Abstract
Administration of cyclosporine A (CsA) after autologous stem cell transplantation elicits an autoimmune syndrome with pathology similar to graft-versus-host disease (GVHD). This syndrome, termed autologous GVHD, is associated with the appearance of autoreactive T cells directed at major histocompatibility class (MHC) class II antigens. In the rat model of autologous GVHD, clonal analysis reveals that the effector T cells are highly conserved and recognize a peptide from the invariant chain peptide presented by MHC class II. Although human autologous GVHD effector T cells share a similar phenotypic specificity, clonality of the response in humans has not been determined. To examine the human effector T-cell response, the T-cell repertoire of peripheral blood lymphocytes was assessed by complementarity-determining region 3 (CDR3) size distribution analysis and T-cell clonotype analysis in 26 patients treated with CsA after transplantation. Autologous GVHD developed in 3 of 4 patients with human leukocyte antigen (HLA)-DRB1*0701, and clonal expansions of β-chain variable region (BV)16+ T cells were shared. Clonal expansions within BV15+ and BV22+ T cells were also detected in 4 of 6 patients with HLA-DRB1*1501 and in 3 of 4 patients with HLA-DRB1*0401, respectively. Sequencing of BV16 cDNA for which the CDR3 size pattern exhibited apparent clone predominance revealed an identical CDR3 peptide sequence in 2 different patients, one with HLA-DRB1*0701 and the other with HLA-DRB1*1502. These findings indicate that the discrete antigen-driven expansion of T cells is involved in autologous GVHD.
Introduction
High-dose chemoradiotherapy combined with autologous stem cell transplantation (ASCT) has been used successfully in the treatment of patients with malignant lymphoma or breast cancer. Clinical studies have demonstrated that among the therapeutic options available for relapsing lymphoma or metastatic breast cancer, ASCT is the most effective in achieving long-term survival.1-4However, clinical trials have failed to demonstrate any advantage of ASCT over conventional chemotherapy in the treatment of patients with either non-Hodgkin lymphoma (NHL) and a slow response to chemotherapy, with disease in remission but poor prognostic factors,5,6or breast cancer with extensive lymph node involvement.7These studies suggest that raising the dose intensity of chemotherapy, facilitated by the use of ASCT, may not necessarily improve the outcome. For patients who do not achieve sustained remission after conventional chemotherapy, novel approaches including immunotherapy are needed.
One such approach is the induction of autologous graft-versus-host disease (GVHD) after ASCT. Autologous GVHD is inducible in recipients of autologous bone marrow by the administration of cyclosporine A (CsA) for a short period after transplantation,8-13 resulting in an autoaggression syndrome that has a dermal pathology similar to that of acute GVHD after allogeneic marrow transplantation. Interestingly, CD8+ T cells that develop during autologous GVHD are also able to lyse myeloma and breast cancer cell lines.14,15T-cell clones associated with autologous GVHD have been isolated in rodents16,17 and are restricted to the T-cell receptor (TCR) β-chain variable region (BV)8.5 allele. These cells recognize MHC class II determinants in association with a peptide from the invariant chain (CLIP).17,18 In humans, the BV15 allele appears to be the dominant TCR in cells infiltrating the skin during autologous GVHD.19 However, clear evidence that antigen-specific T cells are associated with human autologous GVHD has not been obtained. If specific recognition by T cells plays a role in the development of autologous GVHD, clonal expansion of a limited number of T cells should be observed in the peripheral blood (PB) of patients with autologous GVHD.
To evaluate this hypothesis, the T-cell repertoire in peripheral blood lymphocytes (PBL) from 26 patients treated with CsA after ASCT was analyzed. Complementarity-determining region 3 (CDR3) size distribution analysis and sequencing of TCR cDNA were used to determine the clonality of T-cell expansion for each BV family. The CDR3 size pattern of several BV subfamilies that were normal before ASCT became abnormal after ASCT plus CsA treatment, suggesting that clonal or oligoclonal expansion of a limited number of T cells had occurred. Among these BV subfamilies, clonal expansions within BV16+ T cells were shared by 7 of 11 patients in whom autologous GVHD developed and in 5 of 15 patients without clinical evidence of GVHD. Interestingly, both autologous GVHD and clonal expansion within BV16+ T cells developed in 3 of 4 patients with human leukocyte antigen (HLA)-DRB1*0701. Moreover, clonal expansions within BV15+or BV22+ T cells were detected in 4 of 6 patients with HLA-DRB1*1501 and in 3 of 4 patients with HLA-DRB1*0401, respectively. Sequencing of BV16 cDNA for which the CDR3 size profile exhibited apparent clone predominance in autologous GVHD-induced patients revealed significant homology in the joining segment. The same amino acid sequence of the CDR3 domain occurred in 2 different patients with HLA-DRB1*0701 or HLA-DRB1*1502.
