Ring-infected erythrocyte surface antigen (RESA)-positive,Plasmodium falciparum–negative red blood cells (RBCs) are cells from which the malaria parasite has been removed by the host without the destruction of the erythrocyte (“pitting”). The survival of RESA-RBCs in vivo was assessed in 14 severe and 6 uncomplicated falciparum malaria patients. The mean RESA-RBC life of 183 hours (95% confidence interval [CI], 136-246) was longer than the median parasite clearance time of 66 hours (range, 30-108 hours) but shorter than the mean red cell life of 1027 hours (95% CI, 840-1213) (P = .0004), with a median ratio of 0.2:1.0 (range, 0.1-0.7). The estimated median percentage of parasites pitted/body transit was 0.003% (range, 0.001%-0.05%). The rate of rise of the RESA-RBC count during the first 24 hours after antimalarial treatment was significantly faster (P = .036) and the subsequent RESA-RBC survival significantly shorter (P = .017) after treatment with an artemisinin derivative than after treatment with quinine. Parasitization of red cells leads to changes in the erythrocyte that shorten their survival even if the parasite is removed subsequently.
Introduction
The mechanisms responsible for the destruction of red cells during Plasmodium falciparum malaria remain poorly understood. The survival of both infected and uninfected red cells are reduced markedly.1,2 Red cell destruction occurs inevitably at merogony, and it has been assumed to occur during the clearance of parasites by host defenses. However, animal experiments conducted 30 years ago suggested that the spleen can remove intraerythrocytic parasites while leaving the host erythrocyte intact, a process referred to as “pitting,” analogous to the splenic removal of intraerythrocytic particles such as Heinz and Howell-Jolly bodies.3,4 In some patients with falciparum hyperparasitemia, the hematocrit does not fall as much as would be expected if all the parasitized red cells had been destroyed, suggesting that pitting also occurs in man.5 6 Clearance of parasites with red cell salvage would reduce the immediate impact of malaria infection on the red cell count, attenuating anemia.
P falciparum ring–infected erythrocyte surface antigen (RESA), or Pf 155, is deposited from dense granules in the apical part of the merozoite into the cytoplasmic surface of the erythrocyte membrane during parasite invasion. RESA persists in the erythrocyte membrane, enclosing ring-stage parasites.7-10 After fixation of blood films ex vivo, it can be detected easily using an indirect fluorescent antibody technique within the membrane of parasitized erythrocytes. Erythrocytes with residual membrane RESA but no cytoplasmic parasite DNA can be detected readily in the peripheral blood of patients with falciparum malaria.5,6 These cells do not occur in in vitro culture, supporting the hypothesis that host factors are responsible for the removal of the parasites without the destruction of the erythrocyte. Parasites may be removed by the spleen or peripheral phagocytes, be extruded by the red cell, or die and be degraded within the red cell, leaving the RESA behind as a “footprint” indicating the earlier presence of a malaria parasite.5,6 11 Hence, the red cells in a patient with falciparum malaria are composed of 3 distinct pools: infected (parasite+, RESA+) cells, once-infected or pitted (parasite−, RESA+) cells, and never-infected (parasite−, RESA−) cells.
Paradoxically, although all infected red cells are not destroyed during the removal of parasites, many uninfected red cells are destroyed, presumably also in the spleen, and their destruction is the major contributor to malarial anemia.12,13 Recent evidence suggests that reduced uninfected erythrocyte deformability may be important in precipitating their splenic clearance.14-16We hypothesized that the properties of once-infected cells are changed during the residence of the parasite and that they will therefore have reduced survival. We estimated the survival of once-parasitized, parasite−, RESA+ red cells and compared this with the estimated survival of the general red cell pool in the patient, which was composed of infected cells, once-infected cells, and never-infected cells.
Patients, materials, and methods
Patients
The study patients were admitted to Mae Sot Hospital in northwest Thailand. Patients were included if they or their attending relative gave informed written consent, they were aged 16 to 65 years, had single-species asexual P falciparum malaria parasitemia more than 1%, had good likelihood of being able to complete one-month follow-up, did not have a history of significant antimalarial drug intake within the previous 48 hours and, if female, were postmenopausal.
