Stem cell factor (SCF) binds to c-Kit and is an important mediator of survival, growth, and function of hematopoietic progenitor cells and mast cells. Lyn and other Src family members are activated by SCF and associate with phosphorylated tyrosine residues in the c-Kit juxtamembrane region. However, studies using c-Kit mutants incapable of directly recruiting Src family members suggest this kinase family plays a minimal role in c-Kit stimulus-response coupling mechanisms. The objective of this study was to specifically target Lyn and subsequently address its role in SCF-mediated responses of primary hematopoietic progenitor cells and mast cells. To this end, a dominant-inhibitory Lyn mutant and Lyn-deficient mice were used. Transfection of normal murine mast cells with kinase-inactive Lyn impaired SCF-induced growth. Further, SCF-induced proliferation and chemotaxis of Lyn-deficient mast cells were less than for wild-type mast cells. SCF-induced growth of progenitor cells lacking Lyn was also reduced compared with that of wild-type progenitor cells. Impairment of SCF-mediated responses of Lyn-deficient mast cells and progenitor cells did not result from reductions in surface expression of c-Kit. These studies demonstrate that Lyn is required for normal SCF-mediated responses of primary progenitors and for a differentiated lineage.
Introduction
Hematopoiesis is regulated by multiple mechanisms, including interaction of cell surface receptors with growth factors and cytokines. These molecules aid in the survival, proliferation, and differentiation of hematopoietic progenitors. One superfamily of receptors that binds hematopoietic growth factors is the receptor tyrosine kinase (RTK) superfamily (reviewed in 1). The cytokine receptor superfamily also plays an important role in this process (reviewed in 2).
c-Kit is an RTK encoded by the c-kit proto-oncogene (reviewed in3-5). The c-Kit ligand is stem cell factor (SCF). The absence of either SCF or c-Kit is lethal, and decreases in expression or function of ligand or receptor results in macrocytic anemia, mast cell deficiency, aberrations in pigmentation, and sterility (reviewed in 3,6,7). Inappropriate expression or constitutive activation of c-Kit has also been associated with human diseases, including mastocytosis, myeloid leukemia, small-cell lung carcinoma, and gastrointestinal stromal cell tumors.8-12
The critical role of c-Kit in normal physiology and the association of activated mutants with human disease highlights the importance of understanding its mechanism of action. Recent work has demonstrated that SCF activates the Ras-Raf-Map kinase cascade, phosphatidylinositol 3 kinase (PI3K), and, in some lineages, the Jak/Stat pathway (reviewed in 13). SCF induces the association of Src, Lyn, and Fyn with c-Kit and the activation of multiple Src family members.14-20 Reduction in the expression of Lyn using antisense oligonucleotides inhibits SCF-induced proliferation.15 In addition, treatment of cells with PP1, a Src family inhibitor, does not alter the in vitro kinase activity of c-Kit but impairs SCF-induced cell-cycle progression and growth.15,20,21 These data suggest that Lyn has an important function in SCF-induced proliferation in hematopoietic cells. However, several lines of evidence conflict with this hypothesis. First, findings using leukemic cell lines treated with pharmacologic inhibitors and antisense oligonucleotides may not be representative of normal physiology. Second, studies with c-Kit mutants incapable of directly associating with Src family members suggest this kinase family does not play a significant role in SCF-mediated responses.17,19 However, indirect activation of Src family members could be induced through other regions of c-Kit. Third, the phenotype of Lyn-deficient mice is not consistent with the abrogation of SCF-mediated responses, though compensatory mechanisms in vivo could mask highly specific defects in SCF responsiveness.22-24
To understand the role of Lyn in c-Kit stimulus-response coupling mechanisms in primary hematopoietic cells, we examined SCF-mediated responses of Lyn-deficient mast cells and lineage negative (Lin−) progenitor cells. These cells represent differentiated and undifferentiated lineages of hematopoietic cells that are both important target populations for SCF. This is of particular importance because previous studies have shown the protein tyrosine phosphatase Shp1 plays a role in SCF-induced growth of Lin− progenitor cells but not of mast cells.25 Thus, there are lineage-specific differences in SCF signaling pathways. As a second approach to specifically target Lyn, normal bone marrow–derived mast cells were transfected with a kinase-inactive Lyn mutant. Expression of kinase-inactive Src family members has been shown to inhibit Src-mediated responses through a dominant-inhibitory mechanism.
Our studies indicate a reduction in both the frequency and the proliferative potential of SCF-responsive progenitor cells from Lyn-deficient mice. Further, proliferation and chemotaxis of Lyn-deficient mast cells is reduced compared to normal mast cells. Transfection of normal mast cells with a dominant-inhibitory Lyn mutant dramatically inhibits SCF-induced growth. These findings demonstrate that Lyn is required for normal proliferation and chemotaxis responses mediated by c-Kit in progenitor cells and mast cells.
Materials and methods
Animals
Wild-type and Lyn-deficient mice were generated as described and maintained in a pathogen-free environment.24 Animal care was provided in accordance with the procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (publication number 86-23, 1985). Mice between the ages of 8 and 14 weeks were used for the study.
Mast cells
Bone marrow–derived mast cells (BMMCs) were generated as described.26 In brief, bone marrow was harvested from mouse femurs, and mononuclear cells were isolated using lymphocyte separation media (ICN Biomedicals, Aurora, OH). Bone marrow mononuclear cells were maintained at 5 × 105 cells/mL in mast cell growth media consisting of Iscove modified Dulbecco medium (IMDM; JRH Biosciences, Lenexa, KS), 10% fetal calf serum (FCS; Hyclone, Salt Lake City, UT), 2 mM L-glutamine, 1% penicillin-streptomycin, and 30 ng/mL murine interleukin-3 (IL-3) (Peprotech, Rocky Hills, NJ). After 4 to 5 weeks in culture, cytochemistry and fluorescence-activated cell separation (FACS) analysis were performed to confirm the presence of BMMC. FACS analysis of high-affinity immunoglobulin E (IgE) receptors and c-Kit was performed as previously described.27Lyn-deficient mast cells generally express higher levels of c-Kit on the cell surface, and this difference often increases as they mature. Therefore, in some cases, wild-type and Lyn-deficient mast cells were stained for c-Kit, and cells with similar mean fluorescent intensity of c-Kit staining were isolated by FACS and used for studies.
