particles are internalized and trafficked throughout the cytoplasm.
The authors thank Dr Linda Hazlett and Ron Barrett of the electron microscope core facility of Wayne State University School of Medicine for their assistance with the electron microscopy.
Supported by grants AI-27409 (H.R.P.), CA-39064 (R.F.T.), HL-40387 and HL-28207 (A.D.S.), and training grant 5T32-AR07442 (R.G.W.), all from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alan D. Schreiber, Div of Hematology and Oncology, University of Pennsylvania School of Medicine, 705 BRB II/III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail:schreibr@mail.med.upenn.edu.