Severe malaria was defined17 as having one or more of the following criteria: Glasgow coma scale score less than 11 of 15, hematocrit less than 20% with asexual parasitemia more than 100 × 109/L (100 000/μL), bilirubin more than 43 μM/L with asexual parasitemia more than 100 × 109/L (100 000/μL), creatinine more than 264 μM/L with urine output less than 400 mL/24 hr, systolic blood pressure less than 80 mm Hg with cool extremities, asexual parasitemia more than 10%, plasma lactate more than 4 mM/L, glucose less than 2.2 mM/L, or serum bicarbonate less than 15 mM/L.
The study was approved by the Ethical and Scientific Review Committee of the Research Committee, Ministry of Public Health, Royal Government of Thailand.
Initial investigations
On admission, samples for full blood count, parasitemia, reticulocyte count, hemoglobin genotyping, plasma lactate, glucose and quinine dipstick18 and serum creatinine, urea, electrolytes, and liver function tests were taken. Subsequently, samples were taken for parasite counts and hematocrit every 6 hours until parasite clearance, defined as the first negative thick film after counting 200 white cells.
Reticulocytes were counted, on admission and then daily, in thin blood smears after staining with new methylene blue.19Hemoglobin genotyping was performed on the Bio-Rad Variant (Bio-Rad Laboratories, Hertfordshire, United Kingdom) using the β thalassemia short program. The area under the curve for the relationship between time and temperature above 37.0°C (AUCT 37.0) and 37.5°C (AUCT 37.5) was calculated from the weight of cut-out sections from photocopies of the patients' temperature charts.
Measurement of red cell survival and blood volume
Red cell volume was estimated,19,20 and red cell survival was calculated using Method “C” of the International Committee for Standardization in Haematology21 using sterile 51Cr (Amersham International, United Kingdom) as the red cell label.1,2,19,21 22
Venous blood samples (2 mL) were taken from the opposite arm of that which received the 51Cr injection, at 10 minutes and 6, 12, 24, 36, and 48 hours from the end of the injection. Samples for red cell and RESA cell survival and for hematocrit, parasitemia, and reticulocyte count were then taken daily until discharge and then at approximately 7, 14, 21, and 28 days. The gamma emissions of weighed samples of whole blood and plasma were measured, corrected for elution and background radioactivity,21-23 and the number of red cells per gram were calculated using the whole blood hematocrit. Gamma counts were made using a MiniAssay 6-20 Counter (Mini-Instruments, Burnham-on-Crouch, United Kingdom), and at least 10 000 counts were made per sample. All counts were performed on the same day as the blood samples were taken and expressed as a percentage of the 10-minute sample. Mean cell life was determined from the regression equation of the relationship between percentage red cell radioactivity per gram, corrected for isotope decay, and time from injection for all data points after 60 hours, to minimize the effect of an acute hematologic unsteady state.2 Log-linear and linear-linear plots were compared, and the model with the highest correlation coefficient was chosen, provided P ≤ .05. The mean cell life was calculated from log-linear plots as the time at which 37% of the initial activity was reached and for linear-linear plots as the intercept with the x-axis.21 22 This measure reflects the life of all the red cells present at the time of injection, ie, infected, once-infected, and those that either will or will not become infected during subsequent merozoite invasion.
