Multiple myeloma (MM) remains incurable, with a median survival of 3 to 4 years. This study shows direct effects of vascular endothelial growth factor (VEGF) upon MM and plasma cell leukemia (PCL) cells. The results indicate that VEGF triggers tumor cell proliferation via a protein kinase C (PKC)–independent Raf-1–MEK–extracellular signal-regulated protein kinase pathway, and migration via a PKC-dependent pathway. These observations provide the framework for novel therapeutic strategies targeting VEGF signaling cascades in MM.
Introduction
Multiple myeloma (MM) is characterized by the clonal proliferation of malignant plasma cells in the bone marrow (BM) associated with bone loss, renal disease, and immunodeficiency. Malignant transformation in MM progresses from immortalization of plasma cells (monoclonal gammopathy of undetermined significance [MGUS]) to the establishment of MM in the BM with evolution of growth-factor–independent growth and resistance to apoptosis.1 Despite recent new insights into the pathogenesis of MM, it remains incurable with a median survival of approximately 3 to 4 years. New biologically based therapies are therefore urgently needed.
The various growth factors belonging to the vascular endothelial growth factor (VEGF) family (VEGF, placenta growth factor [PlGF], VEGF-B, VEGF-C, and VEGF-D) act as modulators and inducers of angiogenesis in vivo.2-4 The active forms of VEGF are synthesized either as homodimers5,6 or as heterodimers with other VEGF family members, such as PlGF.7 Five molecular forms of VEGF are generated by alternative splicing of its RNA transcripts: VEGF121, VEGF145, VEGF165, VEGF189, and VEGF206. These forms differ primarily in the presence or absence of heparin and heparan-sulfate binding domains, encoded by exons 6 and 7.8 The VEGF121, VEGF165, and VEGF189 forms are abundant and usually produced simultaneously,2 whereas VEGF145 is less common and produced by cells derived from the female reproductive system,9 penis, and kidney.10,11 Recently VEGF145 has also been found in primary effusion lymphomas (PELs) associated with Kaposi sarcoma–associated herpesvirus (or human herpesvirus-8) infection, as well as in prostate carcinoma.12
In solid tumors, VEGF has an important role in the induction of neovascularization correlating with tumor growth and metastatic potential.13-17 However, recent studies also suggest a role for VEGF in hematological malignancies. In MM, for example VEGF is expressed and secreted by tumor cells as well as BM stromal cells (BMSCs).18,19 It may account, at least in part, for the increased microvessel density (MVD) that has been observed in the BM of patients with MM and that correlates with disease progression and poor prognosis.20-22 These data, coupled with the known antiangiogenic properties of thalidomide (Thal),23provided the rationale for the use of Thal to treat MM. Remarkable clinical responses to Thal were observed in 32% of MM patients whose disease was refractory to conventional and high-dose therapy.24 We suggested at the time that the anti-MM activity of Thal against MM may not only be related to inhibition of VEGF-induced BM angiogenesis, but might also include blocking of VEGF-induced direct effects on MM cell growth, survival, and migration.25
The present study shows for the first time that VEGF induces proliferation and triggers migration of human MM cells, suggesting an autocrine VEGF loop in MM. Exogenous VEGF165 activates at least 2 signaling pathways in MM: the Raf-1–MEK–extracellular signal-regulated protein kinase (ERK) pathway mediating proliferation, and a protein kinase C (PKC)–dependent cascade associated with migration. These observations provide the framework for novel therapeutic strategies targeting VEGF in MM.
Materials and methods
Cells and cell culture
The human MM cell line MM.1S (dexamethasone-sensitive),27 patient MM cells, and patient plasma cell leukemia (PCL) cells were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, 10 μg/mL streptomycin, and 2 mM L-glutamine. Human umbilical vein endothelial cells (HUVECs), purchased from Clonetics Biowhittaker (Walkersville, MD) (HUVEC P168) were maintained in EGM-2MV media (Clonetics Biowhittaker).
Isolation of patient's tumor cells
Mononuclear cells were obtained by Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden) separation from peripheral blood samples of a patient with PCL. MM patient cells (96% CD38+CD45RA−) were purified from patient BM samples, as previously described.36
Reverse-transcriptase polymerase chain reaction analyses of VEGF and VEGF-receptors
Total RNA was prepared with Trizol Reagent (Gibco Life Technologies, Rockville, MD) according to the manufacturer's instructions. Complementary DNA (cDNA) was synthesized by means of SuperScript One-Step reverse-transcriptase polymerase chain reaction (RT-PCR) system with Platinum Taq (Gibco Life Technologies). VEGF primers 5′-TCG GGC CTC CGA AAC CAT GA-3′ (sense) and 5′-CCT GGT GAG AGA TCT GGT TC-3′ (antisense), corresponding to sequences in the 3′ and 5′ untranslated regions, were used to amplify the known splice variants: 516 base pairs (bp) of VEGF121; 588 bp of VEGF145; 648 bp of VEGF165; 720 bp of VEGF189; and 771 bp of VEGF206. The thermal cycle profile consisted of denaturating at 94°C for 30 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 1 minute. The samples were amplified for 35 cycles. VEGF receptor sense primer 5′-CAA GTG GCC AGA GGC ATG GAG TT-3′ (corresponding to nucleotides +3262 to +3284) and antisense primer 5′-GAT GTA GTC TTT ACC ATC CTG TTG-3′ (corresponding to nucleotides +3736 to +3759) were used for the amplification of Fms-like tyrosine kinase-1 (Flt-1)37; 5′-CAA CAA AGT CGG GAG AGG AG-3′ (sense) and 5′-ATG ACG ATG GAC AAG TAG CC-3′ (antisense) were used for amplification of kinase insert domain-containing receptor (KDR).18 The thermal cycle profile consisted of denaturating at 94°C for 1 minute, annealing at 60°C for 2 minutes, and extension at 72°C for 3 minutes. The samples were amplified for 32 cycles. We used 20 pg primers. The integrity of messenger RNA of all samples was confirmed by amplification of β-actin. PCR products were separated on a 1% agarose gel and photographed.
