In 93 allograft recipients, the numbers of marrow B-cell precursors on days 80 and 365 correlated with the counts of circulating B cells, suggesting that the posttransplantation B-cell deficiency is at least in part due to insufficient B lymphopoiesis. Factors that could affect B lymphopoiesis were evaluated. The number of marrow B-cell precursors on days 30 and 80 was at least 4-fold lower in patients with grade 2 to 4 acute graft-versus-host disease (GVHD) compared with patients with grade 0 to 1 acute GVHD. The number of B-cell precursors on day 365 was 18-fold lower in patients with extensive chronic GVHD compared with patients with no or limited chronic GVHD. The number of B-cell precursors was not related to CD34 cell dose, type of transplant (marrow versus blood stem cells), donor age, or patient age. It was concluded that posttransplantation B-cell deficiency results in part from inhibition of B lymphopoiesis by GVHD and/or its treatment.
Introduction
Allogeneic hematopoietic cell transplant (HCT) recipients are immunocompromised for more than 1 year after grafting.1,2 Quantitative B-cell deficiency plays a role in the susceptibility of transplant recipients to infections.3 The quantitative B-cell deficiency may be due in part to low B-cell production (B lymphopoiesis) in the marrow of HCT recipients. Quantitative deficiency of B-cell precursors is observed in virtually all patients in the first month and in some patients for more than 1 year after grafting.4-6 We set out to determine which factors might influence B-cell–precursor quantity (index of B lymphopoiesis) in the marrow of HCT recipients. We hypothesized that the following factors might play a role: (1) CD34 cell dose; (2) graft type (marrow versus blood stem cells), as blood stem cell recipients receive 3 times more CD34 cells and 18 times fewer B-cell precursors compared with marrow recipients7; (3) patient or donor age, as young, normal individuals have more B-cell precursors than older individuals8-10; and (4) acute or chronic graft-versus-host disease (GVHD) or its treatment, as chronic GVHD and/or its treatment were associated with low marrow content of B-cell precursors in a small clinical study6 and B-lymphopoiesis was inhibited by glucocorticoids in a murine study.11 12
Study design
Since one of the goals of the study was to determine how B lymphopoiesis was influenced by the graft type, we studied patients randomized to receive either marrow or filgrastim-mobilized blood stem cells.13 Of 135 patients treated randomly with either transplant type at the Fred Hutchinson Cancer Research Center, 123 patients agreed to institutional review board–approved studies of immune reconstitution. Twelve patients died by day 30. One patient received marrow instead of blood stem cells and was excluded from analysis. For 17 patients, no marrow samples were submitted for the quantitation of B-cell precursors. Thus, 93 patients were evaluable for the quantitation of B-cell precursors between 1 and 12 months after transplantation. Their characteristics, including data on CD34 cell dose, graft type, age, and acute and chronic GVHD, are displayed in Table 1.
Marrow was aspirated on approximately days 30, 80, and 365 after transplantation. Cells were stained with CD19 antibody conjugated to phycoerythrin; CD45 antibody conjugated to peridinin-chlorophyll; and CD2, CD3, CD5, and CD33 antibodies conjugated to fluorescein isothiocyanate (Becton Dickinson, San Jose, CA). Erythrocytes were lysed by NH4Cl (0.83%, buffered with KHCO3, pH 7.2). Flow cytometry data acquisition was performed on CytoronAbsolute (Ortho, Raritan, NJ) or FACSCalibur (Becton Dickinson) instruments. Data analysis was performed by means of Winlist software (Verity House, Topsham, ME). Cellular debris and nonviable cells were first eliminated by forward- versus side-scatter gating. Then, the percentage of B-cell precursors (CD19+CD45lowCD2−/CD3−/CD5−/CD33−) of total CD45+ cells was determined.9,14 15 We used the percentage of B-cell precursors among marrow CD45+cells corrected for marrow cellularity (multiplied by the percentage of normal cellularity divided by 100) and for the ratio of myeloid and lymphoid cells to erythrocyte precursors (M:E ratio) since erythrocyte precursors do not express CD45 (multiplied by M:E ratio divided by [1 + M:E ratio]). For example, if 10% of marrow CD45+ cells were B-cell precursors, the hematopoietic cell–to–fat ratio was 25:75 (50% normal), and the M:E ratio was 4:1, then the corrected percentage of B-cell precursors was 10 × 0.5 × 0.8 = 4. Marrow cellularity and M:E ratio were estimated from standard decalcified biopsy sections or aspirated particle sections stained with hematoxylin and eosin. Marrow specimens from patients whose hematologic malignancy relapsed were not evaluated unless relapse was determined only by a polymerase chain reaction.