Detection of RESA+ cells
Heparinized venous blood samples (2 mL) were collected and centrifuged at about 800g for 10 minutes. The plasma and buffy coat were discarded and the packed red cells washed 3 times in phosphate-buffered saline before being resuspended to 50% hematocrit in heat-inactivated fetal calf serum. Thin blood slides were made, air dried, fixed with absolute methanol, and stored at −30°C for about 1 month and then at −80°C for about 3 months until analysis. Thin films were stained for malaria parasites with ethidium bromide (EB) and for RESA with pooled, heated-inactivated malaria immune serum coupled with rabbit antihuman immunoglobulin G (5 μL; Dako, Denmark) conjugated with fluorescein isothiocyanate (FITC) as described previously.5,6 The FITC- and EB-stained parasite-infected red cells and FITC-stained but EB-unstained once-infected red cells were counted per 1000 red cells. If none of the former were detected, 3000 to 5000 red cells were counted. Anti-RESA antibodies were measured by an indirect immunofluorescence assay.5 6
The log-linear and linear-linear relationships between the parasite−RESA+ cell (hereafter, RESA-RBC, ie, excluding parasite+, RESA+ cells) counts, after their peak value, and time were compared and the mean RESA-RBC life calculated as for red cell life. Assuming that no new RESA-RBCs were generated after peripheral blood asexual parasite clearance, this relationship will reflect the loss of RESA-RBCs from the peripheral circulation. If a patient developed a reinfection or recrudescence of falciparum malaria, with a concurrent rise in the density of RESA-RBCs, only the initial slope after parasite clearance was used to calculate mean cell life. The mean residence time (MRT) was calculated as AUMC/AUC where AUC is the area under the RESA-RBC count–time curve and AUMC is the area under the RESA-RBC–time moment curve.24
Treatment
One patient, who was able to take oral medication, received oral dihydroartemisinin (DHA) and artesunate (combined dose 12 mg/kg body weight) and mefloquine (as below) as part of a clinical trial.25 Patients unable to take oral medication were treated, as part of clinical trials to be reported separately, with parenteral artesunate or quinine. Intravenous artesunate (Guilin No. 2 Factory, Guangxi, People's Republic of China), 2.4 mg/kg, was followed by 1.2 mg/kg at 12 hours and then daily until patients were able to take oral artesunate (Guilin No. 1 Factory, People's Republic of China) to give a total artesunate dose of 12 mg/kg over 7 days. Quinine dihydrochloride (Government Pharmaceutical Organisation, Bangkok, Thailand), 20 mg salt per kilogram intravenous loading dose, was followed by 10 mg salt per kilogram 3 times a day intravenously followed by oral quinine sulfate (Government Pharmaceutical Organisation), 10 mg salt per kilogram, 3 times a day to complete a 7-day course.
Patients also received either oral doxycycline hyclate (TO, Bangkok, Thailand), 100 mg twice a day for 7 days, or oral mefloquine (Lariam, Roche), 15 mg/kg immediately with 10 mg/kg after 24 hours. Supportive and drug treatment followed standard guidelines.26Patients received blood transfusions if indicated clinically. If further malaria infections were discovered during follow-up, the patient was treated according to local guidelines.
Statistical analysis
Data were analyzed using SPSS 8.0 (Chicago, IL). Visual inspection and the Shapiro-Wilks test were used to judge whether distributions conformed to the normal distribution, and logarithmic transformations were used when appropriate. Normally distributed data were compared using Student t tests and nonnormally distributed data using the Mann-Whitney U and Wilcoxon signed-rank tests. The Fisher exact test was used to assess categorical variables, and correlations were assessed using Spearman and Pearson correlation coefficients. Forward multiple regression analysis was used for multiple comparisons.
Results
Patients
Admission variables.
A total of 21 patients, 11 Thai and 10 Burmese (20 male, 1 female), were recruited in 1997 and 1998 (Table1). One patient was lost to follow-up, and his data are excluded. Mean (95% confidence interval [CI]) age and body weight were 31.9 years (range, 26.0-37.8) and 51.5 kg (range, 48.9-54.1), respectively. Six patients had uncomplicated malaria, and 14 patients had severe malaria (8 with hyperlactatemia, 8 with hyperbilirubinemia, 7 with hyperparasitemia). None had cerebral malaria as defined as a Glasgow coma scale score less than 11 of 15. No patient had had a splenectomy, but one patient was found to be positive for human immunodeficiency virus antibody (CD4 lymphocytes = 198 cells/μL), and one had non–insulin-dependent diabetes mellitus with peripheral neuropathy. Two of 20 patients had detectable admission serum quinine but at low concentrations (2.5 mg/L or below). Three patients were hemoglobin E heterozygotes, 1 was hemoglobin E homozygous, 1 was β thalassemia heterozygous, and the remaining 15 patients had normal (AA) hemoglobin genotypes.
The geometric mean admission parasitemia was 283.4 × 109/L (283 400/μL) (95% CI, 183.95 × 109/L to 436.616 × 109/L [183 950-436 616/μL]; range 45.216 × 109/L to 1 883.246 × 109/L [45 216-1 883 246/μL]), and the mean admission hematocrit was 0.401 (40.1%) (95% CI, 0.370-0.432 [37.0%-43.2%]; range 0.285-0.540 [28.5%-54.0%]). Of the admission clinical and laboratory measures, only plasma lactate and serum total bilirubin differed significantly between patients with severe and uncomplicated malaria (Tables 1 and2).