Patients, materials, and methods
Patients and autologous blood cell transplantation
Thirty-one patients with breast cancer or malignant lymphoma were enrolled in this study, as previously described.20 21Ten patients with NHL (group A, between 35 and 56 years of age) with poor prognostic factors and 21 patients with metastatic breast cancer (group B, between 18 and 60 years of age) in complete or partial remission were included. This treatment protocol was reviewed and approved by the institutional review board, and informed consent was obtained from all patients.
All patients with NHL (group A) underwent chemotherapy before autologous peripheral blood stem cell transplantation (PBSCT). Before PBSCT, 2 patients underwent conditioning with high-dose chemotherapy consisting of ramimustine (200 mg/m2 intravenously (IV) days −8 and −3), carboplatin (300 mg/m2 IV days −7, −6, −5, −4), etoposide (500 mg/m2 IV days −6, −5, −4), and cyclophosphamide (50 mg/kg IV days −3, −2). The remaining 8 patients underwent a conditioning regimen consisting of cyclophosphamide (50 mg/kg IV days −3, −2) and fractionated total body irradiation at 3 Gy daily for 4 days (total, 12 Gy) in combination with cytarabine (4 g/m2 IV days −4, −3) for 5 patients or etoposide (15 mg/kg IV −4, −3) for 2 patients. Because contamination of the leukapheresis product by lymphoma cells was suspected in 4 patients (patients 2, 3, 4, 6), enrichment of CD34+ cells was performed. All patients with breast cancer (group B) were prepared for autologous bone marrow transplantation by cyclosphosphamide (1.5 g/m2 IV) and thiopeta (200 mg/m2 IV; 4 times daily) before autologous bone marrow rescue.
CsA (2.5 mg/kg·d; Novartis, Hanover, NJ) was administered intravenously to the patients from days 1 through 21 or 28. Recombinant interferon-γ (IFN-γ, 50 μg/m2) was administered subcutaneously to group A patients weekly and (25 μg/m2) to group B patients every other day from days 7 through 28 to induce up-regulation of MHC class II determinants and to enhance tumor cell recognition.14,21 Granulocyte-colony stimulating factor (5 μg/kg·d) was given to the patients beginning on day 1 and continuing until the leukocyte count exceeded 5.0 × 109/L. Control patients underwent ASCT using the same preparative regimens but without the administration of CsA and interferon-γ.20,21 Table 1summarizes the characteristics of the patients. Patients were evaluated daily for evidence of autologous GVHD (erythematous rash) that was confirmed by skin biopsy, as previously described.20 21Twenty-six patients were included in the analysis of T-cell repertoire in the PB. Because autologous GVHD usually occurs during a short period after transplantation, the number of peripheral blood mononuclear cells (PBMCs) was limited. Therefore, not all analyses (ie, CDR3 size distribution analysis and T-cell clonotype analysis) could be performed in all patients.
Cytotoxicity assay
Heparinized PB was collected from the patients before conditioning chemotherapy, and PBMCs were separated using density-gradient centrifugation, suspended in RPMI 1640 medium (Gibco, Grand Island, NY), and cryopreserved at −80°C. For testing autocytotoxic activity, thawed PBMCs were cultured for 5 to 7 days in the presence of 1 μg/mL phytohemagglutinin (Wellcome, Dartford, United Kingdom). After washing with RPMI 1640, the phytohemagglutinin-stimulated lymphoblasts (PHA blasts) were used as targets in a chromium Cr 51 release assay. PHA blasts were suspended for 1 hour in 10 μL 51Cr (400-1200 Ci/g chromium; Dupont NEN Products, Boston, MA). After washing with phosphate-buffered saline, 104 cells were cultured with 8 × 105autologous PBMCs from each patient after ASCT. After 4 hours of incubation, 51Cr release into the medium was measured using a gamma counter. Percentage specific lysis (mean ± SE) was determined from triplicate cultures as follows: 100 × (experimental release cpm − spontaneous release cpm)/(maximum release cpm − spontaneous release cpm). To assess the specificity of the effector T cells, the target cells were pretreated (1 hour at 4°C) with either anti-MHC class II (anti-DR; Becton Dickinson) and anti-MHC class I (DAKO, Carpinteria, CA) monoclonal antibodies or with antihuman CLIP (kindly given by Dr Peter Cresswell). The anti-DR and anti-MHC class I antibodies were used at a final concentration of 1:200. Target cells were pretreated with 1.5 μg antihuman CLIP Fab preparation. After antibody pretreatment, the cells were washed 3 times before assay.
