Transfusions of UV-B–irradiated peripheral blood mononuclear cells (UV-B–PBMCs) from BALB/c (H-2d) mice into CBA (H-2k) mice can induce humoral immune tolerance to H-2d antigens, and the induced tolerance is partially mediated by negative regulatory PBMCs. To further identify which subset of spleen mononuclear leukocytes (MNLs) in the tolerant CBA mice is responsible for the negative regulatory activity, adoptive transfer experiments were conducted using spleen MNLs from the tolerant CBA mice. Results showed that only CD4+ T cells could transfer the negative regulatory activity in a dose-dependent manner. This negative regulatory activity was significantly reduced when CD25+ helper T cells were removed. Further study suggested that inhibition of IL-12 production by UV-B–irradiated PBMCs played a role in the induction of immune tolerance. In vitro study of the cytokine production profile by CBA CD4+ T cells, after stimulation with gamma-irradiated BALB/c spleen cells, revealed an enhanced production of the type 2 T-cell cytokines after tolerance induction. Induction of tolerance also prevented the development of cytotoxic T cells in CBA mice against BALB/c MNLs. Adoptive transfer study suggested that the cellular immune tolerance was also mediated by CD4+ negative regulatory T cells. The induced immune tolerance was nullified after 400 cGy sublethal gamma irradiation. These results suggest that the ex vivo study of cytokine production by T cells may be used to monitor tolerance induction and the selection of gamma radiation dose is critical for potential clinical application of the tolerance induced by UV-B–PBMCs.
Introduction
Transfusions of donor leukocytes containing blood components inactivated with UV light have been shown to induce immunologic tolerance to allografts1 and allogeneic platelets.2,3 On the basis of these observations, we have developed an approach using 4 weekly transfusions of peripheral blood mononuclear cells (PBMCs) inactivated by medium-wavelength UV light (UV-B) to induce humoral immune tolerance to allogeneic major histocompatibility complex (MHC) antigens in a murine model.4 Initial characterization of the induced tolerance indicated that the tolerance is partially mediated by the induction of negative regulatory cells.4 However, the identity of these negative regulatory cells is unknown.
Later, we found that graft-versus-host disease could be prevented in lethally irradiated BALB/c mice if these mice were transplanted with bone marrow and spleen cells from CBA mice tolerized to BALB/c H-2d antigens.5 This finding suggests that cellular immune tolerance might have been induced by transfusions of UV-B–irradiated leukocytes. It appears that the tolerance induction by UV-B–irradiated leukocytes could be clinically useful for the transplantation of allogeneic hematopoietic stem cells and solid organs. Therefore, further investigation is warranted to gain better understanding of the nature of the immune tolerance induced by UV-B–irradiated leukocytes.
We thus conducted a series of experiments to characterize the induced negative regulatory cells, to demonstrate the induction of cellular immune tolerance, to determine the association of tolerance induction with enhanced type 2 T-cell cytokine response, and to investigate the effect of gamma irradiation on the induced tolerance. Results of our study and their clinical implications are reported herein.
Materials and methods
Animals
Eight-week-old syngeneic CBA/CaH-T6J (CBA) mice with H-2k MHC haplotype, BALB/cByJ (BALB/c) mice with H-2d MHC haplotype, and BALB/c-IL-12btm1Jm mice with knockout of IL-12 β chain were purchased from Jackson Laboratory (Bar Harbor, ME). C57BL/6J (BL/6) mice with H-2b MHC haplotype were obtained from the Mouse Core at the Department of Pathology, University of Florida. All mice were housed in a temperature-controlled room (25°C) with a 12-hour light–12-hour dark cycle and fed ad libitum. All animal studies were approved by the Institutional Animal Care and Use Committee.
Antibodies and streptavidin beads
Anti–mouse CD11b(M1/70), CD45R/B220, CD4 (RM4-5), CD8a (53-6.7) Gr-1 (RB6-8C5), CD90.2 (Thy-1.2), and CD25 (PC61) monoclonal antibodies (mAbs) labeled with biotin were purchased from Pharmingen (San Diego, CA). Streptavidin M280 Dynabeads were purchased from Dynal (Lake Success, NY). Murine CD4 enrichment antibody cocktail and other cell separation reagents for the StemSep System were obtained from StemCell Technologies (Vancouver, BC, Canada).