The primary drug treatment was artesunate in 12 patients, DHA in 1, and quinine in 7. Admission clinical and laboratory measures did not differ between the quinine and artemisinin derivative–treated patients (P > .05; Table 3).
Clinical course.
All patients survived. Acute oliguric renal failure, recurrent hypoglycemia, severe hyponatremia, and an ulnar nerve palsy occurred in one patient each. No patient developed blackwater fever, frank hemolysis, or blood loss. One patient received a 410-mL packed RBC transfusion 53 hours after 51Cr injection. The median fall in hematocrit during hospital admission was 0.24 (24%) (range, 0.12-0.37 [12%-37%]), and the minimum hematocrit was reached in a median of 42 (range, 6-264) hours. The mean admission reticulocyte count was 53.16 × 109/L (53 160/μL) (95% CI, 35.199 × 109/L to 71.122 × 109/L [35 199-71 122/μL]), which rose to a mean peak count of 58.668 × 109/L (58 668/μL) (95% CI, 32.319 × 109/L to 85.018 × 109/L [32 319-85 018/μL] ) after a median of 370 hours (range, 72-592). The admission and peak reticulocyte counts and time to peak reticulocyte count did not differ significantly between patients who were treated with quinine or an artemisinin derivative, or between those with severe or uncomplicated malaria, and were not associated significantly with admission hematocrit or the hematocrit at 3 weeks. The admission parasitemia was correlated positively with the subsequent AUCT 37.0 (r = 0.6,P = .005).
Further symptomatic uncomplicated falciparum infections occurred in 4 of 16 patients followed for 28 days, all having presented initially with severe malaria. The median recurrent parasitemia was 0.140 × 109/L (140/μL) (range 0.04 × 109/L to 0.24 × 109/L [40-240/μL]). A further 3 patients are known to have developed falciparum malaria between days 39 and 52. Reinfection and recrudescence were not distinguished.
RESA-RBC acute changes
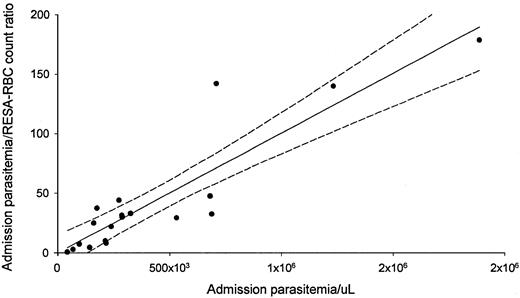
All but one patient had RESA+, parasite−RBCs detected on admission. The median percentage of these RESA red cells was 0.4% (range 0%-1.5%), and the median ratio of parasitized cells to RESA-RBCs on admission was 30:1 (range 1.0:1.0-179:1) (Tables 2 and 3). There was a highly significant linear relationship between the admission parasitemia:RESA-RBC count ratio and admission parasitemia (Figure 1;r = 0.86, P < .001) but not between admission log RESA-RBC counts and parasitemia (P = .08). No relationships were evident with the admission hematocrit or duration of illness. Five of the patients with severe and none of those with uncomplicated malaria had anti-RESA antibodies detected on admission (Table 2).
Admission parasitemia and admission parasitemia:RESA-RBC count ratio are positively correlated.
Data are from 19 patients.
Admission parasitemia and admission parasitemia:RESA-RBC count ratio are positively correlated.
Data are from 19 patients.
The RESA-RBC count rose after drug treatment in all patients to a median of 1.8% (range 0.8%-8.8%) of red cells, equivalent to a geometric mean RESA-RBC density of 66.237 × 109/L (66 237/μL) (95% CI, 48.809 × 109/L to 89.888 × 109/L [48 809-89 888/μL]). The median time to RESA-RBC peak was 46 hours (range 2-548) (Figure2, Tables 2 and 3). The median ratio of admission reticulocyte count to the reticulocyte count at the RESA-RBC count peak was 1.0 (range 0-5.3). There was therefore no significant, potentially confounding reticulocyte response during the period in which parsitized cells were being pitted.