Enzyme-linked immunosorbent assay
Cells were seeded onto 6-well plates at a concentration of 1 × 106 cells per well in RPMI 1640 with 1% FBS. After 48 hours, the supernatants were collected and analyzed by VEGF–enzyme-linked immunosorbent assays (ELISAs) (R&D Systems, Minneapolis, MN). Briefly, 96-well plates were coated with anti–human VEGF antibody overnight, rinsed, and blocked with 300 μL phosphate-buffered saline (PBS), 1% bovine serum albumin (BSA), 5% sucrose, and 0.05% NaN3 for 2 hours. After washing, 100 μL of sample or standards diluted in Tris-Cl, 0.1% BSA, and 0.05% Tween 20 (pH 7.3) were added to the wells and incubated for 2 hours at room temperature (RT). The cells were rinsed again, and biotinylated anti–human VEGF antibody was added for 2 hours at RT. After a further washing step, the wells were incubated with streptavidin horseradish peroxidase for 20 minutes at RT and rinsed again. The reaction was started by the addition of 100 μL H2O2 and tetramethylbenzidine for 30 minutes at RT. After stopping the reaction with 1 M H2SO4, the optical density of each well was detected by means of a microtiter plate reader at 450 nm. Each well was analyzed in triplicate.
Stimulation of cells
For signaling experiments, cells were starved for 15 to 18 hours prior to stimulation with 100 ng/mL VEGF (recombinant human VEGF) (R&D) in medium with 1% FBS overnight and then for 3 hours without FBS. For proliferation assays, cells were starved for 3 hours prior to stimulation with different concentrations of VEGF in medium with 1% to 5% FBS and cultured in starvation medium for 72 hours.
DNA synthesis and cell proliferation assay
Cell growth was assessed by the addition of 0.5 μCi [3H] thymidine per well during the last 9 hours of a 72-hour culture at 37°C. Radioactive labeling was determined by harvesting the cells onto glass-fiber filtermates with an automatic cell harvester (Tomec Harvester 96 Mach III, Hamden, CT) and counting using the Wallac Trilux Betaplate scintillation counter (Turku, Finland).
Cell lysis, immunoprecipitation, and Western blot analysis
Cells were washed 3 times with PBS and lysed with either lysis buffer (10 mM Tris, 50 mM NaCl, Na-pyrophosphate, 1% triton, 1 mM sodium vanadate, 1 mM phenylmethyl sulfonyl fluoride, and protein inhibitor cocktail) (Boehringer Mannheim, Germany) or radioimmunoprecipitation assay lysis buffer. Insoluble material was removed by centrifugation (15 000 rpm for 30 minutes at 4°C). Immunoprecipitation was performed as described previously.38 Briefly, Flt-1 (H-225) and fetal liver kinase-1 (Flk-1) (C-1158) (Santa Cruz Biotechnology, CA) Abs were used to immunoprecipitate Flt-1 and Flk-1, respectively. Immunocomplexes were collected following overnight incubation at 4°C with protein A–Sepharose beads (Sigma, St Louis, MO). For Western blotting, cell lysates (30 to 100 μg per lane) or immunoprecipitates were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis prior to electrophoretic transfer onto Hybond C super (Amersham, Arlington Heights, IL). The blots were probed with Flt-1, Flk-1, Raf-1, phospho-tyrosine–specific 4G10, phospho-ERK, ERK-2, phospho–stress activated protein kinase (SAPK)/c-Jun NH2-terminal kinase (JNK), phospho-p38, p38, phospho–signal transducer and activator of transcription 3 (phospho–STAT 3), and STAT 3 antisera prior to incubation with horseradish peroxidase–conjugated secondary antibodies and exposure to the enhanced chemiluminescence substrate.
Transwell migration assay
Cell migration was assayed by means of a modified Boyden chamber assay, as described previously.39 40 Briefly, starved cells were added on an 8-μm pore size polycarbonate membrane precoated with fibronectin separating the 2 chambers of a 6.5-mm transwell (Costar). VEGF (0.5 to 500 ng/mL), with or without the PKC inhibitor bisindolylmaleimide I, hydrochloride (BIM) (Calbiochem, San Diego, CA) was added to the lower chamber. After 2 to 5 hours, cells that migrated into the lower compartment were counted by means of a Coulter counter ZBII (Coulter Electronics, Bedfordshire, England).
Statistical analysis
Statistical significance of differences observed in VEGF-treated versus control cultures was determined by means of an unpaired Student t test. The minimal level of significance wasP < .05.
Results
VEGF is present in the BM micro-environment of patients with MM and associated with the neovascularization at sites of MM cell infiltration.26 In addition, VEGF is produced by MM cells and triggers interleukin (IL)–6 production from BMSCs, thereby augmenting MM cell growth and survival.19 In this study, we examined whether VEGF also has direct effects on MM cells. As a model system, we used a dexamethasone-sensitive (MM.1S) human MM cell line,27 as well as patient MM and patient PCL cells.
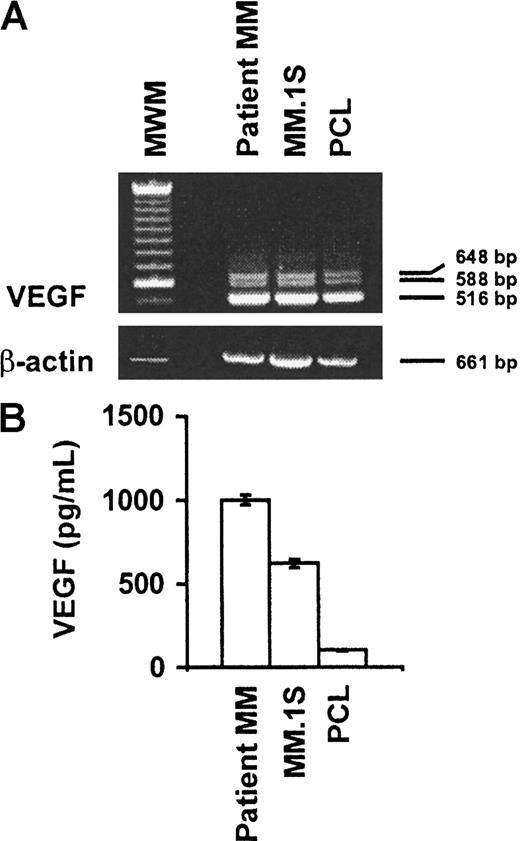
Expression and secretion of VEGF isoforms in MM.1S, patient MM, and patient PCL cells
VEGF is expressed and secreted in MM cell lines, including RPMI 8226, ARH-77, U266, IM-9, and OPM-2,18 19 as well as in BMSCs. In the first series of experiments, we performed RT-PCR analysis to determine whether MM.1S cells, as well as patient MM and patient PCL cells, synthesize VEGF. As shown in Figure1A, 3 predominant bands corresponding to the splice variants VEGF121 (516 bp), VEGF145(588 bp), and VEGF165 (648 bp) were produced by MM.1S, patient MM, and patient PCL cells, respectively.
Expression and secretion of VEGF isoforms in MM.1S, patient MM, and patient PCL cells.