To purify Lin−, c-Kit+ hematopoietic progenitor cells, mononuclear cells were isolated from femur bone marrow as described above. Lineage-committed cells were removed through negative selection. Specifically, bone marrow mononuclear cells were incubated with a cocktail of rat monoclonal antibodies (1 μg/106 cells) specific for murine CD8, CD4, Mac-1, Gr-1, and CD45R/B220 (Pharmingen, San Diego, CA), washed, and incubated with sheep anti–rat IgG coupled to magnetic beads (Dynal, Oslo, Norway). Lineage-committed cells were then removed using a magnet. Lin− progenitor cells were stained with antibody specific for murine c-Kit (Pharmingen) and aseptically sorted using FACS. Sorting was performed using a FACSstar Plus flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Cells that expressed c-Kit were collected for use in subsequent experiments.
Site-specific mutagenesis of wild-type Lyn plasmid DNA
Human Lyn complementary DNA (cDNA) in the pSVL vector (Pharmacia Biotech, Piscataway, NJ) was kindly provided by Dr Becky Vonakis and Dr Henry Metzger (NIAMS/NIH, Bethesda, MD). Site-directed mutagenesis was performed on the human Lyn cDNA using the Stratagene (La Jolla, CA) Quickchange kit according to the manufacturer's directions. Custom oligonucleotide primers (Life Technologies, Rockville, MD) were designed to convert lysine 275 to leucine. Lysine 275 is required for Lyn to bind adenosine triphosphate and subsequent kinase activity.28 29 Mutagenesis was confirmed by DNA sequencing using a Perkin-Elmer (Wellesley, MA) Dye Terminator Cycle Sequencing Reaction kit with AmpliTaq DNA polymerase. The sequence was analyzed using ABI Prism software, version 2.1.0. Immune complex kinase assays were performed on cells transfected with the K275L Lyn mutant and confirmed the loss of kinase activity (data not shown).
Cell transfection
BMMC were incubated for 4 to 6 hours in fresh growth medium, washed twice, and resuspended in IMDM supplemented with 10% FCS, 2 mML-glutamine, and 1% penicillin-streptomycin. Aliquots of cells (107/250 μL) were placed in electroporation cuvettes (4-mm gap) and incubated for 10 minutes at room temperature, and plasmid was added at the indicated concentrations. After a 5-minute incubation at room temperature, cells were electroporated (Bio-Rad Gene Pulser, Hercules, CA) at 280 V and 960 μF. After additional incubation for 5 minutes at room temperature, cells were transferred to a tissue culture flask and resuspended in mast cell growth media. In some experiments, cells were cotransfected with plasmid encoding green fluorescence protein (GFP) to determine the frequency of transfection. This ranged from 15% to 40%, depending on the experiment. Expression of GFP was assessed using a Nikon (Tokyo, Japan) Diaphot microscope. Cells were harvested at the indicated times and lysed in buffer containing 1% Triton X-100, 10 mM Tris-HCl (pH 7.5), 50 mM NaCl, 5 mM EDTA, 30 mM sodium pyrophosphate, 25 mM β-glycerol phosphate, 5 mM sodium orthovanadate, 0.1% P-nitrophenyl phosphate, and 1 mM phenylmethylsulfonyl fluoride.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotting
Proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Immobilon (Millipore, Bedford, MA). Radiolabeled proteins were visualized using autoradiography, and Lyn protein was immunoblotted as described.15 20 Antibody specific for murine Lyn was purchased from Wako Pure Chemical Industries (clone 8; Osaka, Japan), and a monoclonal antibody that recognized human Lyn was purchased from Transduction Laboratories (Lexington, KY).
Proliferation assays, cell-cycle analysis, and chemotaxis
Proliferation was assessed using tritiated thymidine or by cell counts. For tritiated thymidine assays, wild-type and Lyn-deficient mast cells were washed twice and resuspended at 1 × 105cells/mL in serum-free QBSF-58 media (Quality Biological, Gaithersburg, MD). Aliquots of cells (100 μL) were placed in 96-well plates, and 100 μL media alone or media supplemented with murine SCF, murine IL-3, or phorbol 12-myristate 13-acetate (PMA; Sigma, St Louis, MO) were added to triplicate wells at the indicated concentrations. In studies of transfected mast cells, the microtiter plates were incubated with growth factors for 24 hours at 37°C, 5% CO2. Untransfected mast cells were incubated for 60 hours under the same conditions. Each well of the microtiter plate was then pulsed with 1 μCi [3H]-thymidine (6.7 Ci/mM; NEN Life Science Products, Boston, MA) overnight and harvested (Tomtec Mach II semi-automatic cell harvester) onto glass fiber filter paper (Wallac, Turku, Finland). Filters were dried and counted in a liquid scintillation counter (model 1205; Pharmacia Biotech). Proliferation assays with progenitor cells were performed as described above, except that cells were resuspended at 5 × 104 cells/mL in QBSF-60 media (Quality Biological), and the plates were incubated for 4 days before harvest.
Cell-cycle analysis was performed as follows. Murine BMMC were incubated for 18 hours in IMDM supplemented with 10% FCS but no growth factors, and they were harvested at the unstimulated time-point (T = 0). Murine SCF (100 ng/mL) was added, and cells were harvested at the times indicated in the text. For harvest, 2 × 106cells were washed once in cold phosphate-buffered saline (PBS), resuspended in 1 mL 70% methanol in PBS, and stored at 4°C. After cells from all time-points were collected, they were pelleted and resuspended in 1 mL propidium iodide (Sigma) solution (50 μg/mL in 0.1% sodium-citrate and 0.1% Triton-X) and incubated with RNAse A (0.5 μg/mL) for 30 minutes at room temperature. Relative DNA content was analyzed by FACS (Becton Dickinson).
For chemotaxis assays, wild-type and Lyn-deficient mast cells were washed twice with IMDM media without growth factors. Cells were then suspended in IMDM media supplemented with 0.5% bovine serum albumin (migration buffer). A total of 2.5 × 104 cells in 25 μL migration buffer was loaded on the top well of a chemotaxis chamber (96-well Chemotx, 5-μm pore size, 3.2-mm diameter; Neuroprobe, Gaithersburg, MD). The optimal concentration for SCF (100 ng/mL) or PMA (50 ng/mL) in 30 μL was added to the bottom wells of the chemotaxis chamber. Cells were allowed to migrate for 3 hours at 37°C in a 5% CO2 incubator. Cells that migrated were collected from the bottom wells, counted, and expressed as percentages of the total number of cells loaded at the top of the chemotaxis chamber. Chemokinesis was assessed by comparison of the migration of cells in the presence of SCF in both the upper and the lower wells.
Colony assays in methylcellulose
Lin− wild-type and Lyn-deficient progenitor cells selected for the expression of c-Kit were resuspended in serum-free QBSF-60 (Quality Biological). Methylcellulose (Methocult M3100; Stem Cell Technologies, Vancouver, BC, Canada) was diluted to a final concentration of 1% in QBSF-60, 2 mM L-glutamine, and 100 U/mL penicillin-streptomycin. Two milliliters methylcellulose solution, containing 5000 cells in the presence or absence of 100 ng/mL murine SCF, was dispensed into each of 3 small Petri dishes. Petri dishes were incubated for 7 to 10 days at 37°C, 5% CO2. Colonies were then counted and scored for size. Small colonies were defined as 10 to 25 cells, medium colonies as 25 to 100 cells, and large colonies were 100 cells or more.