Results and discussion
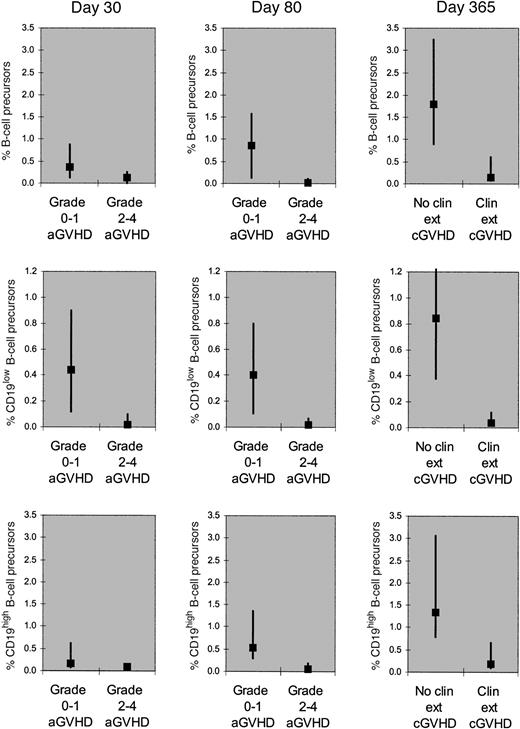
Patients who developed grade 2 to 4 acute GVHD by day 30 had a lower percentage of B-cell precursors on day 30 compared with patients who developed grade 0 to 1 acute GVHD by day 30 (median, 0.1 versus 0.4; P = .03, Mann-Whitney rank-sum test). Patients who developed grade 2 to 4 acute GVHD by day 90 had a lower percentage of B-cell precursors on day 80 compared with patients who developed grade 0 to 1 acute GVHD by day 90 (median, 0 versus 0.9;P < .001). Patients who developed clinical extensive, chronic GVHD in the first posttransplantation year had a lower percentage of B-cell precursors on day 365 compared with patients who developed no or limited chronic GVHD in the first year (median, 0.1 versus 1.8; P < .001) (Figure1, upper panels). We could not separate the effect of acute or chronic GVHD from the effect of glucocorticoids as there were few patients on glucocorticoids without GVHD and few patients off glucocorticoids with GVHD. The GVHD and/or its treatment depressed both early (CD19low) and late (CD19high) B-cell precursors; however, the early precursors appeared depressed to a greater degree (Figure 1, middle and lower panels).
Percentage of B-cell precursors among CD45+marrow cells (corrected for marrow cellularity and for M:E ratio).
Total, CD19low (early), and CD19high (late) B-cell precursors were quantitated (upper, middle, and lower panels, respectively). The corrected percentage of total B-cell precursors on day 30 was lower in patients who developed grade 2 to 4 acute GVHD by day 30 (n = 19) than in patients who did not do so (n = 52). The corrected percentage of total B-cell precursors on day 80 was lower in patients who developed grade 2 to 4 acute GVHD by day 90 (n = 54) than in patients who did not do so (n = 20). The corrected percentage of total B-cell precursors on day 365 was lower in patients who developed clinical extensive, chronic GVHD (n = 28) than in patients who developed no or limited chronic GVHD (n = 23) in the first posttransplantation year. The corrected percentages of the early precursors appear to be influenced by the GVHD and/or its treatment to a greater extent than the late precursors. The squares denote medians and the vertical bars denote 25th to 75th percentiles. In all 9 graphs, the difference between the 2 groups is significant (P < .05 for day 30; P < .01 for day 80;P < .001 for day 365).
Percentage of B-cell precursors among CD45+marrow cells (corrected for marrow cellularity and for M:E ratio).