(A) MM.1S, patient MM, and patient PCL cells express VEGF. Equal amounts of RNA were reverse transcribed to generate cDNA, which was subjected to VEGF-specific PCR amplification by means of paired primers as described in “Materials and methods.” Three splice variants coding for the secreted isoforms VEGF121 (516 bp), VEGF145 (588 bp), and VEGF165 (648 bp) were amplified from all cells. The quality of RNA was confirmed by RT-PCR amplification of β-actin. MWM indicates molecular weight marker. (B) MM.1S, patient MM cells, and patient PCL cells secrete VEGF. Supernatants from equal numbers of cells (1 × 106) were collected after 48 hours and analyzed for VEGF protein by ELISA.
Expression and secretion of VEGF isoforms in MM.1S, patient MM, and patient PCL cells.
(A) MM.1S, patient MM, and patient PCL cells express VEGF. Equal amounts of RNA were reverse transcribed to generate cDNA, which was subjected to VEGF-specific PCR amplification by means of paired primers as described in “Materials and methods.” Three splice variants coding for the secreted isoforms VEGF121 (516 bp), VEGF145 (588 bp), and VEGF165 (648 bp) were amplified from all cells. The quality of RNA was confirmed by RT-PCR amplification of β-actin. MWM indicates molecular weight marker. (B) MM.1S, patient MM cells, and patient PCL cells secrete VEGF. Supernatants from equal numbers of cells (1 × 106) were collected after 48 hours and analyzed for VEGF protein by ELISA.
To assess whether MM.1S, patient MM, and patient PCL cells secrete VEGF protein, cells were seeded onto 6-well plates at a concentration of 1 × 106 cells per well in RPMI 1640 with 1% FBS. VEGF secreted by these tumor cells was measured in 48-hour culture supernatants by ELISA. VEGF secretion ranged from 100 pg/mL (PCL cells) to 1000 pg/mL (MM cells) (Figure 1B). Taken together, these data show that MM.1S cells, patient MM cells, and patient PCL cells both synthesize and secrete VEGF.
VEGF induces proliferation in MM cells
To determine whether VEGF has direct effects on MM cells, we first examined whether VEGF165 induced DNA synthesis in growth-factor–deprived MM.1S and patient MM cells. As shown in Figure2A, VEGF induced a reproducible dose-dependent increase (2-fold) in proliferation in MM.1S cells assayed by [3H] thymidine uptake. To judge the biological significance of this finding, we subsequently studied the effect of VEGF on the proliferation of patient MM cells derived from 5 patients. As shown in Figure 2B, VEGF also induced a dose-dependent increase (1.3- to 1.4-fold) in proliferation in these cells.
Effect of VEGF on MM cell proliferation.
MM.1S (panel A) and MM cells isolated from 5 patients (panel B) (3 × 104 cells per well) were cultured in RPMI 1640 medium with 5% (MM.1S) or 10% (patient MM cells) FBS in 96-well assay plates. The cells were then either left untreated or treated with 1 to 1000 ng/mL of VEGF165. Cell growth was assessed by addition of 0.5μCi [3H] thymidine [3H (dT)] per well during the last 9 hours of 72-hour cultures at 37°C. Radioactive labeling was determined by harvesting the cells onto glass-fiber filtermates with an automatic cell harvester (Tomec Harvester 96 Mach III) and counting by means of the Wallac Trilux Betaplate scintillation counter. Data represent means and SDs for triplicate samples. Statistical significance for the proliferation of MM.1S cells was determined by analysis of variance followed by an unpaired Student t test: *P < .005; **P < .001, as compared with control (without VEGF).
Effect of VEGF on MM cell proliferation.
MM.1S (panel A) and MM cells isolated from 5 patients (panel B) (3 × 104 cells per well) were cultured in RPMI 1640 medium with 5% (MM.1S) or 10% (patient MM cells) FBS in 96-well assay plates. The cells were then either left untreated or treated with 1 to 1000 ng/mL of VEGF165. Cell growth was assessed by addition of 0.5μCi [3H] thymidine [3H (dT)] per well during the last 9 hours of 72-hour cultures at 37°C. Radioactive labeling was determined by harvesting the cells onto glass-fiber filtermates with an automatic cell harvester (Tomec Harvester 96 Mach III) and counting by means of the Wallac Trilux Betaplate scintillation counter. Data represent means and SDs for triplicate samples. Statistical significance for the proliferation of MM.1S cells was determined by analysis of variance followed by an unpaired Student t test: *P < .005; **P < .001, as compared with control (without VEGF).
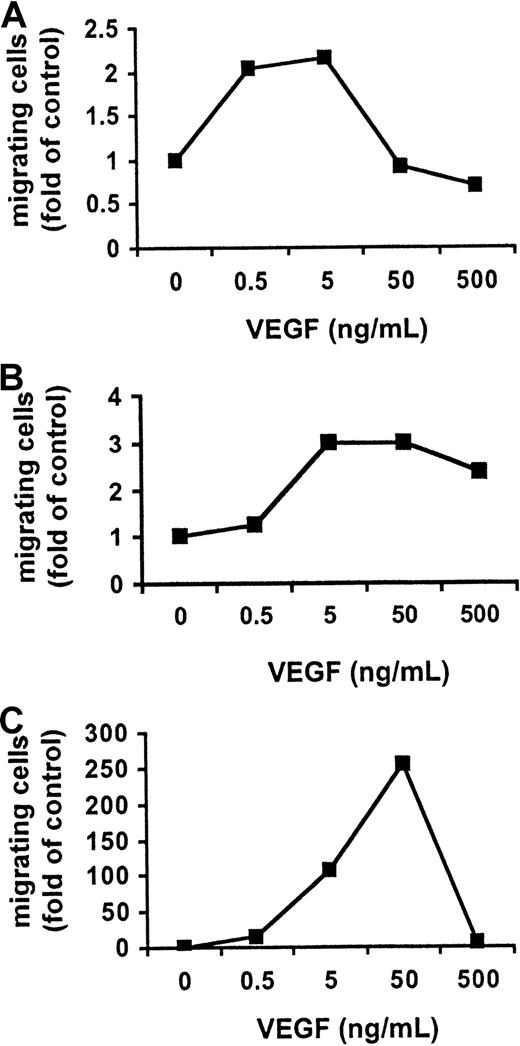
VEGF triggers migration of MM and PCL cells
Since it was recently reported that VEGF stimulates migration of monocytes and macrophages,28-30 we next examined whether VEGF also triggers migration in MM.1S, patient MM, and patient PCL cells. Cell migration was assayed by measuring the transfilter migration activity of MM.1S cells seeded on membranes precoated with fibronectin. The addition of VEGF to conditioned medium in the lower chamber induced a dose-dependent cell migration of growth-factor–deprived MM.1S cells, patient MM cells, and patient PCL cells in the upper transwell chamber. Maximal activation of migration in MM.1S cells (2-fold) and patient MM cells (3-fold) was induced by 5 ng/mL VEGF165 (Figure 3A-B), whereas maximal activation of migration in patient PCL cells (approximately 250-fold) was induced by 50 ng/mL VEGF165(Figure 3C). VEGF165 (5 ng/mL) markedly enhanced the migration in patient PCL cells (approximately 100-fold) relative to MM.1S cells, suggesting an important role of VEGF in the transition of MM to PCL.