RNA extraction and cDNA preparation
Heparinized PB was collected from the patients after informed consent was obtained and PBMCs were separated using density-gradient centrifugation. Total RNA was extracted from PBMCs, and 1 μg RNA was reverse transcribed into cDNA in a reaction primed with oligo (dT) 12-18 by using SuperScript II reverse transcriptase, as recommended by the manufacturer (Gibco-BRL, Bethesda, MD).
CDR3 size distribution analysis
Conditions for the generation of CDR3 size distribution analysis have been reported elsewhere.22-24 Briefly, cDNA was polymerase chain reaction (PCR) amplified through 35 cycles (94°C for 1 minute, 55°C for 1 minute, 72°C for 1 minute) with a primer specific to 24 different BV subfamilies (BVs1-20,25BVs21-2423) and a fluorescent BC primer.25Analysis of BV10 and BV19 was excluded from this study because these are pseudogenes.26 One microliter amplified products was mixed with 2 μL 100% formamide, heated at 90°C for 3 minutes and electrophoresed in a 6.75% denaturing polyacrylamide gel. The distribution of the CDR3 size within the amplified product of each BV subfamily was analyzed using an automatic sequencer (Applied Biosystems Division, Foster city, CA) equipped with the computer program Genescan Software (Applied Biosystems Division) allowing determination of the fluorescence intensity of each band. CDR3 size patterns that failed to exhibit a bell-shaped distribution owing to the appearance of prominent peaks with or without a reduced peak number (less than 5 peaks) were judged abnormal. This judgment was made by 3 different investigators to minimize interindividual differences.
Cloning and sequencing of PCR-amplified cDNA
The PCR products of BV16 or BV22 cDNA were cloned into a pCR II-TOPO Vector system (Invitrogen, Carlsbad, CA). Thirty colonies containing the insert fragment were randomly selected and sequenced using an ABI pRISM cycle Sequencing Kit (PerkinElmer, Norwalk, CT) and an automatic DNA sequencer (ABI 373; PerkinElmer). The amino acid sequence of the CDR3 region was deduced using the DNASIS-Mac v3.2 software (Hitachi Software, Yokohama, Japan).
Results
Relation between autologous GVHD and HLA
Clinical GVHD developed in 13 of 31 (41.9%) patients. Relations between certain HLA phenotypes and autoimmune diseases have been previously described.27 For example, the expression of HLA-DRB1*1501 or *0410, respectively, was found to be closely associated with susceptibility to aplastic anemia or idiopathic thrombocytopenic purpura.28 29 Therefore, the HLA phenotypes of 31 patients undergoing ASCT and receiving CsA to induce autologous GVHD were examined. Autologous GVHD developed in 3 of 4 patients with HLA-DRB1*0701.