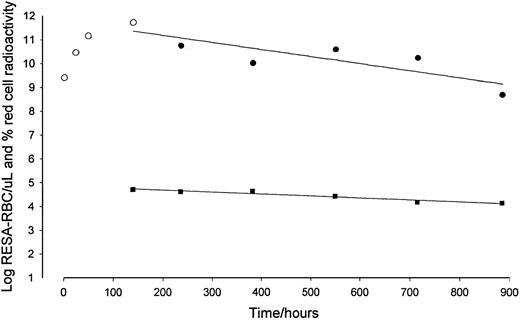
The log RESA-RBC count declines faster than the log red cell activity.
Data from one patient; red cell activity (▪), RESA-RBC count before and at peak (○), and from peak (●).
The log RESA-RBC count declines faster than the log red cell activity.
Data from one patient; red cell activity (▪), RESA-RBC count before and at peak (○), and from peak (●).
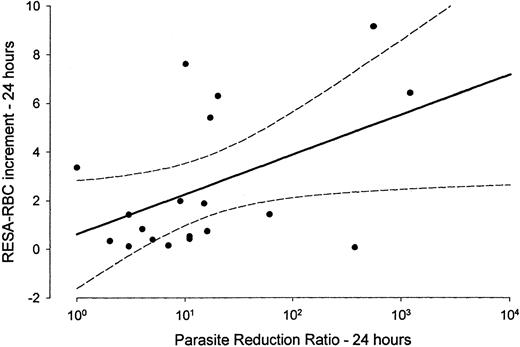
The median fractional increment in RESA-RBCs, from admission to 24 hours, was approximately 5 times greater for patients treated with an artemisinin derivative than those treated with quinine (Figure3, Table 3). The fractional RESA-RBC increment to 24 hours was correlated positively (r = 0.63,P = .004) with the log parasite reduction ratio at 24 hours (Figure 4). The overall median fractional increase in the RESA-RBC count, from admission to the RESA-RBC peak, was 4.0 (range 0.3-17.0) (Tables 2 and 3).
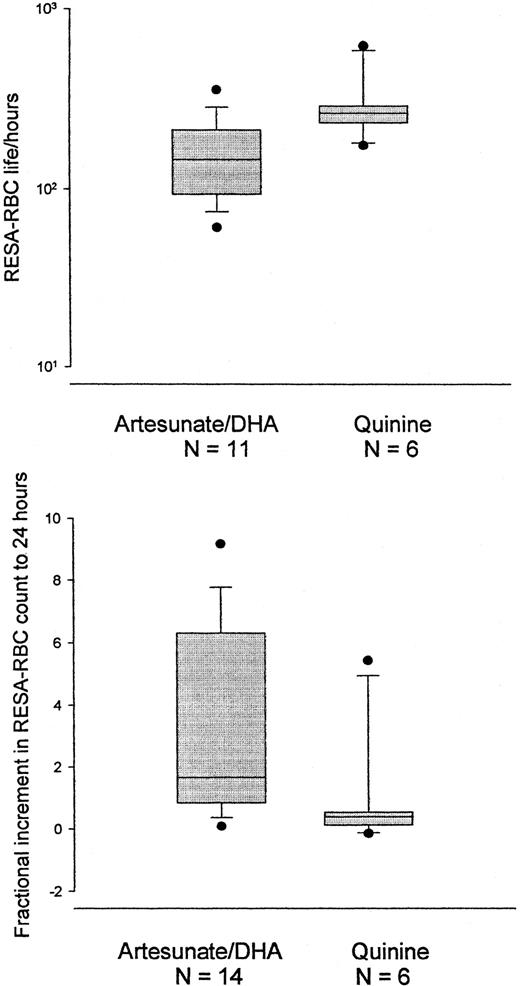
Mean life and fractional increase for RESA-RBC.
The log RESA-RBC mean life (upper) is shorter and the RESA-RBC fractional increase between admission and 24 hours (lower) is higher for patients treated with an artemisinin derivative than for patients treated with quinine. Median, 10th, 25th, 75th, and 90th percentiles and outliers are shown.
Mean life and fractional increase for RESA-RBC.
The log RESA-RBC mean life (upper) is shorter and the RESA-RBC fractional increase between admission and 24 hours (lower) is higher for patients treated with an artemisinin derivative than for patients treated with quinine. Median, 10th, 25th, 75th, and 90th percentiles and outliers are shown.