Results
SCF-induced growth of Lyn-deficient progenitor cells is reduced
SCF activates Lyn in the megakaryoblastic cell line Mo7e and in fetal liver cells.15 20 These findings led us to hypothesize that Lyn plays an important role in SCF-mediated responses of progenitor cells. To test this hypothesis, we examined the capacity of Lin− hematopoietic progenitor cells from Lyn-deficient animals to respond to SCF in vitro.
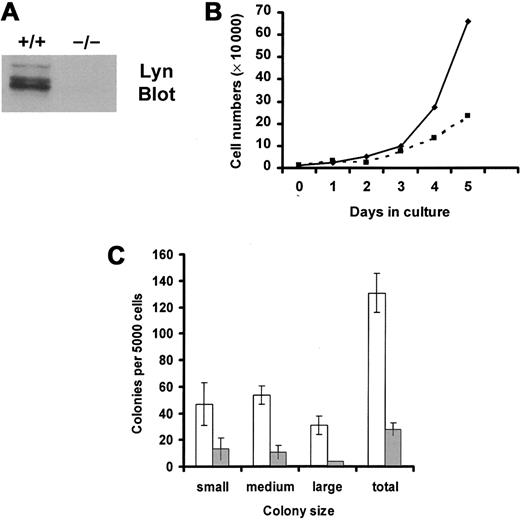
Lin− progenitors were isolated from murine bone marrow mononuclear cells by negative selection and were selected for the expression of murine c-Kit using FACS. Percentages of progenitor cells that expressed c-Kit in the resultant population of sorted cells were 96% of wild-type cells and 98% of Lyn-deficient cells. Mean fluorescence intensities of c-Kit expression were 95 relative units for wild-type progenitor cells and 108 for Lyn-deficient progenitor cells. Thus, FACS selection of Lin− progenitor cells that express c-Kit permitted the comparison of equivalent numbers of potential SCF responders in the knockout and wild-type groups. Figure1A demonstrates that Lyn protein was not detected in tissues from Lyn-deficient mice.
SCF-induced growth of Lyn-deficient progenitor cells is reduced compared with wild-type controls.
(A) Lyn-deficient mice do not express Lyn protein. Lysates were generated from wild-type (+/+) and Lyn-deficient (−/−) mast cells. Equivalent cell units were resolved by SDS-PAGE, transferred to Immobilon, and immunoblotted with antibody specific for murine Lyn. (B) Lyn-deficient progenitor cells (▪) proliferated more slowly than wild-type cells (♦) in response to SCF. Mononuclear cells from bone marrow from wild-type and Lyn-deficient mice were isolated using lymphocyte separation media. Lin− progenitor cells were purified by negative selection, as described in “Materials and methods.” These cells were stained with antibody specific for murine c-Kit and were isolated using FACS. The resultant population of Lin−, c-Kit+(LinnegKitpos) progenitor cells was resuspended in QBSF-60 (serum-free) media supplemented with 100 ng/mL murine SCF, and the numbers of viable cells were counted each day. Data are representative of results from 3 experiments. (C) SCF-induced colony formation of Lyn-deficient progenitors (░) is reduced compared with normal cells (■). Wild-type and Lyn-deficient LinnegKitpos progenitor cells were resuspended in 1% methylcellulose with QBSF-60 media in the presence or absence of murine SCF (100 ng/mL). Five thousand cells were plated per 35-mm2 Petri dish. After an 8-day incubation, the size and number of colonies were determined for each group. Data represent the mean and standard deviation of triplicate samples. No colonies formed in the absence of SCF. Small colonies were defined as those containing 10 to 25 cells, medium colonies as those containing 25 to 100 cells, and large colonies as those containing 100 cells or more.
SCF-induced growth of Lyn-deficient progenitor cells is reduced compared with wild-type controls.
(A) Lyn-deficient mice do not express Lyn protein. Lysates were generated from wild-type (+/+) and Lyn-deficient (−/−) mast cells. Equivalent cell units were resolved by SDS-PAGE, transferred to Immobilon, and immunoblotted with antibody specific for murine Lyn. (B) Lyn-deficient progenitor cells (▪) proliferated more slowly than wild-type cells (♦) in response to SCF. Mononuclear cells from bone marrow from wild-type and Lyn-deficient mice were isolated using lymphocyte separation media. Lin− progenitor cells were purified by negative selection, as described in “Materials and methods.” These cells were stained with antibody specific for murine c-Kit and were isolated using FACS. The resultant population of Lin−, c-Kit+(LinnegKitpos) progenitor cells was resuspended in QBSF-60 (serum-free) media supplemented with 100 ng/mL murine SCF, and the numbers of viable cells were counted each day. Data are representative of results from 3 experiments. (C) SCF-induced colony formation of Lyn-deficient progenitors (░) is reduced compared with normal cells (■). Wild-type and Lyn-deficient LinnegKitpos progenitor cells were resuspended in 1% methylcellulose with QBSF-60 media in the presence or absence of murine SCF (100 ng/mL). Five thousand cells were plated per 35-mm2 Petri dish. After an 8-day incubation, the size and number of colonies were determined for each group. Data represent the mean and standard deviation of triplicate samples. No colonies formed in the absence of SCF. Small colonies were defined as those containing 10 to 25 cells, medium colonies as those containing 25 to 100 cells, and large colonies as those containing 100 cells or more.
We next compared SCF-induced growth of wild-type and Lyn-deficient progenitor cells for a 5-day culture period in serum-free media. These data, shown in Figure 1B, demonstrated a reduction in the growth rate of Lyn-deficient progenitor cells in response to SCF. Reductions in SCF-induced proliferation could have resulted from a decrease in the numbers of cells that responded to SCF, a decrease in the proliferative potential of responding cells, or both. To assess these possibilities, colony formation in methylcellulose was assessed. Although no colonies were formed by either group in the absence of SCF, SCF induced smaller colonies of Lyn-deficient progenitors than the normal progenitors (Figure 1C). Further, the total number of colonies formed after culture in SCF was also decreased in the Lyn-deficient progenitors and in the normal progenitor cells (28 vs 131 colonies, respectively). This indicates that expression of Lyn increased the frequency of SCF-responsive progenitor cells and increased the proliferative potential. Similar results were obtained when SCF-induced colony formation was assessed in single-cell colony assays in liquid culture. Wild-type progenitors formed 150 colonies, of which 22 were large, after a 10-day incubation in SCF. In contrast, Lyn-deficient progenitors formed only 2 large colonies of 46 total colonies.