Induction of immune tolerance
Immune tolerance was induced in CBA mice by 4 weekly intravenous injections of 2 × 105 UV-B–irradiated BALB/c PBMCs in 100 μL phosphate-buffered saline (PBS) through a tail vein under light anesthesia of methoxyflurane (Pitman-Moore, Mundelein, IL). UV-B–irradiated PBMCs were prepared as reported previously.4 A week after the last transfusion of UV-B–irradiated leukocytes, serum samples were collected to assess the development of antibodies to donor MHC antigens by flow cytometry.3 4 If the CBA mice did not develop any anti–H-2d antibodies after 4 weekly transfusions of UV-B–irradiated BALB/c PBMCs, they became tolerized. To ensure the development of tolerance, 2 transfused CBA mice were randomly selected and challenged with 2 weekly transfusions of 1 × 105untreated BALB/c PBMCs. As expected, none of the tolerized CBA mice developed antibody to H-2d after 2 challenging transfusions with BALB/c leukocytes. All control naive CBA mice developed anti–H-2d antibody.
Immunofluorescent flow cytometry for detection of antibodies to H-2 antigen
The development of antibodies to donor H-2 antigen was determined by immunofluorescence flow cytometry.3 Briefly, BALB/c spleen PBMCs (1.5 × 105 cells) suspended in 20 μL PBS containing 0.1% sodium azide and 1% bovine serum albumin (PBS-azide-BSA) were incubated with 10 μL serum at room temperature for 45 minutes. Cells were washed 3 times and stained with 30 μL 40× diluted fluorescein-labeled goat anti–mouse IgG-Fc antibody (Sigma, St Louis, MO) for 30 minutes. After 3 washes, cells were suspended in 0.5 mL PBS-azide-BSA. Mean fluorescence intensity (MFI) of 1 × 104 cells were measured in arbitrary units using a FACScan flow cytometer and Lysis-II software (Becton Dickinson, Palo Alto, CA). Pooled preimmune serum was used as a negative control, and pooled immune serum was used as a positive control. Based on the results of our previous study,3 a serum sample that produced an MFI greater than 1.5 times the MFI of the pooled preimmune serum was considered positive for antibody to donor H-2 antigens. Our previous study also showed that the MFI of antibodies were well correlated with antibody titers measured by an enzyme immunoassay.
Isolation of different subsets of spleen T cells
Spleen MNLs were prepared by Ficoll-Hypaque gradient centrifugation4 and suspended in PBS containing 1% fetal calf serum (PBS-FCS) to a concentration of 20 000 cells/μL after 2 washes with PBS. Different subsets of spleen cells were isolated by negative selection through the incubation of cells with cocktails of biotin-labeled monoclonal antibodies at 4°C for 30 minutes. For isolation of T cells, the antibody cocktail included anti-CD45R/B220 mAb, anti-CD11b mAb, and anti–Gr-1 mAb. Anti-CD8 or anti-CD4 mAb was added to this cocktail for the isolation of CD4+ or CD8+ T cells, respectively. For preparation of spleen non-T cells, biotinylated anti-Thy1.2 mAb was used to deplete T cells. The final concentration of each mAb was 5 μg/mL. After incubation with biotinylated antibodies, the cells were washed twice with 5 mL ice-cold PBS and resuspended in the original volume of PBS-FCS. Washed M-280 streptavidin beads (7 × 108 beads/mL in PBS) were added to the cell suspension. The ratio of beads to cells was 5:1. Cells and streptavidin beads were incubated in sterile polypropylene tubes at 4°C under constant rocking for 40 minutes. Incubation was stopped by the addition of a half-volume of PBS. Cells coated with streptavidin beads were magnetically removed. Cells without magnetic beads were harvested, washed once with 3 mL PBS, and resuspended in PBS or appropriate culture media. Purity of the isolated cells was assessed by immunofluorescence flow cytometry. Purity of the isolated T cells was always between 85% and 90%.