The log parasite reduction ratio 0-24 hours is positively correlated with the RESA-RBC increment at 24 hours.
Data are from 19 patients.
The log parasite reduction ratio 0-24 hours is positively correlated with the RESA-RBC increment at 24 hours.
Data are from 19 patients.
For the 7 patients, all treated with an artemisinin derivative, with more than 2 RESA-RBC counts before and including the peak value and with a linear relationship between time and RESA-RBC counts, the median net rate of increase of RESA-RBCs was 785 cells/μL whole blood per hour (range 241-6226). The median admission red cell volume of these 7 patients was 957 × 103/μL (range 734 × 103 to 2850 × 103). Therefore, the median “pitting rate” per patient can be estimated to be 21 × 108 cells per person per hour (range 5 × 108 to 126 × 108). Assuming a circulation time of 45 seconds, the estimated median pitting rate per transit is 26 × 106 cells per person per transit (range 6 × 106 to 177 × 106). If the density of parasites delivered to the spleen is the same as the density in the peripheral blood, the median percentage of parsitized cells pitted per body transit is estimated to be 0.003% (range 0.001%-0.05%).
The median ratio of rise in RESA-RBC count from admission to peak to fall in parasite count from admission to the RESA-RBC peak was 0.2:1.0 (range 0.1:1.0-3.8:1.0). In 3 patients, the proportion exceeded 1 (median 1.3, range 1.01-3.8), ie, in 3 patients the increment in RESA-RBCs exceeded the fall in parasitemia. These patients did not differ significantly from the remaining 17 in measures of disease severity, organ involvement, or drug therapy (P > .05).
RESA-RBC survival
The geometric mean RESA-RBC cell life was 183 hours (95% CI, 136-246) and did not differ significantly between patients with severe and uncomplicated malaria (Figure 2, Table 2). These calculations were based on a median of 7.5 data points (range 4-22) from and including the peak RESA-RBC count with a median correlation coefficient of 0.90 (range 0.64-0.99). The patient given a blood transfusion received it before the RESA-RBC peak, and therefore the RESA-RBC mean life was calculated as described above. The best fits were linear-linear and log-linear for the data sets from 1 and 16 patients, respectively. Neither relationship fitted 3 data sets, and 1 patient was lost to follow-up; these 4 patients were excluded from RESA-RBC mean life calculations. The mean RESA-RBC MRT for all patients was 282 hours (95% CI, 233-332) and did not differ significantly between patients with severe or uncomplicated malaria.
The presence of RESA antibodies was unrelated to admission or peak RESA-RBC counts, time to RESA-RBC peak, or to mean RESA-RBC life (P > .05). In 8 of the 9 patients followed for at least 28 days without malaria recurrence, RESA-RBCs were still detectable after 4 weeks with a geometric mean cell count of 10 481/μL (95% CI, 4436-24 763). The geometric mean RESA-RBC count at 4 weeks in the 7 patients who had a subsequent recrudescence of falciparum malaria was 15 314/μL (95% CI, 7768-30 193), which was not significantly different from those who were cured (P = .5). Of the 4 patients who developed further episodes of falciparum malaria during the 4-week follow-up, a concurrent rise in the RESA-RBC count was apparent in 2.
There were no significant differences in admission RESA-RBC count, mean RESA-RBC life, or RESA-RBC MRT between patients with elevated (more than 4 mM/L) and normal plasma lactate, the presence of anti-RESA antibodies or hemoglobinopathy, or the recurrence of falciparum malaria. The geometric mean RESA-RBC mean cell life was significantly longer after treatment with quinine at 282 hours (95% CI, 181-440) than after an artemisinin derivative at 145 hours (95% CI, 102-205) (P = .017) (Figure 3, Table 3).
There was no evident relationship between RESA-RBC life, admission RESA-RBC count, red cell mean cell life, AUCT 37.0, AUCT 37.5, and measures of disease severity, such as parasitemia, serum creatinine, and spleen size. Forward multiple regression analysis including drug treatment, RESA-RBC fractional increment at 24 hours, hemoglobinopathy, and admission plasma lactate as independent variables suggested that only drug treatment had a significant effect (P = .02, β = 0.569) on mean RESA-RBC life, explaining 32% of its variability.