Effect of VEGF on MM and PCL cell migration.
VEGF activates cell migration in MM and PCL cells. Growth-factor–deprived MM.1S (panel A), patient MM (panel B), and patient PCL (panel C) cells were plated on a polycarbonate membrane (8-μm pore size) in a modified Boyden chamber and exposed for 4 hours to VEGF (0.5 to 500 ng/mL) added to the lower chamber. At the end of the treatments, cells on the lower part of the membrane were counted with a Coulter counter ZBII. The results shown for MM.1S cells are representative of 3 independent experiments.
Effect of VEGF on MM and PCL cell migration.
VEGF activates cell migration in MM and PCL cells. Growth-factor–deprived MM.1S (panel A), patient MM (panel B), and patient PCL (panel C) cells were plated on a polycarbonate membrane (8-μm pore size) in a modified Boyden chamber and exposed for 4 hours to VEGF (0.5 to 500 ng/mL) added to the lower chamber. At the end of the treatments, cells on the lower part of the membrane were counted with a Coulter counter ZBII. The results shown for MM.1S cells are representative of 3 independent experiments.
Flt-1 is expressed and activated by VEGF in MM cells
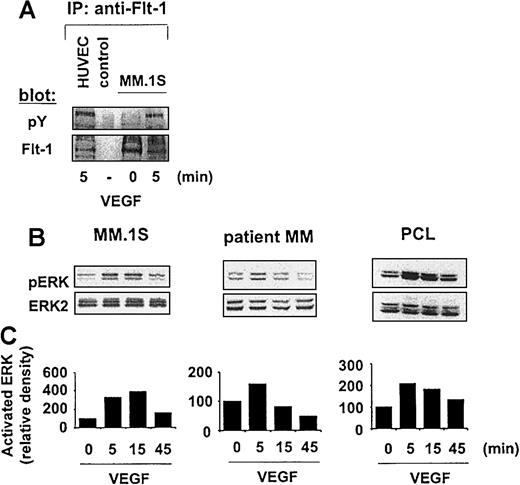
We next sought to identify signaling pathways mediating these effects. Comparable transcript and protein expression of high-affinity VEGF receptor (VEGFR)–1/Flt-1 were detected by RT-PCR and immunoprecipitation, respectively, in MM.1S, patient MM cells, and patient plasma cells (data not shown). Despite low-level expression of Flt-1, VEGF triggered an up-regulation of tyrosine phosphorylation of Flt-1 in MM cell line MM.1S cells (Figure4A).
Effect of VEGF on the Raf-MEK-ERK pathway.
(A) MM.1S cells were starved overnight in RPMI 1640 with 1% FBS and for 3 hours in RPMI 1640 with no FBS. After stimulation of MM.1S cells with 100 g/mL VEGF165 for 5 minutes, Flt-1 immunoprecipitates (IP) from whole cell lysates were analyzed by Western blotting with the use of antisera against phospho-tyrosine residues. Equal loading was confirmed by immunoblotting with antisera directed against Flt-1, and whole cell lysate of VEGF-stimulated HUVECs was similary immunoblotted as a positive control. Nonspecific protein binding and detection were excluded by incubating protein A–Sepharose (PAS) beads with lysis-buffer and Flt-1 antibody only (control). (B) MM.1S, patient MM, and patient PCL cells, pretreated as described in panel A, were stimulated with 100 ng/mL VEGF165 for 5 minutes, 15 minutes, and 45 minutes. Whole cell lysates (40 μg) were analyzed by Western blotting with antisera against phospho-ERK (pERK). Immunoblotting for ERK-2 confirmed equal protein loading. (C) Densitometry was used to quantitate pERK activity in panel B. The data shown are representative of 3 separate experiments.
Effect of VEGF on the Raf-MEK-ERK pathway.
(A) MM.1S cells were starved overnight in RPMI 1640 with 1% FBS and for 3 hours in RPMI 1640 with no FBS. After stimulation of MM.1S cells with 100 g/mL VEGF165 for 5 minutes, Flt-1 immunoprecipitates (IP) from whole cell lysates were analyzed by Western blotting with the use of antisera against phospho-tyrosine residues. Equal loading was confirmed by immunoblotting with antisera directed against Flt-1, and whole cell lysate of VEGF-stimulated HUVECs was similary immunoblotted as a positive control. Nonspecific protein binding and detection were excluded by incubating protein A–Sepharose (PAS) beads with lysis-buffer and Flt-1 antibody only (control). (B) MM.1S, patient MM, and patient PCL cells, pretreated as described in panel A, were stimulated with 100 ng/mL VEGF165 for 5 minutes, 15 minutes, and 45 minutes. Whole cell lysates (40 μg) were analyzed by Western blotting with antisera against phospho-ERK (pERK). Immunoblotting for ERK-2 confirmed equal protein loading. (C) Densitometry was used to quantitate pERK activity in panel B. The data shown are representative of 3 separate experiments.
Flt-1 immunoprecipitates of whole cell lysates of VEGF-stimulated HUVECs immunoblotted for Flt-1 served as a positive control. Reprobing of the membrane with antisera directed against Flt-1 confirmed equal protein loading. In contrast, KDR/VEGFR-2 the human homolog of Flk-1, was not detected (data not shown). Taken together, these data suggest that Flt-1 is a functional signaling receptor for VEGF in MM.1S cells.
VEGF activates the Raf–MEK–ERK pathway
We next sought to identify signaling molecules downstream of the receptor activated by VEGF. The activation of ERK-1 and ERK-2 mediates DNA-synthesis,31,32 and we have shown that IL-6–induced proliferation of MM cells is mediated by this pathway.33To assess whether VEGF activates ERK in MM cells, we starved MM.1S, patient MM cells, and patient PCL cells overnight in RPMI 1640 with 1% FBS and then for 3 hours in RPMI 1640 with no FBS. In time-course stimulation experiments, 100 ng/mL VEGF165 triggered specific phosphorylation of ERK-1 and ERK-2 in MM.1S cells, in patient MM cells, and in patient PCL cells (Figure 4B). Equivalent amounts of ERK protein were confirmed by immunoblotting with anti–ERK-2 antibody. ERK phosphorylation, quantitated by densitometry, demonstrated variations in the time of maximal activation (Figure 4C). Similar results were observed in Hs Sultan and RPMI-8226 MM cells (data not shown).