CD4+ spleen T cells used for some adoptive transfer experiments were prepared from spleens by negative selection using a CD4+ T-cell enrichment cocktail from StemCell Technologies. The purity of CD4+ T cells isolated by this method is between 93% and 95%. To deplete T cells positive for both CD4 and CD25, the isolated CD4+ T cells were incubated with biotinylated anti–murine CD25 mAb at 5 μg/mL and were processed by following the protocol from StemCell Technologies. Flow cytometry analysis showed that there were no detectable CD25+ T cells (less than 0.005%).
Adoptive transfer experiments
To investigate the role of T cells in the tolerance induced by UV-B–irradiated MNLs, T cells from spleen MNLs of tolerant or naive CBA mice were isolated by negative selection as described above. Isolated spleen T cells suspended in PBS were injected into tail veins of naive CBA mice. Non-T cells prepared from spleen MNLs by the depletion of T cells with anti-Thy 1.2 magnetic beads were used as additional control. Two days after the injection, CBA mice were challenged with 2 weekly transfusions of a submaximal antigenic dose (4 × 104) of untreated BALB/c peripheral MNLs. One week after the second challenging transfusion, serum samples were collected and assayed for anti–H-2d antibodies by immunofluorescence flow cytometry. In a separate experiment, CBA mice adoptively transferred with the isolated spleen CD4+ T cells from naive or tolerant CBA mice were challenged with peripheral MNLs from BL/6 or BALB/c mice. Serum samples from the challenged CBA mice were collected and assayed for the development of anti–H-2b or anti–H-2d antibodies.
Cytotoxic T-lymphocyte assay
Naive and tolerant CBA mice were immunized with 2 weekly transfusions of a fully immunogenic dose of BALB/c PBMCs (2 × 105 cells per transfusion). One week after the second transfusion, spleen T cells were harvested and suspended in Iscoves modified Dulbecco medium (IMDM) containing 7% FCS, 2 mM L-glutamine, 50 μM mercaptoethanol, and antibiotics (IMDM-7). Cytotoxic T-lymphocyte (CTL) activity to H-2d– or H-2b–positive lymphoblasts was measured by using the JAM assay.6 Lymphoblast targets were prepared by stimulation of spleen MNLs (1.5 × 106 cell/mL) from H-2dBALB/c or H-2b BL/6 mice with concanavalin A (Con-A) (2.5 μg/mL) for 48 hours in IMDM-7 media and labeled with3H-thymidine (9.25 × 104 Bq/mL) overnight. Viable Con-A stimulated lymphoblasts were isolated by Ficoll-Hypaque centrifugation at 800g for 20 minutes. After 2 washes, the lymphoblasts were suspended in IMDM-7 and diluted to 100 cells/μL. Ten thousand labeled lymphoblast targets were incubated with different numbers of T cells for 5 hours in a tissue culture incubator. Cells were harvested using a PHD cell harvester (Brandel, Gaithersburg, MD). Degrees of cytotoxic killing were determined as described.6 A similar CTL assay was conducted in the CBA mice adoptively transferred with 5 × 106 spleen CD4+ T cells from naive or tolerant CBA mice. Two days after the adoptive transfer of CD4+ T cells, the CBA mice were challenged with 2 weekly transfusions of BALB/c or BL/6 PBMCs as described above. A week after the second challenge transfusion, spleen T cells were harvested from 2 mice of each group for the CTL assay.
Mixed leukocyte culture for cytokine production profile
Spleen T lymphocytes (4 × 105) from naive, tolerant, and immunized CBA mice were cultured with 6 × 105 BALB/c spleen cells pretreated with 25 Gy gamma ray or 50 μg/mL mitomycin C in 200 μL RPMI medium containing 10 mM HEPES, 2 mM L-glutamine, 50 μM 2-mercaptoethanol, and 1% heat-inactivated human AB serum in a 96-well flat-bottom plate.7 Supernatants were harvested and pooled daily from triplicate wells and were assayed for cytokines by enzyme-linked immunoassays.