Transfection with kinase-inactive Lyn inhibits SCF-induced growth of normal mast cells
SCF is also critical in the development and function of mast cells. Therefore, we next examined the role of Lyn in SCF-mediated responses of this differentiated lineage by transiently transfecting normal murine BMMCs with a kinase-inactive (K275L) human Lyn mutant and examining SCF-induced proliferation. Figure2A (lower panel) demonstrates that SCF-induced growth of murine mast cells transfected with kinase-inactive human Lyn was dramatically reduced compared to cells transfected with the vector control. Immunoblotting with antiserum specific for human Lyn demonstrated that the human Lyn protein was expressed in transfected cells (Figure 2A, upper panel). In some experiments, such as the one shown in Figure 2B, SCF-induced growth was slightly enhanced after transfection of mast cells with wild-type Lyn plasmid DNA. However, this mild effect was not observed in all experiments. Immunoblotting with antisera specific for human Lyn protein demonstrated that transfected cells expressed similar amounts of wild-type and mutant Lyn protein (Figure 2B, upper panel). These results indicate that the inhibition of SCF-induced growth by the kinase-inactive Lyn mutant does not result from toxicity associated with expressing high levels of Lyn protein.
Ectopic expression of a kinase-inactive Lyn mutant inhibits SCF-induced proliferation.
(A) Transfection of normal mast cells with kinase-inactive Lyn inhibits SCF-induced growth. Normal murine BMMCs were transfected with either kinase-inactive (KI) human Lyn (▴) or vector (V) control (▪) DNA. Twenty-four hours after transfection, lysates were prepared, and equivalent cell units were resolved by SDS-PAGE, transferred to Immobilon, and immunoblotted with antibody specific for human Lyn (upper panel). At the same time, cells were also assessed for SCF-induced [3H]-thymidine incorporation in QBSF-58 media (lower panel). Data represent the mean and standard deviation of triplicate wells. (B) Kinase-inactive Lyn inhibits SCF-induced growth, but wild-type Lyn does not. Normal murine BMMCs were transfected with K275L human Lyn (KI), vector control (V), or wild-type (WT) Lyn. Twenty-four hours after transfection, lysates were generated, resolved by SDS-PAGE, transferred to Immobilon, and immunoblotted with antibody specific for human Lyn (upper panel). Cells were also assessed for [3H]-thymidine incorporation in response to 100 ng/mL SCF (lower panel). Data represent the mean and standard deviation of triplicate wells.
Ectopic expression of a kinase-inactive Lyn mutant inhibits SCF-induced proliferation.
(A) Transfection of normal mast cells with kinase-inactive Lyn inhibits SCF-induced growth. Normal murine BMMCs were transfected with either kinase-inactive (KI) human Lyn (▴) or vector (V) control (▪) DNA. Twenty-four hours after transfection, lysates were prepared, and equivalent cell units were resolved by SDS-PAGE, transferred to Immobilon, and immunoblotted with antibody specific for human Lyn (upper panel). At the same time, cells were also assessed for SCF-induced [3H]-thymidine incorporation in QBSF-58 media (lower panel). Data represent the mean and standard deviation of triplicate wells. (B) Kinase-inactive Lyn inhibits SCF-induced growth, but wild-type Lyn does not. Normal murine BMMCs were transfected with K275L human Lyn (KI), vector control (V), or wild-type (WT) Lyn. Twenty-four hours after transfection, lysates were generated, resolved by SDS-PAGE, transferred to Immobilon, and immunoblotted with antibody specific for human Lyn (upper panel). Cells were also assessed for [3H]-thymidine incorporation in response to 100 ng/mL SCF (lower panel). Data represent the mean and standard deviation of triplicate wells.
Cotransfection studies with GFP indicated that the efficiency of transfection of mast cells ranged from 15% to 40%. This seems low, given the magnitude of the effect of the kinase-inactive Lyn mutant. However, because the GFP and Lyn constructs were in different vectors, the frequency of transfection of the Lyn construct might have been higher than the GFP construct. Further, it is possible that the amount of kinase-inactive Lyn required to alter cellular responses was lower than the threshold of detection for GFP using immunofluorescence.
Reductions in SCF-mediated growth and chemotaxis of Lyn-deficient mast cells
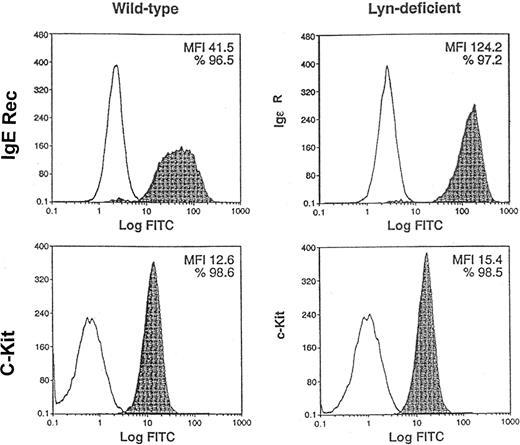
As a second approach to address the role of Lyn in SCF-mediated responses of mast cells, we used BMMCs from normal and Lyn-deficient mice. Previous studies have suggested that Lyn is not essential for IL-3–induced development of BMMCs in vitro.26 Indeed, we confirmed that 5-week culture of bone marrow mononuclear cells in IL-3 generated wild-type and Lyn-deficient BMMCs (data not shown). Figure3 demonstrates that similar percentages of wild-type and Lyn-deficient cells expressed high-affinity IgE receptors (96.5% and 97.2%, respectively) and c-Kit (98.6% and 98.5%, respectively). Mean fluorescence intensity (MFI) of c-Kit staining of Lyn-deficient mast cells was approximately 20% higher than wild-type mast cells (15.4 and 12.6, respectively). In contrast, Lyn-deficient mast cells had a striking increase in the MFI of the high-affinity IgE receptor compared with wild-type mast cells (124.2 and 41.5, respectively). This demonstrates that similar percentages of wild-type and Lyn-deficient mast cells express c-Kit and suggests that Lyn-deficient mast cells have a slight increase in the numbers of c-Kit receptors on the cell surface. Interestingly, the expression of c-Kit on the surface of the Lyn-deficient mast cells increased with the age of the cultures. Overall, these studies indicated that BMMC could be generated from the bone marrow of wild-type and Lyn-deficient mice and that these mast cells were suitable for use in subsequent experiments comparing SCF-mediated responses.
Similar percentages of Lyn-deficient mast cells express c-Kit as wild-type mast cells.