Red cell survival
The mean red cell life was 1027 hours (95% CI, 840-1213; range 421-1734) and did not differ significantly between patients with uncomplicated and severe malaria or between those given artesunate or quinine (Figure 2, Tables 2 and 3). The median number of data points after and including 60 hours from 51Cr injection was 7 (range 4-15) with a median correlation coefficient of 0.98 (range 0.84-0.99). The best fits were log-linear for data from 11 patients and a linear-linear relationship for 8 patients. There was no significant linear relationship in the data from one patient, which were excluded. The mean red cell survival for the patient who received a blood transfusion was calculated using the slope derived from the posttransfusion regression equation and the intercept from the pretransfusion regression equation.
There were no significant associations between mean red cell life and lactate, hemoglobin genotype, antimalarial used, or recurrent infection. Mean red cell life was correlated positively with hematocrit on admission (r = 0.65, P = .003) and negatively with parasite clearance time (r = −0.57,P = 0.013). Forward multiple regression analysis of mean red cell survival with duration of illness, admission parasitemia, hematocrit, reticulocyte count, serum urea and bilirubin, and parasite clearance time as independent variables yielded significant correlations with admission parasitemia (P = .003, β = −0.609) and admission hematocrit (P = .024, β = 0.024), jointly explaining 66% of the variability in mean red cell life.
RESA-RBC survival was significantly shorter than the total red cell mean cell life (P = .0004, n = 16). The median ratio of mean life span of RESA-RBCs to red cells, for severe and uncomplicated malaria patients combined, was 0.2:1.0 (range 0.1:1.0-0.7:1.0; Table 2).
Discussion
The mean red cell survival in patients with uncomplicated and severe malaria was 49 and 40 days, respectively, consistent with previous studies of Thai adults with malaria and considerably shorter than the survival of heterologous red cells in healthy Thai adults.1,2,20 There are 4 potential problems in applying the 51Cr technique to patients with malaria. First, the51Cr technique may overestimate mean red cell survival because chromium is taken up preferentially by younger cells,22 although this will be balanced by the propensity for falciparum malaria to infect and destroy younger red cells and the suppression of reticulocytosis in acute malaria.27Second, the calculated survival is dependent on the elution correction factor,23 and whether these corrections are appropriate for red cells rigidified by malaria infection16 is unknown. Third, a hematologic steady state, which is a condition for the valid use of this technique, is unlikely to apply acutely. We attempted to minimize this by calculating the red cell survival from 60 hours after51Cr injection.2 Fourth, ascorbic acid is a potent antioxidant and may protect labeled red cells susceptible to oxidant damage, especially those with abnormal hemoglobin types,28 thereby overestimating red cell survival. There is also evidence that, paradoxically, ascorbic acid acts as a pro-oxidant within parasitized erythrocytes,29 potentially enhancing parasite killing.
Our estimate of red cell survival reflects the survival of 3 pools of red cells within the blood: those that are infected and subsequently destroyed at merogony or by the host humoral and cellular responses; the pitted, once-infected red cells; and those that were never infected. Recent models using large data sets from patients with falciparum malaria suggest that the destruction of the never-infected red cells is the major contributor (about 81%-92%) to the loss of red cells.12,13 The principal mechanism involved in the destruction of never-infected red cells is probably the splenic clearance of red cells with reduced deformability, induced by an as yet unidentified mechanism.14-16 Additional possible explanations include immune hemolysis30 and the attachment of merozoites to the external surface of erythrocytes.13The observed negative relationship between peripheral parasitemia and red cell survival presumably reflects higher parasite loads leading to enhanced destruction of both parasitized and unparasitized cells. The positive relationship between red cell life and admission hematocrit presumably reflects the loss of red cells before treatment, resulting in a lower hematocrit.
It is likely that the main site of both pitting and subsequent destruction of RESA-RBCs is the spleen.3 Whether the in vitro observations that human red cells can extrude malaria parasites31 or that peripheral phagocytic cells can remove parasites from human red cells11 are relevant in vivo remains to be determined. The faster rise in RESA-RBC count after treatment with an artemisinin derivative than after quinine probably reflects the greater efficacy of artemisinin derivatives in killing or damaging circulating ring-stage parasites.6 The spleen presumably pits circulating artemisinin-damaged parasites as they become particulate within the erythrocytes. Quinine has much less effect because it acts predominantly on the more mature, sequestered parasites, which do not circulate. The alternative hypothesis, that artemisinin derivatives have a direct effect on splenic function, is unlikely.