Cytotoxicity assay
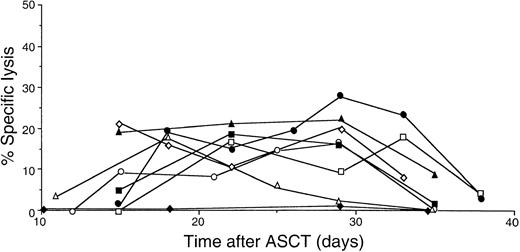
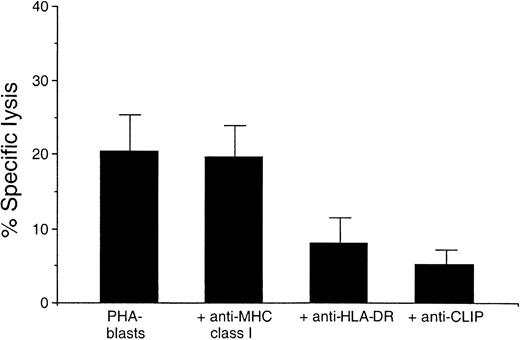
Autocytotoxic activity of lymphocytes obtained at different time points after ASCT was determined against autologous lymphocytes using the 51Cr release assay. Autocytotoxic activity of PBMCs was detectable in all patients treated with both CsA and IFN-γ. Figure1 illustrates the profile of autocytotoxic activity of lymphocytes obtained at different time points after ASCT. Lymphocytes with autocytotoxic activity often appeared at day 12, remained until day 35, and were no longer detected after day 36. Autocytotoxic activity peaked between days 15 and 28 and occurred in a concentration-dependent manner.14 30 Such autocytotoxic lymphocytes were never detected in ASCT patients who did not receive CsA (Figure 1, patient 4). To determine whether the cytotoxicity mediated by post-transplantation lymphocytes is restricted by certain HLA molecules, blocking studies using monoclonal antibodies were performed (Figure 2). Specific lysis (20.4 ± 5.2; n = 3; mean ± SD) by the autocytotoxic effector T cells was blocked by the addition of anti-DR (7.4 ± 4.2) and anti-CLIP antibodies (4.3 ± 2.6), but not by anti-MHC class I antibody (19.4 ± 4.5).
Changes in cytotoxicity of PBMCs for autologous PHA blasts after ASCT.
The cytotoxicity for autologous PHA blasts obtained at different time points after ASCT was determined using the standard 51Cr release assay with an effector–target ratio of 80. ■, patient 1; ▵, patient 2; ○, patient 3; ⋄, patient 4; ▪, patient 6; ▴, patient 7; ●, patient 8; ♦, a patient not administered CsA and IFN-γ.
Changes in cytotoxicity of PBMCs for autologous PHA blasts after ASCT.
The cytotoxicity for autologous PHA blasts obtained at different time points after ASCT was determined using the standard 51Cr release assay with an effector–target ratio of 80. ■, patient 1; ▵, patient 2; ○, patient 3; ⋄, patient 4; ▪, patient 6; ▴, patient 7; ●, patient 8; ♦, a patient not administered CsA and IFN-γ.
Blocking of cytotoxicity against autologous PHA-stimulated lymphoblasts by PBMCs with monoclonal antibodies.
Lysis of the target cells was measured using a standard51Cr release assay at an 80:1 effector–target ratio. Cytotoxicities are presented as mean ± SD of 3 patients (patients 3, 7, 8). CLIP, class II–associated invariant chain peptide. HLA, human leukocyte antigen.
Blocking of cytotoxicity against autologous PHA-stimulated lymphoblasts by PBMCs with monoclonal antibodies.
Lysis of the target cells was measured using a standard51Cr release assay at an 80:1 effector–target ratio. Cytotoxicities are presented as mean ± SD of 3 patients (patients 3, 7, 8). CLIP, class II–associated invariant chain peptide. HLA, human leukocyte antigen.
CDR3 size distribution of TCRBV cDNA of PBL
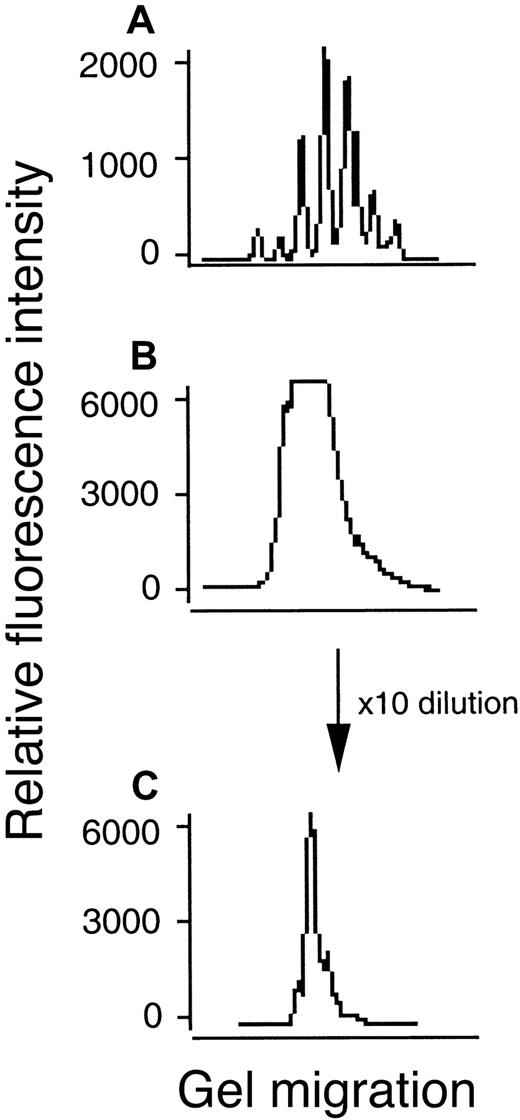
cDNA of 24 different BV subfamilies was amplified using a fluorescent BC primer, and the CDR3 size distribution of each BV subfamily was compared among healthy controls and the patients. The specificity of each T-cell was determined by the amino acid sequence of the TCR β-chain CDR3 region, corresponding to diversity (D) region, upstream and downstream nucleotides (N), and joining (J) region genes. Diversity of CDR3 within the T-cell repertoire facilitates recognition of a variety of antigens. CDR3 cDNA can be categorized in groups, each differing by 3 nucleotides, constituting peptides from 6 to 12 amino acids in length.22 31 Sequence analysis of electrophoretic