Pooled mononuclear cells from the bone marrow (n = 2) of wild-type or Lyn-deficient mice were cultured in murine IL-3 for 5 weeks. FACS analysis was performed to compare the expression of c-Kit and high-affinity IgE receptors, 2 mast-cell markers, on the cell surface. Nonshaded peaks represent cells stained with isotype-matched control antibody. Shaded peaks represent cells stained with either IgE or c-Kit antibodies, as indicated. MFI represents the average level of high-affinity IgE receptor or c-Kit expression on the cell surface. Data are representative of the results of triplicate experiments. FITC indicates fluorescein isothiocyanate.
Similar percentages of Lyn-deficient mast cells express c-Kit as wild-type mast cells.
Pooled mononuclear cells from the bone marrow (n = 2) of wild-type or Lyn-deficient mice were cultured in murine IL-3 for 5 weeks. FACS analysis was performed to compare the expression of c-Kit and high-affinity IgE receptors, 2 mast-cell markers, on the cell surface. Nonshaded peaks represent cells stained with isotype-matched control antibody. Shaded peaks represent cells stained with either IgE or c-Kit antibodies, as indicated. MFI represents the average level of high-affinity IgE receptor or c-Kit expression on the cell surface. Data are representative of the results of triplicate experiments. FITC indicates fluorescein isothiocyanate.
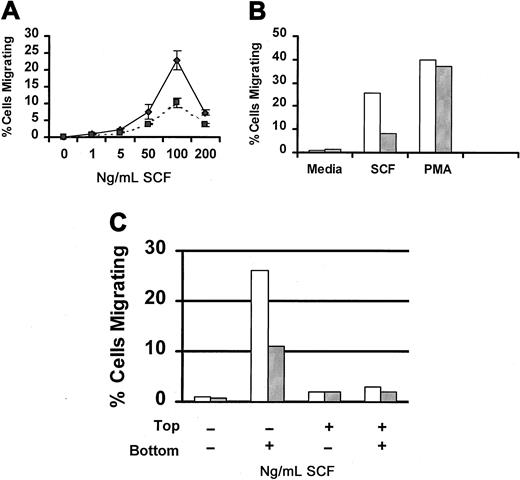
SCF is a potent chemoattractant for mast cells (reviewed in3 30). Therefore, we next examined the capacity of Lyn-deficient mast cells to migrate in response to SCF. Figure4A compares SCF-induced migration of wild-type and Lyn-deficient mast cells. Although both populations of cells exhibited the classic bell-shaped dose-response curve, the number of Lyn-deficient mast cells that migrated in response to SCF was lower than wild-type mast cells at each concentration of SCF examined. To determine whether this defect in migration was specific for SCF, we examined migration in response to PMA, a direct activator of protein kinase C that works independently of Src family members. Figure 4B demonstrates that PMA-induced migration was similar for Lyn-deficient and wild-type mast cells. We next examined SCF-induced chemokinesis of wild-type and Lyn-deficient mast cells. Figure 4C demonstrates that SCF did not induce chemokinesis of either wild-type or Lyn-deficient mast cells.
SCF-induced migration of Lyn-deficient mast cells is reduced.
(A) Impaired migration of Lyn-deficient mast cells is dose-related. Wild-type (♦) or Lyn-deficient (▪) mast cells were assessed for migration in response to the indicated concentration of murine SCF using a modified Boyden chamber as described in “Materials and methods.” Data represent the mean and standard deviation of triplicate samples and are representative of the results of 4 independent experiments. (B) PMA-induced migration of BMMCs from Lyn-deficient mice is normal. Migration of BMMCs from wild-type (■) or Lyn-deficient (░) mice in response to murine SCF (100 ng/mL) or PMA (50 ng/mL). All samples were performed in triplicate, and results are representative of 2 independent experiments. (C) SCF-induced migration of BMMCs is not the result of chemokinesis. Migration of BMMCs from wild-type (■) or Lyn-deficient (░) mice was performed as described above, except that the percentage of migrating cells was assessed in the presence of media or SCF in the top or bottom wells of the chemotaxis chamber, as indicated.
SCF-induced migration of Lyn-deficient mast cells is reduced.
(A) Impaired migration of Lyn-deficient mast cells is dose-related. Wild-type (♦) or Lyn-deficient (▪) mast cells were assessed for migration in response to the indicated concentration of murine SCF using a modified Boyden chamber as described in “Materials and methods.” Data represent the mean and standard deviation of triplicate samples and are representative of the results of 4 independent experiments. (B) PMA-induced migration of BMMCs from Lyn-deficient mice is normal. Migration of BMMCs from wild-type (■) or Lyn-deficient (░) mice in response to murine SCF (100 ng/mL) or PMA (50 ng/mL). All samples were performed in triplicate, and results are representative of 2 independent experiments. (C) SCF-induced migration of BMMCs is not the result of chemokinesis. Migration of BMMCs from wild-type (■) or Lyn-deficient (░) mice was performed as described above, except that the percentage of migrating cells was assessed in the presence of media or SCF in the top or bottom wells of the chemotaxis chamber, as indicated.
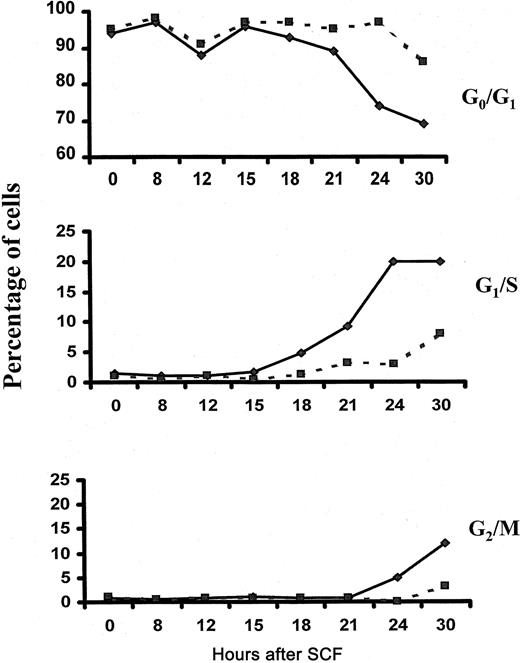
To determine whether Lyn is required for SCF-induced cell-cycle progression, we performed cell-cycle analysis of wild-type and Lyn-deficient mast cells after stimulation with SCF. Figure5 demonstrates that 3% of Lyn-deficient mast cells entered S phase after a 24-hour stimulation with SCF and that 8% were in S phase after a 30-hour stimulation. In contrast, 20% of wild-type controls were in S phase after culture for 24 and 30 hours in SCF. These data indicate that the onset of S phase is delayed in mast cells lacking Lyn and that fewer cells actually reach S phase compared to wild-type controls. Similarly, the onset of the G2/M phase was delayed in Lyn-deficient mast cells, and the numbers of cells reaching mitosis was reduced.