To confirm that VEGF triggers ERK activation, we used anti–human VEGF antibody (anti-VEGF) (R&D) to neutralize human VEGF bioactivity, as well as PD98059 as a specific MEK-1 inhibitor. As can be seen in Figure5B, VEGF-induced ERK phosphorylation in MM.1S and patient MM cells (Figure 5A) was inhibited by 60-minute pretreatment with anti-VEGF antibody (Figure 5A), confirming specific VEGF signaling. ERK phosphorylation by VEGF was also inhibited by 60-minute pretreatment with PD98059 (Figure 5C), implicating MEK-1 in VEGF signal transduction in MM cells (Figure 5C). We further observed that VEGF induced electric mobility shifting of the MEK kinase Raf-1, consistent with Raf-1 activation, which was abrogated by anti-VEGF antibody (data not shown).
Inhibition of VEGF-triggered ERK activation by blockade of VEGF and MEK-1.
MM.1S and patient MM cells pretreated as described in Figure 4A were either stimulated with 100 ng/mL VEGF165 alone (panel A) or treated for 30 minutes with 30 μg/mL anti–human VEGF antibody (anti-VEGF) (panel B) or for 1 hour with 50 μM PD89059 (panel C) prior to VEGF stimulation. Cells were solubilized, and whole cell lysates (40 μg) were analyzed by Western blotting with antisera against dually phosphorylated MAPK (ERK-1, ERK-2). Equal loading was confirmed by immunoblotting the membranes with antisera against ERK-2.
Inhibition of VEGF-triggered ERK activation by blockade of VEGF and MEK-1.
MM.1S and patient MM cells pretreated as described in Figure 4A were either stimulated with 100 ng/mL VEGF165 alone (panel A) or treated for 30 minutes with 30 μg/mL anti–human VEGF antibody (anti-VEGF) (panel B) or for 1 hour with 50 μM PD89059 (panel C) prior to VEGF stimulation. Cells were solubilized, and whole cell lysates (40 μg) were analyzed by Western blotting with antisera against dually phosphorylated MAPK (ERK-1, ERK-2). Equal loading was confirmed by immunoblotting the membranes with antisera against ERK-2.
Besides ERK, we further investigated whether other members of the mitogen activated protein (MAP) kinase family, including SAPK/JNK and p38 (stress activated protein kinase 2), are also activated by VEGF. IL-6 activates STAT 3, which mediates antiapoptotic effects in MM cells,34 35 and we therefore also tested whether VEGF activates the STAT 3 pathway. Neither p38 and SAPK/JNK nor STAT3 were phosphorylated in MM cells triggered by VEGF (data not shown). Taken together, these data show that VEGF triggers ERK, but not p38, SAPK/JNK, and STAT 3 signaling in MM cells.
VEGF-induced proliferation of MM cells is inhibited by a MEK-1 inhibitor
Maximal stimulation of proliferation of MM.1S cells was observed at VEGF concentrations of 100 ng/mL and 1000 ng/mL, respectively (Figure 2A). To support the functional importance of the ERK pathway in mediating tumor cell proliferation, we measured [3H] thymidine incorporation in these cells triggered by VEGF in the presence or absence of the MEK-1 inhibitor PD98059 (25 μM). Treatment with PD98059 alone did not inhibit basal MM cell proliferation (Figure6); however, VEGF-triggered increases in DNA synthesis in MM.1S cells were inhibited by PD98059. In contrast, the PKC inhibitor BIM (2 μM) did not block proliferation. As a further proof that proliferation is PKC independent, we next determined whether VEGF-induced ERK activation is abrogated by the blockade of PKC. We assayed ERK activation induced by 100 ng/mL VEGF165in MM.1S, as well as patient PCL cells, in the presence or absence of 2 μM of the PKC inhibitor BIM. The inhibition of PKC did not block VEGF-induced MEK-1–ERK signaling (data not shown). Taken together, these data suggest that proliferation is transduced via MEK-1, but not via PKC, cascades.
MEK-1 dependency of VEGF-induced proliferation.
MM.1S cells were starved for 3 hours in RPMI 1640 with 5% FBS. Cells were incubated with 100 or 1000 ng/mL VEGF165 with or without PD98059 (25μM) or BIM (2μM). For the last 9 hours of 72-hour cultures, 0.5μCi [3H] thymidine was added, and radioactivity incorporated into DNA was determined by scintillation counting (Wallac Trilux Betaplate scintillation counter) of automatically harvested cells (Tomec Harvester 96 Mach III). Results are expressed as fold-proliferation of untreated cells (control). The data are means of 3 wells, and the experiment was repeated twice.
MEK-1 dependency of VEGF-induced proliferation.
MM.1S cells were starved for 3 hours in RPMI 1640 with 5% FBS. Cells were incubated with 100 or 1000 ng/mL VEGF165 with or without PD98059 (25μM) or BIM (2μM). For the last 9 hours of 72-hour cultures, 0.5μCi [3H] thymidine was added, and radioactivity incorporated into DNA was determined by scintillation counting (Wallac Trilux Betaplate scintillation counter) of automatically harvested cells (Tomec Harvester 96 Mach III). Results are expressed as fold-proliferation of untreated cells (control). The data are means of 3 wells, and the experiment was repeated twice.