patterns that result from these groups has revealed that T-cell subfamilies in which clonal expansion did not occur have a CDR3 size distribution that was characteristically bell shaped (Figure3A). In contrast, when clonal expansion of a few T cells occurred, the bell-shaped distribution was skewed with the appearance of a sharp peak (Figure 3C). All CDR3 size distribution patterns from each BV subfamily in PBL from a healthy control andStaphylococcus enterotoxin B (SEB) stimulated PBL from a healthy control showed the normal pattern for polyclonality (data not shown).
CDR3 size distribution analysis of BV14 or BV16 cDNA from PBLs in patient 22.
The cDNA amplified using primers specific to BV16 subfamilies coupled with a fluorescent Cβ primer was analyzed for size with an automated DNA sequencer and Genescan software. Fluorescence intensity (y axis) is plotted against CDR3 size (x axis). (A) BV14 indicating normal pattern. (B) BV16 indicating abnormal pattern with high-fluorescence intensity. (C) Abnormal pattern after 10 times dilution of panel B indicating monoclonal proliferation of T cells.
CDR3 size distribution analysis of BV14 or BV16 cDNA from PBLs in patient 22.
The cDNA amplified using primers specific to BV16 subfamilies coupled with a fluorescent Cβ primer was analyzed for size with an automated DNA sequencer and Genescan software. Fluorescence intensity (y axis) is plotted against CDR3 size (x axis). (A) BV14 indicating normal pattern. (B) BV16 indicating abnormal pattern with high-fluorescence intensity. (C) Abnormal pattern after 10 times dilution of panel B indicating monoclonal proliferation of T cells.
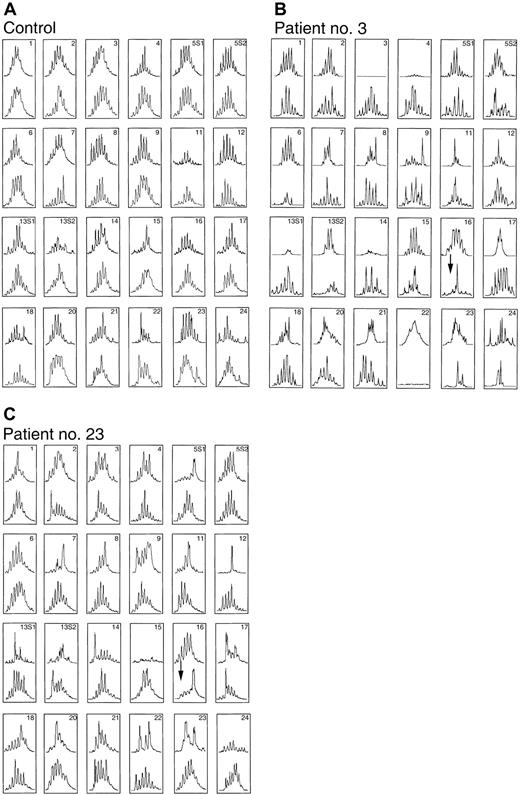
Figure 4 shows the CDR3 size distribution pattern for each BV subfamily before ASCT (upper) and after ASCT (lower) at the onset of autologous GVHD in patients 3 and 23 and in another ASCT patient who did not receive CsA (control). In these patients, abnormalities were found in the CDR3 size distribution pattern for more than half of the subfamilies, most likely because of past chemotherapy and blood transfusion. On the other hand, at 26 days after transplantation for the ASCT patient who did not receive CsA, all subfamilies with skewed distributions before transplantation (upper) had switched to polyclonal patterns, suggesting reconstitution of the T-cell compartment (lower). Most subfamilies that gave a skewed distribution before transplantation had changed from oligoclonality to polyclonality, even in patients with autologous GVHD. However, this analysis also revealed that the BV16 family and some other BV families, which showed normal patterns before ASCT, developed CDR3 distribution peaks consistent with monoclonal expansion. Although it cannot be excluded that these abnormal CDR3 patterns were due to a delayed immune reconstitution, these patterns did not occur in healthy controls or in ASCT patients who were not administered CsA. Although the frequency of BV families using anti-TCR BV monoclonal antibodies on an flow cytometry system was compared between patients (patients 3 and 6), immunofluorescent monoclonal antibody staining did not demonstrate significant changes in the patients before or after transplantation (data not shown). Previous studies indicate that clonotypic expansions do not necessarily elevate the overall frequencies of TCR BV families.32
Changing in CDR3 size patterns associated with autologous GVHD before ASCT (upper) and day 26 after ASCT at the onset of autologous GVHD (lower).