This study demonstrates that once-infected or pitted red cells survive approximately one-sixth as long as the combined pool of red cells present at the start of treatment. More severe malarial anemia is observed in children than adults, perhaps because of greater splenic clearance of poorly deformable red cells in children.15 If this mechanism is correct, the mean life of RESA-RBCs would be expected to be shorter in children than in adults.
The reduced survival of once-parasitized or pitted red cells may be explained by RESA binding to spectrin, a major component of the red cell cytoskeleton.32,33 Inherited abnormalities of spectrin have been shown to reduce the deformability of red cells, leading to shortened red cell survival, which is attenuated by splenectomy.34 RESA contains a chaperone-like motif, suggesting that RESA may protect spectrin from heat stress.35 Hence, the dispersion of RESA into the red cell membrane may protect the parasitized red cell but reduce the subsequent survival of the pitted cell. Alternatively, the spleen and/or peripheral phagocytes may recognize other aspects of RESA cells that mark them as different, such as relative spherocytosis, which may arise if the spleen removes some of the erythrocyte membrane during pitting, and reduced hemoglobin concentration. It is unlikely that the host humoral immune system recognizes RESA directly in once-infected cells, because RESA is not expressed on the surface of ex vivo cells,6 32 and in this study there was no difference in RESA cell survival between patients with or without anti-RESA antibodies.
The only significant determinant of RESA-RBC survival identified here is the drug used to treat malaria. The artemisinin derivatives were associated with a significantly shorter RESA-RBC life. The artemisinin derivatives may damage the parsitized red cell membranes directly. DHA, the active metabolite of the artemisinin derivatives, binds to spectrin in the red cell membrane and could alter its properties.36However, artesunate has been shown recently to have no significant effect on erythrocyte deformability.6 Alternatively, the faster and earlier net generation of pitted cells associated with treatment by an artemisinin derivative may shorten their life expectancy. RESA-RBCs generated early in the course of the illness may be more severely damaged, in the deranged pro-oxidant metabolic and immunologic milieu associated with the acute illness, than those generated as the patient recovers. They also encounter a more active spleen with a reduced recognition threshold for detecting red cell abnormalities.1,30,37,38 Alternatively, the high rate of cells presented for pitting acutely may overload the ability of the spleen to remove parasites efficiently, leading to increased membrane damage, analogous to the failure of Fc receptor–mediated red cell clearance in patients with high parasitemia.37 38 This is supported by the log-linear relationship between the parasite reduction ratio at 24 hours (PRR24) and RESA-RBC increment at 24 hours (Figure 4).
An unexpected excess of ring-form parasites in the brain microvasculature of patients dying from falciparum malaria has been demonstrated postmortem.39 That ring stages may pool or sequester in the deep tissues was supported by the demonstration that the proportion of RESA cells that appear in the circulation after treatment can exceed the fall in parasite count during the same period.6
Although the assumption that all parsitized red cells are destroyed is incorrect,12,13 20 RESA-RBCs are found at too low a density to lead to an important overestimation of the proportion of red cells lost acutely. However, because pitted erythrocytes are derived from cells parasitized with ring stages, which consume little hemoglobin, it is likely that these salvaged cells can contribute to the transport and delivery of oxygen and carbon dioxide, attenuating the consequences of parasitism.
We are grateful to the patients, to the Mae Sot Hospital director and nurses (especially Jantipa Sutham) and physicians and Dr Yupin Suputtamongkol for their help, and to Thanongsak Teewarakulpana for translation and facilitating patient follow-up. We thank Professor John Clegg for hemoglobin genotyping and Drs Michele van Vugt, Mayfong Mayxay, Brian Angus, and George Watt for their clinical assistance.
This study was part of the Wellcome Trust—Mahidol University—Oxford Tropical Medicine Research Programme, funded by the Wellcome Trust of Great Britain.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nicholas J. White, Faculty of Tropical Medicine, Mahidol University, 420/6 Rajvithi Rd, Bangkok 10400, Thailand; e-mail:fnnjw@diamond.mahidol.ac.th.