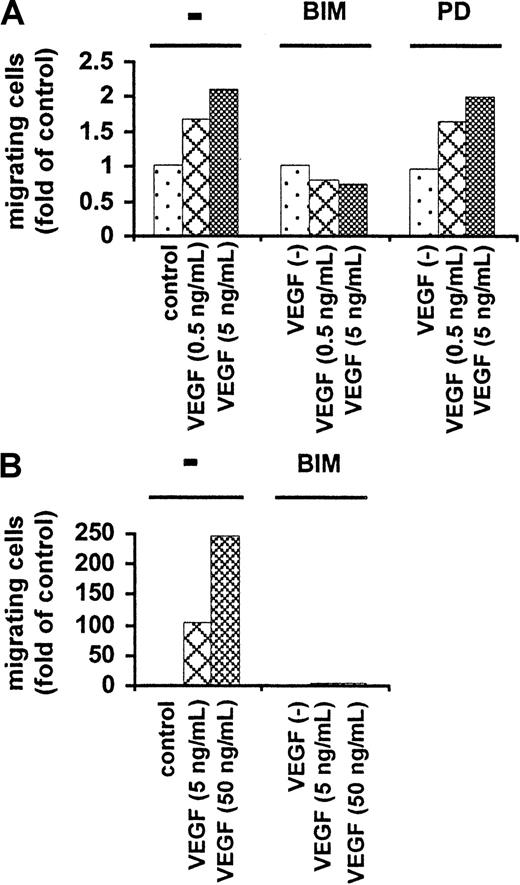
VEGF-triggered migration of MM and PCL cells is PKC dependent
The involvement of PKC in migration was next investigated. Growth-factor–deprived MM.1S cells were preincubated at 37°C for 60 minutes with BIM and then tested for their ability to migrate in the presence of those VEGF concentrations shown to trigger maximal migration (Figure 3). Figure 7A shows that PKC blockade with BIM, but not MEK-1 blockade with PD89059, abrogates migration in MM.1S cells. Similar results were observed in IM-9 MM cells (data not shown). The abrogation of VEGF-induced migration by BIM (5 and 50 ng/mL) was subsequently also demonstrated in patient PCL cells (Figure 8B). These results indicate the presence of VEGF-induced PKC-dependent, but ERK-independent, migration.
PKC dependency of VEGF-induced migration.
Growth-factor–deprived MM.1S cells (panel A) were pretreated with or without BIM or PD89059, plated on a polycarbonate membrane (8-μm pore size) in a modified Boyden chamber, and exposed for 4 hours to VEGF (0.5 and 5 ng/mL) added to the lower chamber. At the end of the treatments, cells on the lower part of the membrane were counted with a Coulter counter ZBII. Growth-factor–deprived patient PCL cells (panel B) were pretreated with BIM, and migration induced by VEGF (5 or 50 ng/mL) was analyzed as described above. Results are expressed as the fold migration of untreated cells (control). Representative results from 2 separate experiments are shown.
PKC dependency of VEGF-induced migration.
Growth-factor–deprived MM.1S cells (panel A) were pretreated with or without BIM or PD89059, plated on a polycarbonate membrane (8-μm pore size) in a modified Boyden chamber, and exposed for 4 hours to VEGF (0.5 and 5 ng/mL) added to the lower chamber. At the end of the treatments, cells on the lower part of the membrane were counted with a Coulter counter ZBII. Growth-factor–deprived patient PCL cells (panel B) were pretreated with BIM, and migration induced by VEGF (5 or 50 ng/mL) was analyzed as described above. Results are expressed as the fold migration of untreated cells (control). Representative results from 2 separate experiments are shown.
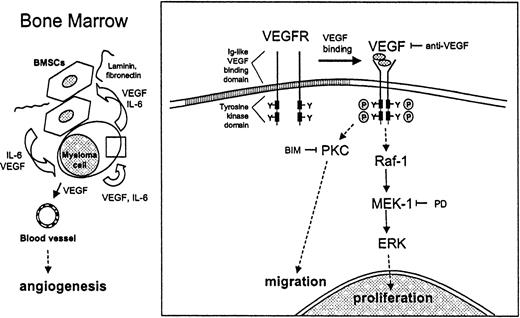
VEGF-mediated proliferation and migration of MM cells.
VEGF stimulates proliferation and migration of MM cells in a paracrine and autocrine pathway. Within the bone marrow, VEGF is produced by both MM cells and BMSCs. IL-6 secreted by BMSCs enhances the production and secretion of VEGF by MM cells. Conversely, VEGF secreted by MM cells enhances IL-6 production by BMSCs. Binding of MM cells to BMSCs increases both IL-6 and VEGF secretion. Besides stimulating angiogenesis in the BM, VEGF directly triggers cell growth and migration of MM cells via the Raf-1–MEK-1–ERK cascade and a PKC-dependent ERK-independent pathway, respectively.
VEGF-mediated proliferation and migration of MM cells.
VEGF stimulates proliferation and migration of MM cells in a paracrine and autocrine pathway. Within the bone marrow, VEGF is produced by both MM cells and BMSCs. IL-6 secreted by BMSCs enhances the production and secretion of VEGF by MM cells. Conversely, VEGF secreted by MM cells enhances IL-6 production by BMSCs. Binding of MM cells to BMSCs increases both IL-6 and VEGF secretion. Besides stimulating angiogenesis in the BM, VEGF directly triggers cell growth and migration of MM cells via the Raf-1–MEK-1–ERK cascade and a PKC-dependent ERK-independent pathway, respectively.
Discussion
This study shows that VEGF, in addition to its known stimulation of BM angiogenesis, also has direct effects on MM cells. As a model system, we used the MM.1S MM cell line, as well as patient MM and patient PCL cells. Our studies suggest that VEGF stimulates proliferation and migration of MM cells in both autocrine and paracrine mechanisms (Figure 8). Within the BM, VEGF is produced by both MM cells and BMSCs.18,19 IL-6 secreted by BMSCs enhances the production and secretion of VEGF by MM cells; conversely, VEGF secreted by MM cells enhances IL-6 production by BMSCs.19 Binding of MM cells to BMSCs enhances both IL-6 and VEGF secretion. Our studies show that VEGF directly triggers growth and migration of MM cells via the Raf-1–MEK-1–ERK cascade and a PKC-dependent, ERK-independent pathway, respectively.
Our studies first demonstrated that VEGF is produced and secreted by MM cells, suggesting an autocrine VEGF loop in MM. Many hematopoietic tumor cell lines, as well as freshly isolated hematological tumor cells including MM cells, have been reported to produce and secrete VEGF,18,19,41,42 consistent with an autocrine VEGF pathway. Our previous studies have demonstrated that IL-6 production by BMSCs is stimulated by the adhesion of MM cells,43-45 and more recent studies show increased VEGF secretion in BMSCs after tumor cell binding. Adhesion of MM cells to BMSCs would therefore enhance the VEGF autocrine loop. VEGF stimulates IL-6 in BMSCs,18 19suggesting that VEGF produced by MM cells can augment IL-6 secretion by BMSCs, thereby also augmenting MM growth and survival indirectly.
Our studies first showed the expression of VEGF121 (516 bp), VEGF145 (588 bp), and VEGF165 (165 bp) isoforms in MM.1S, patient MM cells, and patient PCL cells, respectively. Interestingly, we could also detect VEGF145, a rarely expressed and sparsely studied VEGF form recently found in PELs and prostate cancer.12 Whether the expression of this splice form plays a role in the pathogenesis of MM is unknown. We next investigated the secretion of VEGF by these cells. MM.1S, patient MM cells, and patient PCL cells secrete VEGF in the range of 100 to 1000 pg/mL (Figure 1B). Taken together, these data show that VEGF is produced and secreted by MM.1S, patient MM, and patient PCL cells and suggest both paracrine and autocrine VEGF-mediated effects on MM cells.