Enzyme-linked immunoassays for IL-4, IL-5, IL-10, and interferon-γ
Each well in an immunoassay plate was coated with 50 μL of 5 μg/mL anti–mouse IL-4, IL-5, or IL-10 antibody in PBS-azide overnight at 4°C. Plates were washed 3 times using PBS-azide-0.2% Tween-20, or PBS-a-Tween, and were blocked with 150μL PBS containing 10% newborn calf serum for 30 minutes at room temperature. Thereafter, 50 μL cytokine standards and unknown samples were added and incubated overnight at 4°C. After incubation, plates were washed and incubated sequentially with 50 μL of 5 μg/mL biotinylated anti-cytokine antibody diluted in PBS-Tween-1% BSA, streptavidin-peroxidase (Sigma, St Louis, MO), and o-phenylenediamine dihydrochloride peroxidase substrate (Sigma). Paired anti–IL-4, –IL-5, and –IL-10 antibodies and recombinant murine IL-4, IL-5, and IL-10 standards were obtained from Pharmingen (San Diego, CA). Reagents for interferon γ (IFN-γ) immunoassay were obtained from Endogen (Woburn, MA), and the assay was performed according to the manufacturer's instructions. Sensitivities of IL-4, IL-5, IL-10, and IFN-γ assays were 0.2 pg/mL, 0.2 pg/mL, 5 pg/mL, and 50 pg/mL, respectively.
Results
Characterization of negative regulatory cells induced by transfusions of UV-B–PBMCs
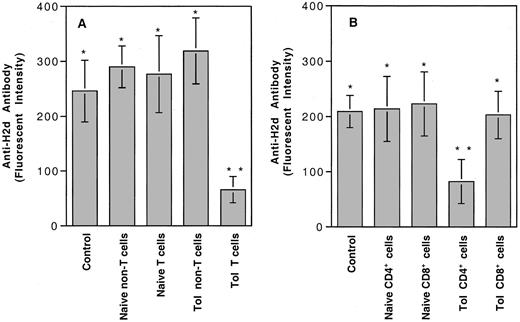
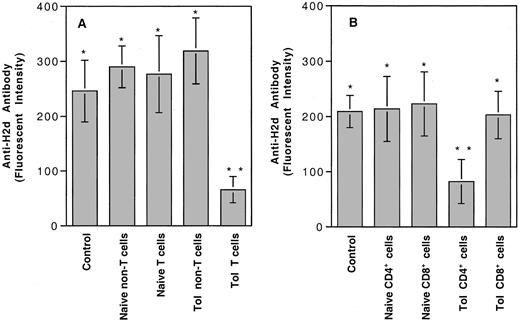
Our earlier study showed that 4 weekly transfusions of UV-B–irradiated PBMCs from BALB/c (H-2d) mice into CBA (H-2k) mice could induce humoral immune tolerance to H-2d antigens.4 Tolerance appeared to be partially mediated by the induction of negative regulatory cells.4 To identify and further characterize the subpopulation of lymphocytes from the tolerant CBA mice responsible for the negative regulatory activity, we injected each naive CBA mouse intravenously with 6 × 106 spleen T or non-T cells from naive or tolerant CBA mice. Adoptively transferred mice were challenged with 2 weekly transfusions of a submaximal antigenic dose of BALB/c PBMCs (4 × 104 cells per transfusion). The development of anti–H-2d antibody was measured by immunofluorescence flow cytometry. As shown in Figure 1A, only naive CBA mice treated with spleen T cells from tolerant CBA mice showed significantly suppressed production of anti–H-2dantibody. Further adoptive transfer study indicated that the negative regulatory activity could be transferred only by CD4+ T cells (Figure 1B). Negative regulatory activity was proportional to the number of CD4+ T cells transferred to a naive CBA mouse (Figure 2).
Adoptive transfer experiments for identification of negative regulatory cells in tolerized CBA mice.
(A) Six million cells from spleens of naive or tolerant CBA mice were injected into each naive CBA mouse. (B) Five million CD4+ T cells were injected into each naive CBA. Control, naive CBA mice with no transfer of spleen cells. Types of spleen cells used for injection are shown at the bottom. There were 4 mice in each treatment group. Each value represents the mean ± SD. Analysis of variance showed that only the mice treated with tolerant T cells (**, panel A) (P ≤ .0001) or tolerant CD4+ T cells (**, panel B) (P ≤ .0023) were significantly different from the remaining groups. There were no statistical differences among groups marked with asterisks.
Adoptive transfer experiments for identification of negative regulatory cells in tolerized CBA mice.