Total, CD19low (early), and CD19high (late) B-cell precursors were quantitated (upper, middle, and lower panels, respectively). The corrected percentage of total B-cell precursors on day 30 was lower in patients who developed grade 2 to 4 acute GVHD by day 30 (n = 19) than in patients who did not do so (n = 52). The corrected percentage of total B-cell precursors on day 80 was lower in patients who developed grade 2 to 4 acute GVHD by day 90 (n = 54) than in patients who did not do so (n = 20). The corrected percentage of total B-cell precursors on day 365 was lower in patients who developed clinical extensive, chronic GVHD (n = 28) than in patients who developed no or limited chronic GVHD (n = 23) in the first posttransplantation year. The corrected percentages of the early precursors appear to be influenced by the GVHD and/or its treatment to a greater extent than the late precursors. The squares denote medians and the vertical bars denote 25th to 75th percentiles. In all 9 graphs, the difference between the 2 groups is significant (P < .05 for day 30; P < .01 for day 80;P < .001 for day 365).
Peripheral blood stem cell recipients and marrow recipients did not significantly differ in the percentage of total B-cell precursors on day 30, 80, or 365. Also, there was no significant correlation between CD34 cell dose, patient age, or donor age and the percentage of B-cell precursors on either day 30 or day 80 (Spearman rank correlation test). Similarly, there was no significant correlation between CD34 cell dose or patient age and the percentage of B-cell precursors on day 365. However, there was an inverse correlation between the percentage of B-cell precursors on day 365 and donor age (r = −0.32; P = .02). In multivariate analysis, the percentage of B-cell precursors on day 365 was significantly associated with clinical extensive chronic GVHD (P < .001) and not with donor age (linear regression analysis of log-transformed percentages).
We also evaluated whether a low percentage of B-cell precursors was associated with low circulating B-cell counts (determined as described [mononuclear cells expressing CD19 or CD20 and not brightly expressing CD3, CD13, CD14, CD16, CD56, CD10, or CD34]7 on days 30, 80, 180, and 365 after transplantation). The following correlations were found: (1) the percentage of B-cell precursors on day 80 and B-cell counts on day 80 (Spearman correlation coefficientr = 0.25; P = .057); (2) the percentage of B-cell precursors on day 80 and B-cell counts on day 180 (r = 0.35; P = .031); and (3) the percentage of B-cell precursors on day 365 and B-cell counts on day 365 (r = 0.50; P = .002).
The results show that between days 80 and 365 after transplantation, the size of the circulating B-cell pool depends on B lymphopoiesis and that GVHD and/or its treatment is the major factor influencing B lymphopoiesis. CD34 cell dose, graft type (blood stem cells versus marrow), patient age, and donor age do not affect B lymphopoiesis significantly.
In the first 3 months after grafting, blood stem cell recipients have significantly higher counts of circulating B cells than marrow recipients.7,16 Because the percentage of B-cell precursors on days 30 and 80 was similar in blood stem cell and marrow recipients, the higher circulating B-cell count in blood stem cell recipients is most likely due to the higher content of mature B cells in blood stem cell grafts compared with marrow grafts,7,16 17 rather than to increased B lymphopoiesis.
There are several possible mechanisms by which patients with GVHD have decreased B lymphopoiesis. The first is through production of cytokines from activated cells that may inhibit B lymphopoiesis. Examples include interferon-γ produced by T cells18 or interleukin-1 produced by macrophages during the GVHD-associated “cytokine storm.”12,19,20 The second possible mechanism is the destruction of marrow stromal cells, which support B lymphopoiesis,21 by donor lymphocytes (graft-versus-stroma reaction).22,23 The third mechanism is suppression of B lymphopoiesis through treatments given for GVHD, eg, glucocorticoids.11 12 Although some of these mechanisms might be amenable to a therapeutic intervention, significant improvement of B lymphopoiesis is likely to be achieved only through effective GVHD prophylaxis.
Supported by National Institutes of Health grants CA68496, AI46108, CA18221, and CA18029.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jan Storek, FHCRC, D1-100, 1100 Fairview Ave N, Seattle, WA 98109-1024; e-mail: jstorek@fhcrc.org.