MVD has been observed in the BM of MM patients with disease progression and poor prognosis.20-22 In this report we found additionally (1) that VEGF induces modest increments in proliferation in MM.1S and patient MM cells and (2) that VEGF triggers migration of MM.1S and patient PCL cells. Interestingly, maximal activation of migration was much higher in patient PCL than in MM.1S cells, suggesting an important role of VEGF in the transition of MM to PCL. The complex phenomenon of cell migration involves both cytoskeleton-regulated cell motility and cell adhesion,46and in ongoing studies we are examining the differential expression of proteins in MM and PCL cells that are associated with these functions.
These data are also consistent with recent observations that VEGF not only has effects on endothelial cells, but also triggers biological responses in tumor cells. For example, VEGF appears to be a critical cytokine modulating the growth (approximately 3-fold) and spread of Kaposi sarcoma cells.47-52 Additionally, VEGF has been shown to increase proliferation (approximately 2-fold) and migration of leukemic cells.53 The presence of VEGF receptors on hematologic malignancies, as well as these actions on tumor cells triggered by VEGF, may indicate its important role in hematologic malignancies including MM. When coupled, these data strongly indicate a role of VEGF in tumor cells in general and in MM cells in particular.
Having demonstrated these distinct biological sequelae of VEGF in MM cells, we began to delineate the responsible signaling pathways. VEGF mediates its activity mainly via 2 receptor tyrosine kinases (RTKs): Flt-154 and KDR.55 These VEGF-RTKs, which bind VEGF, are single-pass transmembrane receptors with 7 immunoglobulinlike loops in the extracellular domain.56Although Flt-1 has been found on several hematopoietic tumor cell lines, to date neither KDR nor Flt-1 has been identified on MM cells.18 We found the high-affinity VEGF receptor Flt-1 in MM.1S; in contrast, KDR transcript and protein expression were not detected by RT-PCR and immunoprecipitation, respectively. Activation of VEGF-RTKs occurs through ligand binding, which facilitates receptor dimerization and autophosphorylation of tyrosine residues in the cytoplasmic portion. By immunoprecipitating cell lysates with Flt-1 antibody, we subsequently showed that VEGF triggered tyrosine-specific phosphorylation of the receptor. Interestingly, further supporting the activation of Flt-1 by VEGF, phosphorylation was also seen in coimmunoprecipitated proteins (data not shown). Studies are presently underway to define these proteins and delineate their function. RT-PCR analysis confirmed expression of Flt-1 comparable to that in MM.1S cells in patient MM cells and patient PCL cells (data not shown). Owing to a restricted number of these cells, we were not able to extract sufficient protein to perform biochemical studies similar to those in MM cell line MM.1S cells. Although the present report shows the expression and activation of Flt-1 in MM.1S cells, recent studies have found either no or only weak expression of Flt-1 and KDR on MM cell lines and primary cells.18 19 It therefore remains possible that an additional biologically active VEGF receptor, distinct from Flt-1 and KDR or of mutant forms of these RTKs, is expressed on MM cells.
To characterize downstream signaling molecules, we first investigated whether VEGF activated the ERK pathway in MM cells. The activation of ERK-1 and ERK-2 mediates DNA-synthesis.31 32 In time-course experiments using VEGF165, specific phosphorylation of ERK-1 and ERK-2 was observed in MM.1S, patient MM, and patient PCL cells. Although cascades activated by VEGF are similar, activation kinetics varied in those cells. Phosphorylation was inhibited by pretreatment with anti–human VEGF antibody, confirming selective ERK activation by VEGF. ERK phosphorylation was also inhibited by pretreatment with MEK-1 inhibitor PD89059, also implicating MEK-1 in the VEGF signaling cascade of MM cells. In addition, we observed VEGF-induced electrophoretic mobility shifting of Raf-1 in MM.1S cells that was inhibited with anti–human VEGF antibody, indicating VEGF-triggered Raf-1 activation. Neither the other prominent MAPK pathways (p38, SAPK) nor the STAT 3 kinase pathway were activated by VEGF. To support the importance of the ERK pathway in mediating VEGF-triggered DNA synthesis in MM cells, [3H] thymidine incorporation assays using 100 and 1000 ng/mL of VEGF, in the absence or presence of PD89059, were carried out in MM.1S cells. Proliferation induced by VEGF was blocked by PD89059, but not by the PKC inhibitor BIM. Migration of MM as well as of PCL cells induced by VEGF was inhibited by means of the PKC inhibitor BIM but not by PD89059. Importantly, PKC inhibition, in contrast to the MEK-1 inhibition, could not prevent the phosphorylation of ERK-1 and ERK-2. These data indicate the separation of VEGF-induced pathways causing distinct biological sequelae, proliferation through a PKC-independent Raf-1–MEK-1–ERK pathway, and migration through a PKC-dependent pathway.
This study adds another facet to the complex pathophysiological events in the BM micro-environment of MM: VEGF, besides stimulating angiogenesis, has direct effects on MM cells. Moreover, the range of VEGF target cells within the BM compartment of MM is probably even broader. Recently, the ability of VEGF to inhibit the maturation of dendritic cells57 through inhibition of nuclear factor κ B activation was demonstrated.58 Further, it was shown that VEGF increases osteoclastic bone-resorbing activity by enhancing their survival.59 The direct effects of VEGF on tumor cells, coupled with its effects in the BM micro-environment, suggest VEGF as a target for novel therapies to improve outcome in MM.
Dr T. Roberts (Dana-Farber Cancer Insitute, Boston, MA) for the gift of phospho-tyrosine and Raf-1 antibodies; Dr D. Frank (Dana-Farber Cancer Institute) for the gift of phospho–STAT 3 antibody; Ms. G. Young and L. Mazor for their technical assistance; and Dr H. Ludwig (Wilhelminenspital, Vienna, Austria) for helpful discussions.
Supported by National Institutes of Health grant PO-1 78378, and the Doris Duke Distinguished Clinical Research Scientist Award (K.C.A).
F.E.D. and S.L. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth C. Anderson, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail:kenneth_anderson@dfci.harvard.edu.