Fewer numbers of Lyn-deficient mast cells reach S phase after stimulation with SCF.
Wild-type (—) and Lyn-deficient (- - -) mast cells were incubated in 100 ng/mL murine SCF. At the times indicated, cells were assessed for cell-cycle progression as described in “Materials and methods.” The experiment was performed 3 times, and the data shown are representative of the results of each.
Fewer numbers of Lyn-deficient mast cells reach S phase after stimulation with SCF.
Wild-type (—) and Lyn-deficient (- - -) mast cells were incubated in 100 ng/mL murine SCF. At the times indicated, cells were assessed for cell-cycle progression as described in “Materials and methods.” The experiment was performed 3 times, and the data shown are representative of the results of each.
Reductions in the numbers of cells capable of replicating DNA in response to SCF should also be reflected in SCF-induced tritiated thymidine incorporation. Figure 6A indicates that SCF-induced tritiated thymidine incorporation of the Lyn-deficient mast cells was 45% lower than wild-type mast cells. In contrast, tritiated thymidine incorporation of Lyn-deficient mast cells in response to PMA was greater than wild-type mast cells (Figure 6B). These data indicate that Lyn-deficient mast cells are capable of responding normally to mitogens other than SCF.
SCF-induced proliferation of Lyn-deficient mast cells is reduced.
(A) SCF-induced DNA synthesis of Lyn-deficient mast cells is reduced compared to wild-type control cells. Bone marrow–derived mast cells from wild-type (■) and Lyn-deficient (░) mice were incubated for 60 hours in QBSF-58 media in the presence or absence of murine SCF (100 ng/mL). SCF-induced [3H]-thymidine incorporation was assessed as described in “Materials and methods.” All data represent the mean and standard deviation of quadruplicate samples. (B) PMA-induced growth of Lyn-deficient mast cells is normal. Bone marrow–derived mast cells from wild-type (■) and Lyn-deficient (░) mice were incubated in media or PMA (250 ng/mL). SCF-induced [3H]-thymidine incorporation was assessed as described in “Materials and methods.” All data represent the mean and standard deviation of quadruplicate samples.
SCF-induced proliferation of Lyn-deficient mast cells is reduced.
(A) SCF-induced DNA synthesis of Lyn-deficient mast cells is reduced compared to wild-type control cells. Bone marrow–derived mast cells from wild-type (■) and Lyn-deficient (░) mice were incubated for 60 hours in QBSF-58 media in the presence or absence of murine SCF (100 ng/mL). SCF-induced [3H]-thymidine incorporation was assessed as described in “Materials and methods.” All data represent the mean and standard deviation of quadruplicate samples. (B) PMA-induced growth of Lyn-deficient mast cells is normal. Bone marrow–derived mast cells from wild-type (■) and Lyn-deficient (░) mice were incubated in media or PMA (250 ng/mL). SCF-induced [3H]-thymidine incorporation was assessed as described in “Materials and methods.” All data represent the mean and standard deviation of quadruplicate samples.
Ectopic expression of wild-type Lyn enhances SCF-induced growth of Lyn-deficient mast cells
To determine whether the reduction in SCF-induced growth of Lyn-deficient mast cells was directly related to the absence of Lyn, we examined whether expression of exogenous Lyn reconstituted SCF-induced proliferation. Lyn-deficient mast cells were transfected with wild-type human Lyn DNA, or vector control, and assessed for tritiated thymidine incorporation after culture for 24 hours in SCF. Immunoblotting with antiserum specific for human Lyn demonstrated that the human Lyn protein was expressed in transfected cells (data not shown). Figure7A demonstrates that ectopic expression of Lyn protein led to increases in SCF-induced growth compared with the Lyn-deficient cells transfected with vector control. In fact, SCF-induced proliferation of Lyn-deficient mast cells transfected with wild-type Lyn was similar to the proliferation of wild-type mast cells transfected with vector controls.
Reduction in SCF-induced tritiated thymidine incorporation of Lyn-deficient mast cells can be reconstituted by ectopic expression of wild-type Lyn.
(A) Transfection with wild-type Lyn restores SCF-induced proliferation of Lyn-deficient mast cells to normal. Wild-type (WT) and Lyn-deficient mast cells (KO) were transfected with Lyn wild-type (WT) or vector control DNA. Transfected mast cells were incubated for 24 hours in QBSF-58 media containing the indicated concentration of SCF and then assessed for [3H]-thymidine incorporation as described in “Materials and methods.” All data represent the mean and standard deviation of quadruplicate samples. (B) Reconstitution of SCF-induced proliferation of Lyn-deficient cells is dependent on Lyn kinase activity. Lyn-deficient mast cells (KO) were transfected with wild-type Lyn (WT), kinase-inactive Lyn (KI), or vector control DNA. Transfected mast cells were incubated for 24 hours in the indicated concentration of SCF and then assessed for [3H]-thymidine incorporation, as described in “Materials and methods.” All data represent the mean and standard deviation of quadruplicate samples.
Reduction in SCF-induced tritiated thymidine incorporation of Lyn-deficient mast cells can be reconstituted by ectopic expression of wild-type Lyn.
(A) Transfection with wild-type Lyn restores SCF-induced proliferation of Lyn-deficient mast cells to normal. Wild-type (WT) and Lyn-deficient mast cells (KO) were transfected with Lyn wild-type (WT) or vector control DNA. Transfected mast cells were incubated for 24 hours in QBSF-58 media containing the indicated concentration of SCF and then assessed for [3H]-thymidine incorporation as described in “Materials and methods.” All data represent the mean and standard deviation of quadruplicate samples. (B) Reconstitution of SCF-induced proliferation of Lyn-deficient cells is dependent on Lyn kinase activity. Lyn-deficient mast cells (KO) were transfected with wild-type Lyn (WT), kinase-inactive Lyn (KI), or vector control DNA. Transfected mast cells were incubated for 24 hours in the indicated concentration of SCF and then assessed for [3H]-thymidine incorporation, as described in “Materials and methods.” All data represent the mean and standard deviation of quadruplicate samples.
To determine whether the reconstitution of SCF-induced growth of Lyn-deficient mast cells required Lyn kinase activity, cells were transfected either with vector control, kinase-inactive Lyn, or wild-type Lyn. Figure 7B demonstrates that expression of the kinase-inactive mutant, K275L Lyn, in Lyn-deficient mast cells reduced SCF-induced growth compared with the proliferation of cells transfected with vector control. Thus, increases in SCF-induced growth of Lyn-deficient mast cells by ectopic expression of Lyn is dependent on its kinase activity.