(A) Six million cells from spleens of naive or tolerant CBA mice were injected into each naive CBA mouse. (B) Five million CD4+ T cells were injected into each naive CBA. Control, naive CBA mice with no transfer of spleen cells. Types of spleen cells used for injection are shown at the bottom. There were 4 mice in each treatment group. Each value represents the mean ± SD. Analysis of variance showed that only the mice treated with tolerant T cells (**, panel A) (P ≤ .0001) or tolerant CD4+ T cells (**, panel B) (P ≤ .0023) were significantly different from the remaining groups. There were no statistical differences among groups marked with asterisks.
Dose-response study of the negative regulatory activity of CD4+ T cells.
Different numbers of CD4+ T cells isolated from the tolerant CBA mice were injected into naive CBA mice. After adoptive transfer of the CD4+ T cells, the treated mice were challenged with 2 weekly intravenous injections of 4 × 104 BALB/c PBMCs. One week after the second challenge transfusion, serum samples were harvested and assayed for anti–H-2d antibody activities by immunofluorescence flow cytometry. There were 3 mice in each treatment group. Each value represents the mean ± SD.
Dose-response study of the negative regulatory activity of CD4+ T cells.
Different numbers of CD4+ T cells isolated from the tolerant CBA mice were injected into naive CBA mice. After adoptive transfer of the CD4+ T cells, the treated mice were challenged with 2 weekly intravenous injections of 4 × 104 BALB/c PBMCs. One week after the second challenge transfusion, serum samples were harvested and assayed for anti–H-2d antibody activities by immunofluorescence flow cytometry. There were 3 mice in each treatment group. Each value represents the mean ± SD.
Because CD4+CD25+ T cells have been shown to mediate the suppressive activity in peripheral self-tolerance,8-10 we investigated how depletion of CD25+ T cells affected the negative regulatory activity of CD4+ helper T cells. The results shown in Figure3A indicated that the negative regulatory activity was significantly reduced after the removal of CD25+ helper T cells. To determine whether the negative regulatory cells in CBA mice tolerant of BALB/c H-2dantigens could suppress the primary humoral immune response to other H-2 antigens, we adoptively transferred naive CBA mice with different subsets of spleen CD4+ T cells from naive or tolerant CBA mice. Adoptively transferred and control CBA mice were challenged with BALB/c H-2d– or BL/6 H-2b–positive peripheral leukocytes, as described. Results of this experiment indicated that the CD4+C25+ negative regulatory cells from the tolerant CBA mice could also suppress the humoral immune response to H-2b antigens (Figure 3B).
Effect of depletion of CD25+ cells on the negative regulatory activity of CD4+ T cells from the tolerant CBA mice.
(A) Each naive CBA mouse was treated with 2.5 × 106spleen CD4+ T cells with or without depletion of CD25+ cells from naive or tolerant CBA mice. No CD4+ T cells were given to the CBA mice in the control group (Control). Thereafter, all CBA mice were challenged with BALB/c PBMCs, and the development of anti–H-2d antibody was determined as described. Analysis of variance showed that only the mice treated with tolerant CD4+ T cells without depletion of CD25+ cells (Tol.CD4+ cells) had anti–H-2d antibody activity significantly lower than in the other 4 groups (P < .04). Anti–H-2dantibody activities in the CBA mice treated with tolerant CD4+ with depletion of CD25+ T cells (CD4+/CD25−) were not significantly different than in the control and in those treated with naive CD4+with or without depletion of CD25+ T cells (P ≥ .40). There were 4 mice in each group. (B) In a separate experiment, each naive CBA mouse was adoptively transferred with 5 × 106 spleen CD4+ T cells with or without depletion of CD25+ cells from naive CBA mice or CBA mice tolerant to BALB/c H-2d antigens. Adoptively transferred and control CBA mice were challenged with H-2b–positive BL/6 PBMCs, as described. Analysis of variance showed that only CBA mice adoptively transferred with the tolerant CD4+ T cells (Tol. CD4+ cells) developed anti–H-2b antibody activities significantly lower than in the other 3 groups (P ≤ .02). There were 4 mice in each group.
Effect of depletion of CD25+ cells on the negative regulatory activity of CD4+ T cells from the tolerant CBA mice.