![Fig. 2. Effect of VEGF on MM cell proliferation. / MM.1S (panel A) and MM cells isolated from 5 patients (panel B) (3 × 104 cells per well) were cultured in RPMI 1640 medium with 5% (MM.1S) or 10% (patient MM cells) FBS in 96-well assay plates. The cells were then either left untreated or treated with 1 to 1000 ng/mL of VEGF165. Cell growth was assessed by addition of 0.5μCi [3H] thymidine [3H (dT)] per well during the last 9 hours of 72-hour cultures at 37°C. Radioactive labeling was determined by harvesting the cells onto glass-fiber filtermates with an automatic cell harvester (Tomec Harvester 96 Mach III) and counting by means of the Wallac Trilux Betaplate scintillation counter. Data represent means and SDs for triplicate samples. Statistical significance for the proliferation of MM.1S cells was determined by analysis of variance followed by an unpaired Student t test: *P < .005; **P < .001, as compared with control (without VEGF).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/2/10.1182_blood.v98.2.428/5/m_h81411288002.jpeg?Expires=1767840502&Signature=GH9GB4rxnIAJG7VF1bDHQ7vFHPzO~lJfSIX2572OeVLORPVkGy5Ge76Er07j5cP4EKKs4hchRpGkrAGV8qzFPprpfsEg9hGE~F7r2k-Vs-m3pN7-lYTP8x9frRJS50QV~xn7cOaqx9-KC45uLky1fWT3ggAocf9k-NLP-YAj68dQ0TDcHxFzAGrUbA6QA0cjLibWKzV5ElKXJUqUgtU2ImnvrxYqm0AQlQ~8taNd9MtlTyeyTWeL-JiJ9z~4tqM~bnfsLXzmd-e1n-gpluwYrvXjAHIATl5vNVGIcJeBS26ja5~u~oM6jrflIBNlNRAFwxPqtGVd7QMgewQ~OHL4Lw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 6. MEK-1 dependency of VEGF-induced proliferation. / MM.1S cells were starved for 3 hours in RPMI 1640 with 5% FBS. Cells were incubated with 100 or 1000 ng/mL VEGF165 with or without PD98059 (25μM) or BIM (2μM). For the last 9 hours of 72-hour cultures, 0.5μCi [3H] thymidine was added, and radioactivity incorporated into DNA was determined by scintillation counting (Wallac Trilux Betaplate scintillation counter) of automatically harvested cells (Tomec Harvester 96 Mach III). Results are expressed as fold-proliferation of untreated cells (control). The data are means of 3 wells, and the experiment was repeated twice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/2/10.1182_blood.v98.2.428/5/m_h81411288006.jpeg?Expires=1767840502&Signature=dbh3tDY92LOM8PdoX3J27mzSGbdowgx8iGEw6SlaP-SLB6ryv4cH2N6ZZx8wOGTWTziwoLFt79whfEZuQguh4OJTnthA4PXoa3Pxd0uSnb07wfrrmmvyzxRHImWFaR0Y0fi2pw7SXZO9BDCPObOnkdSeSXtNxo-vvHXwvpQi0nGShKN4opkU5TSVcY~qbbgb0RQWDKIHngEXVQYAxIo2TGSFKkJC41GZYwsMRCv~3gKW7o9L51NuQCH6tvNnwOrgVHvDSbdCyVAB~X1OA6L9BDfDjjNcnK9j5zslM2QKRN2W3LZbcMMNO1J0Cb285eLy~shefQRW~o7iwznu2UdLCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Fig. 2. Effect of VEGF on MM cell proliferation. / MM.1S (panel A) and MM cells isolated from 5 patients (panel B) (3 × 104 cells per well) were cultured in RPMI 1640 medium with 5% (MM.1S) or 10% (patient MM cells) FBS in 96-well assay plates. The cells were then either left untreated or treated with 1 to 1000 ng/mL of VEGF165. Cell growth was assessed by addition of 0.5μCi [3H] thymidine [3H (dT)] per well during the last 9 hours of 72-hour cultures at 37°C. Radioactive labeling was determined by harvesting the cells onto glass-fiber filtermates with an automatic cell harvester (Tomec Harvester 96 Mach III) and counting by means of the Wallac Trilux Betaplate scintillation counter. Data represent means and SDs for triplicate samples. Statistical significance for the proliferation of MM.1S cells was determined by analysis of variance followed by an unpaired Student t test: *P < .005; **P < .001, as compared with control (without VEGF).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/2/10.1182_blood.v98.2.428/5/m_h81411288002.jpeg?Expires=1767840503&Signature=HinmOJEUJLama1exIfssKJuc2IzSkmBj69MyAWzUo8T2bJojfIrRosoAs3t6UFzm~VQ~2ZdbmyVnqfNsjhbujHKq2ehXxqlJJ70jxo1t5uXm-zfbgu5JtOIFPN2hv3fW4KS5DjUZ6tPHhMs~YgFqvJNxmLJ8cyernkT1YoPYFUEkd6NNNSfKGDIW34pkbsScT-TmyQPle0NNWgAbE86xop9uAV3KMndOqPhD0-piH5k-DhFarrsajWj8-T80Olzrq5dG8z7pH0a5J-kPNaMbiLMe0rU~DvGreklmbPRx13GIpyeq~34-UHNfxOnGkHLiYwfPI1fRmgMwHUrSdpHvoA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Fig. 6. MEK-1 dependency of VEGF-induced proliferation. / MM.1S cells were starved for 3 hours in RPMI 1640 with 5% FBS. Cells were incubated with 100 or 1000 ng/mL VEGF165 with or without PD98059 (25μM) or BIM (2μM). For the last 9 hours of 72-hour cultures, 0.5μCi [3H] thymidine was added, and radioactivity incorporated into DNA was determined by scintillation counting (Wallac Trilux Betaplate scintillation counter) of automatically harvested cells (Tomec Harvester 96 Mach III). Results are expressed as fold-proliferation of untreated cells (control). The data are means of 3 wells, and the experiment was repeated twice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/2/10.1182_blood.v98.2.428/5/m_h81411288006.jpeg?Expires=1767840503&Signature=Whsznu876yLv6nesHsyV4Sby~dEg8Vvb4~5ifADKzvto12bWFjnfxGXqjLzhh4NNfBgZjcRAQyHMBP~QEfUimXXZQpF3rxDoHq9dW52S7slnhSaXSj4jZkgSI9Wnqqef9LM0Z0ER4aIXBDdpnV4lsLEyla-4C5UJHgOX8EJYhm3BwRm7Ipn5RDec8dfj1Cw8AUNpKcxAwfYUtHrlTLXalysFRvceR3ZrbA~WF8phU-fjXJgV96SyByWhyEAq1z537XBFup4xMn-v7~U~ap4JZ3TrYE-c8dX7rOBM~c4bcZZ~wcFRL3CyUyK2o1MvDd7I3a-1joguQZKgKijYrQnRlA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)