CDR3 size patterns of PBLs in the patients who did not receive CsA (control; panel A) and those in whom autologous GVHD developed (patients 3 and 23; panels B and C, respectively).
Changing in CDR3 size patterns associated with autologous GVHD before ASCT (upper) and day 26 after ASCT at the onset of autologous GVHD (lower).
CDR3 size patterns of PBLs in the patients who did not receive CsA (control; panel A) and those in whom autologous GVHD developed (patients 3 and 23; panels B and C, respectively).
Selection of TCR-β diversity after transplantation
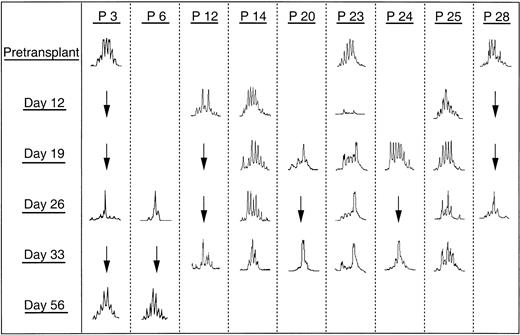
CDR3 size distribution in the PBL at different time points was analyzed and compared with the patterns obtained before transplantation, at the onset of autologous GVHD after transplantation, and at the time of resolution of autologous GVHD. Table2 summarizes BV subfamilies showing abnormal CDR3 size pattern after transplantation. In each case, a remarkable change in the CDR3 size pattern of some BV subfamilies was observed at the onset of autologous GVHD. For these patients, particularly BV16, single peaks were detected, suggesting monoclonal T-cell expansion. Clonal expansions within BV16+ T cells were shared by 7 of 11 patients in whom autologous GVHD developed and in 5 of 15 patients without clinical evidence of GVHD. Figure5 illustrates these changes in the CDR3 size pattern of BV16 for 9 patients after transplantation. Although BV 16 spectratypes obtained before transplantation exhibited more than 6 peaks, some patients still showed the skewed pattern (Figure 5, P3 and P23). Interestingly, these skewed changes appeared from day 19 until day 33 in patient 23, in whom autologous GVHD developed, and in patients in whom clinical signs of GVHD did not develop. In 2 patients in whom samples were available, these changes were lost at 56 days after transplantation, and the CDR3 size pattern for all subfamilies was restored to normal (Figure 5, P3 and P6).
Changes in the CDR3 size distribution pattern of BV16 cDNA at different time points.
GVHD developed in 6 patients (patients 3, 6, 14, 20, 25, 28). Three patients (patients 12, 23, 24) did not have clinical signs of autologous GVHD.
Changes in the CDR3 size distribution pattern of BV16 cDNA at different time points.
GVHD developed in 6 patients (patients 3, 6, 14, 20, 25, 28). Three patients (patients 12, 23, 24) did not have clinical signs of autologous GVHD.