Discussion
Previous studies have shown that SCF activates Src family members; however, little is known about the role of these kinases in the biologic responses of primary hematopoietic cells.15,17,19 20 Here we provide several lines of evidence demonstrating that Lyn contributes to SCF-mediated responses of mast cells and progenitor cells. First, the expression of a kinase-inactive Lyn protein reduced SCF-induced proliferation of wild-type mast cells (Figure 2). Second, SCF-induced growth of Lyn-deficient progenitor cells was reduced compared with wild-type progenitors (Figure 1). Third, SCF-induced migration and proliferation of Lyn-deficient mast cells was reduced compared with wild-type mast cells (Figures 4-7). Additionally, our data demonstrate that reductions in the growth of Lyn-deficient cells are specific for SCF and relate directly to a deficiency in the expression of Lyn protein. PMA-induced proliferation of Lyn-deficient mast cells and progenitors was greater than that of wild-type mast cells (Figure 6B and data not shown), and proliferation of Lyn-deficient mast cells could be reconstituted by ectopic expression of wild-type Lyn (Figure 7).
The reduction in SCF-induced proliferation of Lyn-deficient mast cells and Lin− progenitor cells was not caused by decreases in surface expression of receptor. c-Kit was expressed on similar percentages of wild-type and Lyn-deficient mast cells and on progenitor cells (Figure 3 and data not shown). Further, Lyn-deficient mast cells and progenitor cells increase in the intensity of c-Kit staining compared with wild-type mast cells and progenitors. Interestingly, the magnitude of this difference is increased as the Lyn-deficient mast cells age. This could result from a selective growth advantage of Lyn-deficient mast cells expressing higher amounts of c-Kit on the surface. In addition, the absence of Lyn may reduce the internalization of c-Kit. Our previous work has shown that Src family members, including Lyn, are involved in the trafficking of c-Kit in hematopoietic cells.21 Internalization of the high-affinity IgE receptor may also be altered in Lyn-deficient mast cells. Lyn-deficient mast cells had a striking increase in high-affinity IgE receptors on the cell surface compared with wild-type mast cells (Figure 3).
One major binding site for Src family members on murine c-Kit has been mapped to tyrosine 567.17 Correspondingly, tyrosine 568 of human c-Kit coprecipitates Src family members, and tyrosine 570 contributes to maximal interaction.16,19Studies19 with Y568F human c-Kit suggested that Src family members contribute minimally to SCF-induced proliferation of transfected porcine aortic endothelial cells, and Besmer et al17 found the SCF-induced growth of mast cells transfected with a Y567F murine c-Kit mutant was only partially inhibited. Our findings with mast cells and progenitor cells demonstrate that Src family members make a more significant contribution to SCF-mediated responses than previously thought. This may be mediated, in part, by regions of human c-Kit other than tyrosines 568 and 570 and likely occurs indirectly. Similarly, studies of c-Kit mutants incapable of directly recruiting PI3 kinase suggested a minimal role for this lipid kinase in c-Kit stimulus-response coupling mechanisms.17,31,32 However, bone marrow–derived mast cells from p85PI3K-deficient mice do not respond to SCF, whereas FcΕRI-mediated responses are intact.33Thus, differences in results using receptor mutants and approaches specifically targeting a particular signal transduction component have previously been noted.
Studies have found that SCF-induced growth of mast cells treated with the Src family inhibitor PP1 is inhibited approximately 40%.17 This is a less dramatic inhibition than we observed in SCF-mediated responses of Lyn-deficient cells or wild-type mast cells transfected with kinase-inactive Lyn. However, the concentration of PP1 used (20 ng/mL) was low and might have been a suboptimal dose. Indeed, our previous work has demonstrated a 70% inhibition of SCF-induced proliferation of Mo7e cells at 10 μM PP1, a concentration shown to inhibit Lyn autophosphorylation activity in vitro, but not that of c-Kit.15 21
Lyn-deficient mice have normal T cells but exhibit severe B-cell abnormalities.22-24 Defects in IgE-mediated mast-cell function have also been reported.22 As they age, a striking splenomegaly develops in these animals, in part, from extramedullary hematopoiesis.34 Therefore, the phenotype of Lyn-deficient mice is not consistent with dramatic aberrations in c-Kit signaling. It is likely that SCF synergy with other growth factors compensates for the defects in SCF-mediated responses of Lyn-deficient mast cells and progenitor cells in vivo. In this regard, the difference in SCF-mediated responses of Lyn-deficient and wild-type cells in vitro, was most dramatic when studies were performed in serum-free media. Less notable differences in SCF-mediated responses of wild-type and Lyn-deficient cells were observed in studies performed in media supplemented with fetal calf serum (data not shown). Synergistic interaction of growth factors in vivo may explain why Lyn-deficient mice generate mast cells and are not anemic.
Our data demonstrate that Lyn contributes to SCF-mediated proliferation of mast cells and hematopoietic progenitor cells; however, Lyn is not essential for these responses. Lyn-deficient cells still migrate and proliferate in response to SCF, albeit to a limited extent (Figures 1,2, 4-7). In addition, the transfection of normal mast cells with kinase-inactive Lyn does not completely eliminate SCF-induced growth (Figure 2). One potential explanation of these findings is that other Src family members compensate for the loss of Lyn. Indeed, SCF activates multiple Src-related kinases, including Fyn.17,19 In this regard, SCF-induced growth of Lyn-deficient mast cells transfected with kinase-inactive Lyn was reduced compared with that of cells transfected with vector control (Figure 7B). These data support a role for multiple Src family members in SCF-mediated responses. SCF also activates the Ras-Raf-MAP kinase cascade, the Jak/Stat pathway, and PI3 kinase. Studies with pharmacologic inhibitors, c-Kit receptor mutants, and p85PI3K-deficient mice have shown that the PI3 kinase pathway contributes to SCF-mediated survival and growth of mast cells.17 31-33 Thus, the activation of Src-independent signal transduction pathways also likely accounts for the capacity of Lyn-deficient hematopoietic cells to respond to SCF.
Lyn has been reported to act as both a positive and a negative regulator of various cell stimuli. Corey et al29demonstrated that Lyn is critical for G-CSF–mediated growth of a lymphoblastoid cell line. In contrast, Lyn plays a role in the negative regulation of BCR, IgE, and IL-4 receptor signaling.22-24,35-37 Therefore, the cellular context is critical in determining whether Lyn contributes to the positive or the negative regulation of signaling pathways. The present study demonstrates that Lyn is important in the SCF-induced proliferation and chemotaxis of primary mast cells and in the proliferation of normal hematopoietic progenitor cells. Constitutive activation of Lyn has been reported in leukemic cell lines and primary leukemic blast cells.38 Elucidation of Lyn-dependent signaling pathways leading to proliferation may aid in the development of therapies for myeloid leukemias.