Deduced amino acid sequence of CDR3 region of BV16 cDNA
These findings indicated that clonal expansion of a limited number of T cells is common in autologous GVHD-induced patients. To directly demonstrate the clonal expansion of a limited number of T cells, the amplified products of the BV16 cDNA derived from 10 patients (patients 6, 8, 12, 20, 22, 23, 24, 25, 28, 29) were cloned and sequenced. Table3 summarizes the amino acid sequence of each clone. Interestingly, some patients exhibited a high frequency of clones with a specific N-D-N region—RYRID (9/10) in patient 8, QVSRA (9/19) in patient 12, RTGTTS in patient 20, AIGP (11/22) in patient 22, AYRDRRL (18/26) in patient 23, RIETYP (8/14) in patient 24, QDFPSGSWE (7/19) in patient 25, AIGP (14/18) in patient 28, and HRGQG (9/15) in patient 29. Such high frequencies of certain clones are compatible with the results of CDR3 size distribution. Surprisingly, AIGP in patient 22 matches the sequence in patient 28 with the same DRB1*0701. Similarly, the most frequent amino acid sequence, RYRID, in patient 6 occurred in patient 8; both had the same HLA-DRB1*1502. All these clones expressed a common TCR BJ element, BJ2S1. The CDR3 region interacts directly with the peptide epitope.33,34 These observations suggest that at time of onset of autologous GVHD, clonal T-cell expansion against a common antigen occurs in the PB. Of further interest was the observation of AYRDRRL (15/19) and RIETYP (8/14) in patients 23 and 24, respectively, who did not have clinical signs of autologous GVHD though they received CsA after transplantation. Interestingly, these high-frequency clones have the same codon (cgc tat orata) in the first and second portions of the hypervariable CDR3 region (Table 4), a finding that is unlikely to reflect selection by antigen.33 34 It appears that clonal T-cell expansion occurred even in patients administered CsA after transplantation, though they did not subsequently have clinical autologous GVHD.
Relation between clonal expansion within certain BV family and HLA phenotype
Autologous GVHD and clonal expansions within BV16+ T cells developed in 3 of 4 patients with HLA-DRB1*0701. Clonal expansions within BV15+ T cells were also shared by 4 of 6 patients with HLA-DRB1*1501 during the early post-transplantation period (days 12-19), and clonal expansions within BV22+ T cells were shared by 3 of 4 patients with HLA-DRB1*0401 during the late post-transplantation period (days 26-33). However, only one patient in each of these groups had clinical evidence of autologous GVHD. Although clonal expansions occurred within BV16, BV15, and BV22, only the expression of HLA-DRB1*0701 and not of HLA-DRB1*1501 or HLA-DRB1*0401 appears to be related to the development of autologous GVHD. Table5 summarizes the amino acid sequence of TCR BV22 cDNA derived from PB of 5 patients (patients 11, 15, 18, 20, 25). Although high frequencies of certain clones were compatible with the results of CDR3 size distribution, identification of a common N-D-N amino acid sequence was not observed among 3 different patients with HLA-DRB1*0401 (patients 11, 18, 20).
Discussion
The present analysis of T-cell repertoire in the PB of the patients with autologous GVHD reveals several new findings regarding the autoreactive T-cell repertoire in the pathophysiology of human autologous GVHD. Patients with autologous GVHD exhibited CDR3 size abnormalities suggestive of clonal predominance. These abnormalities did not appear in healthy controls or in ASCT patients not administered CsA. A subset of these changes overlaps the autocytotoxic activity (days 11-35), and skewed spectratype patterns of BV16 were recognized until day 33. This monoclonal T-cell expansion is in accordance with the appearance of autocytotoxic T cells. The abnormal CDR3 size patterns of patients cannot be attributed to a delay in the reconstitution of T cells because they were not detected in the ASCT patients not administered CsA. Furthermore, the skewed pattern cannot be detected on resolution of autologous GVHD beyond 56 days after transplantation. Thus, clonal expansion of a limited number of T cells in the PB appears to be related to the pathophysiology of autologous GVHD.
Although the mechanism of autologous GVHD is unclear, recent studies suggest that CD8+ effector lymphocytes recognize HLA-class II determinants10-12 and that there is an infiltration of BV15+ T cells in skin lesions.19 Additionally, the molecule CLIP appears to play a major role as the target antigen in autologous GVHD.17,18 CLIP is a peptide associated with MHC class II β chain and occupies the MHC class II groove.35,36 Certain TCR BV elements, including BV15, interact directly with the superantigen SEB.37,38 It is thought that HLA-DR–bound CLIP stimulates autologous GVHD T cells through the SEB binding site in a superantigen-like fashion, resulting in the induction of T-cell cytotoxicity against MHC class II–positive cells.17,18 Post-transplantation autocytotoxic activity was inhibited by the pretreatment of target cells with anti-CLIP and anti–HLA-DR antibodies. These results suggest that the MHC class II–CLIP complex is one of major target antigens for the autoreactive T cells induced with CsA. A vigorous cellular immune reaction, dominated by activated proliferating CD8+ cytotoxic T lymphocytes, is mounted during autologous GVHD. It is not yet clear from functional studies whether most of these activated T cells are antigen-specific expanded or result from nonspecific, antigen-independent “bystander” activation. If all effector cells in autologous GVHD were activated by a superantigen, antigen-specific proliferation of T cells in PB should not be observed at the onset of autologous GVHD.23 39 Evaluation of PBL at time of onset of autologous GVHD by CDR3 size distribution analysis, however, revealed that there was a clonal expansion of certain T-cell families. Therefore, autologous GVHD appeared to be dependent on the recognition of self-antigen. In the present study, clonal expansion of BV16+ T cells in particular was observed in 7 of 11 patients with autologous GVHD. Moreover, homology analysis of the CDR3 amino acid sequence revealed a common motif, AIGP or RYRID, in patients with HLA-DRB1*0701 or HLA-DRB1*1502, respectively. Taken together, our results suggest the possibility that this T-cell family is stimulated by a common antigen in association with MHC class II–CLIP and is associated with the onset of autologous GVHD. The BV16+ T cells expressing the same CDR3 motif probably recognize a common peptide related to the pathophysiology of autologous GVHD. These findings are compatible with the results in the rat model of autologous GVHD, indicating a common target antigen.
Of interest, autocytolytic activity can be detected in most patients who were treated with CsA plus IFN-γ, regardless of whether they had clinical evidence of autologous GVHD. In accord, a comparable clonal predominance of this BV16 family was detected in 5 of 15 patients without clinical evidence of autologous GVHD. Similarly, clonal expansions within the BV15+ or BV22+ population of T cells were also shared by 4 of 6 patients with HLA-DRB1*1501 and by 3 of 4 patients with HLA-DRB1*0401, respectively. It appears that the resultant specific clonal expansion of autoreactive T cells can occur without the presentation of clinical disease. Clonal expansion of T cells expressing BV15 or BV22, in the context of a given HLA-DR (DRB1*0401 or 1501), is of interest because recent studies suggest that these alleles can mediate rheumatoid arthritis or immune-mediated aplastic anemia.40,41 In 2 patients in whom autologous GVHD did not develop but who were administered CsA after transplantation, homology analysis of the CDR3 amino acid sequence revealed the high-frequency expression of the CDR3 motifs, AYRDRRL and RIETYP. Moreover, these clones expressed the same codon (cgc ata) in the first and second portions of the CDR3 region, a finding that may not reflect specific selection by antigen.33,34 It is possible that the appearance of autocytotoxic lymphocytes in the PB is associated with clonal T-cell expansion after CsA treatment without clinical evidence of autologous GVHD. The failure of clinical autologous GVHD to develop may be attributed to peripheral regulatory cells that are transferred with the stem cell inoculum.15,16 These findings are consistent with the demonstration that rigorous T-cell depletion of the stem cell inoculum is associated with a greater frequency of autologous GVHD.20 CDR3 size distribution analysis of TCR BV on PBLs in the present study reveals that a limited number of T cells clonally expand in the autologous GVHD-induced patients administered CsA after transplantation. It seems likely that antigen-driven T-cell expansion plays an essential role in the pathogenesis of autologous GVHD in these patients. The development of autologous GVHD is associated with clonal expansion of a limited number of T cells that may recognize some antigens, including the CLIP MHC class II complex.17 18Further studies using dendritic cells presenting CLIP are needed to clarify whether this peptide MHC class II complex induces proliferation of T cells with these specific CDR3 motifs. The association of autologous GVHD with certain DRB1 alleles may be related to the clonal expansion of T cells expressing restricted BV genes; however, conformation would require a much larger population of patients.
Supported by grants CA15396, CA82583, and AI 24319 from the National Institutes of Health.
Submitted January 9, 2001; accepted March 17, 2001.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yuji Miura, Oncology Center, Johns Hopkins University School of Medicine, Bunting-Blaustein Cancer Research Bldg, Rm 484, 1650 Orleans St, Baltimore, MD 21231; e-mail:ymiura@mail.jhmi.edu.