We thank Dr Douglas Lowy for review of this manuscript and for many helpful discussions. We also thank Dr Becky Vonakis and Dr Henry Metzger for providing human Lyn cDNA, and we thank Louise Finch in the Flow Cytometry Laboratory for her efforts and expertise.
Supported in part by National Institutes of Health grant HL54476.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Diana Linnekin, Basic Research Laboratory, Division of Basic Sciences, National Cancer Institute–Frederick, Bldg 567, Rm 226, Frederick, MD 21702; e-mail: dlinnekin@mail.ncifcrf.gov.

![Fig. 2. Ectopic expression of a kinase-inactive Lyn mutant inhibits SCF-induced proliferation. / (A) Transfection of normal mast cells with kinase-inactive Lyn inhibits SCF-induced growth. Normal murine BMMCs were transfected with either kinase-inactive (KI) human Lyn (▴) or vector (V) control (▪) DNA. Twenty-four hours after transfection, lysates were prepared, and equivalent cell units were resolved by SDS-PAGE, transferred to Immobilon, and immunoblotted with antibody specific for human Lyn (upper panel). At the same time, cells were also assessed for SCF-induced [3H]-thymidine incorporation in QBSF-58 media (lower panel). Data represent the mean and standard deviation of triplicate wells. (B) Kinase-inactive Lyn inhibits SCF-induced growth, but wild-type Lyn does not. Normal murine BMMCs were transfected with K275L human Lyn (KI), vector control (V), or wild-type (WT) Lyn. Twenty-four hours after transfection, lysates were generated, resolved by SDS-PAGE, transferred to Immobilon, and immunoblotted with antibody specific for human Lyn (upper panel). Cells were also assessed for [3H]-thymidine incorporation in response to 100 ng/mL SCF (lower panel). Data represent the mean and standard deviation of triplicate wells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/2/10.1182_blood.v98.2.343/5/m_h81411293002.jpeg?Expires=1768386458&Signature=Wu2iNAcI0bLIYNFf~z1WBofglZccJmqKPfNfVl45dkyFljHcvzQyd8Y0Wfs00WtiznIUZT1xc2bK1afdkFvrn6Fv2jJZFCGkv425QqnqKRzMKylFEE88LgkdOWSXooAjU1oHCOB13yzPizobO0BQe7B~Tx7gTPNjrmP0TF8JLnFxAxMrPAJC~BPr5s3SyFzp1yksIh6poNBOyD1bn-khsN0OaqsE76c1hB6lxB-Ft-qSOk3V2im0rNrpRjZbfBSlqrMFFsvgvJX0T4evIz7-Euf27-kDr278CUffiTm8uEnYs3bP9~rVKrYdZSDfqRfPz0Pd7wtv0d45kC3FLuBi5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. SCF-induced proliferation of Lyn-deficient mast cells is reduced. / (A) SCF-induced DNA synthesis of Lyn-deficient mast cells is reduced compared to wild-type control cells. Bone marrow–derived mast cells from wild-type (■) and Lyn-deficient (░) mice were incubated for 60 hours in QBSF-58 media in the presence or absence of murine SCF (100 ng/mL). SCF-induced [3H]-thymidine incorporation was assessed as described in “Materials and methods.” All data represent the mean and standard deviation of quadruplicate samples. (B) PMA-induced growth of Lyn-deficient mast cells is normal. Bone marrow–derived mast cells from wild-type (■) and Lyn-deficient (░) mice were incubated in media or PMA (250 ng/mL). SCF-induced [3H]-thymidine incorporation was assessed as described in “Materials and methods.” All data represent the mean and standard deviation of quadruplicate samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/2/10.1182_blood.v98.2.343/5/m_h81411293006.jpeg?Expires=1768386458&Signature=oQnfe3FB~v990beKpcIGMkymBYOx5sf5G5k69VDR99dRLn~TXNeUM8NBv3UADsTpZlHkknPD4clIiJBJi11g1-r1zGknux3ukllOts~NCywNxPf971FmKEzfi3NmaK2be-ghOSBBRDMO2HOcl7LP8cR7UcUuQ8nAWOF3NcHPTh0CwuJ~iAOIAWjqWONBnm2gMGvwYpiZAi9OHndZjdY5ay0rXFCbNmdNgAfu3IReiuO6eTh4wGATGthtUdWH5ZMD9fFfNrop~jj6HOv1AbSK4N8k8lw4WhgIXHgNmFqD18ykwQ3FnZucczIsSq8xS20hqtUz6oH5otYHdFanpYfq8Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Reduction in SCF-induced tritiated thymidine incorporation of Lyn-deficient mast cells can be reconstituted by ectopic expression of wild-type Lyn. / (A) Transfection with wild-type Lyn restores SCF-induced proliferation of Lyn-deficient mast cells to normal. Wild-type (WT) and Lyn-deficient mast cells (KO) were transfected with Lyn wild-type (WT) or vector control DNA. Transfected mast cells were incubated for 24 hours in QBSF-58 media containing the indicated concentration of SCF and then assessed for [3H]-thymidine incorporation as described in “Materials and methods.” All data represent the mean and standard deviation of quadruplicate samples. (B) Reconstitution of SCF-induced proliferation of Lyn-deficient cells is dependent on Lyn kinase activity. Lyn-deficient mast cells (KO) were transfected with wild-type Lyn (WT), kinase-inactive Lyn (KI), or vector control DNA. Transfected mast cells were incubated for 24 hours in the indicated concentration of SCF and then assessed for [3H]-thymidine incorporation, as described in “Materials and methods.” All data represent the mean and standard deviation of quadruplicate samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/2/10.1182_blood.v98.2.343/5/m_h81411293007.jpeg?Expires=1768386458&Signature=mbMKsDbN0vF~Gx22vScvGaD-Qn6KKEm1iMwWXrMj-UTWh632Hda07Q7VNte5r7DRy4KXVCp3hlWFsyYTjoAKJ-mu75IJ~Kw3AeHG8XUCLmHC-hjgnX6zmMkpsm700i1PYeTy9LRGb7K3FobYRz5jGKMxS7dWJsljMgLJ~aRlZyI2etieV2rxMicNOEx1FILG3RhDQ0UXEIYiZ4FDJWc-ZdR5Mr5ZRx9W1APEZyT2rc-2a1CQiif~8Bj4W07zE-11UnbdemBkeyPjTSWEdmJHvCsjJICqGg~lI0GCUaFpj4s1mWcb5D5znhJXofzI0dGvEF3M~B5Eb7d1Ftk5M~o-Ag__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