(A) Each naive CBA mouse was treated with 2.5 × 106spleen CD4+ T cells with or without depletion of CD25+ cells from naive or tolerant CBA mice. No CD4+ T cells were given to the CBA mice in the control group (Control). Thereafter, all CBA mice were challenged with BALB/c PBMCs, and the development of anti–H-2d antibody was determined as described. Analysis of variance showed that only the mice treated with tolerant CD4+ T cells without depletion of CD25+ cells (Tol.CD4+ cells) had anti–H-2d antibody activity significantly lower than in the other 4 groups (P < .04). Anti–H-2dantibody activities in the CBA mice treated with tolerant CD4+ with depletion of CD25+ T cells (CD4+/CD25−) were not significantly different than in the control and in those treated with naive CD4+with or without depletion of CD25+ T cells (P ≥ .40). There were 4 mice in each group. (B) In a separate experiment, each naive CBA mouse was adoptively transferred with 5 × 106 spleen CD4+ T cells with or without depletion of CD25+ cells from naive CBA mice or CBA mice tolerant to BALB/c H-2d antigens. Adoptively transferred and control CBA mice were challenged with H-2b–positive BL/6 PBMCs, as described. Analysis of variance showed that only CBA mice adoptively transferred with the tolerant CD4+ T cells (Tol. CD4+ cells) developed anti–H-2b antibody activities significantly lower than in the other 3 groups (P ≤ .02). There were 4 mice in each group.
Cytokine production profile by spleen T cells from tolerant CBA mice
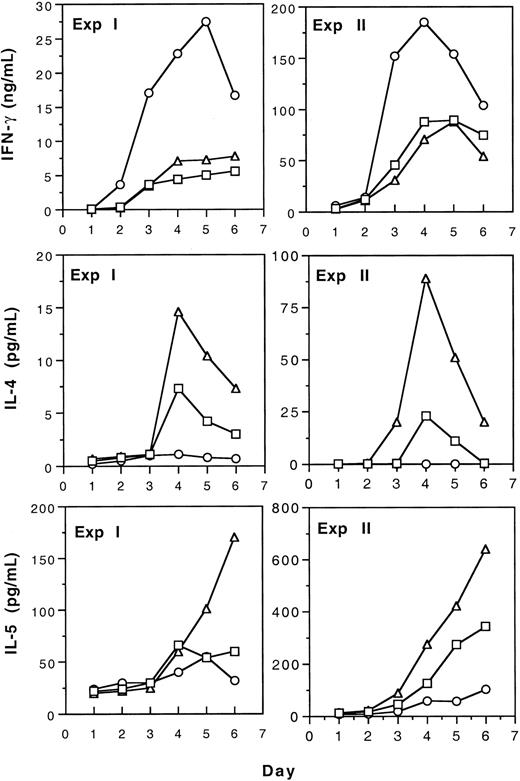
Production of type 1 (IFN-γ) and type 2 (IL-4 and IL-5) cytokines by spleen T cells from naive, tolerant, and immunized CBA mice was studied by measurement of cytokines in the media of mixed leukocyte culture (MLC). The results of 2 separate experiments shown in Figure 4 indicate that tolerance induction by transfusion of UV-B–irradiated leukocytes resulted in the increased production of type 2 cytokines by T cells. In contrast, immunization to allogeneic leukocytes led to enhanced type 1 and reduced type 2 cytokine production. Polarization to type 2 cytokine production after tolerance induction was confirmed in another experiment using CD4+ spleen T cells as responders in MLC (Table 1). In addition, we found that after tolerance induction, the secretion of IL-10 was also increased (Table 1).
Production of IL-4, IL-5, and IFN-γ by T cells from tolerant, immunized, and naive CBA spleen T cells in mLC.
Spleen T cells from tolerant (▵), immunized (○), and naive (■) CBA mice were stimulated with BALB/c spleen cells treated with 25 Gy gamma irradiation (experiment ) or 50 μg/mL mitomycin C (experiment 2) in mLC. Supernatants were harvested and pooled from triplicate wells daily and assayed for cytokines as described.
Production of IL-4, IL-5, and IFN-γ by T cells from tolerant, immunized, and naive CBA spleen T cells in mLC.
Spleen T cells from tolerant (▵), immunized (○), and naive (■) CBA mice were stimulated with BALB/c spleen cells treated with 25 Gy gamma irradiation (experiment ) or 50 μg/mL mitomycin C (experiment 2) in mLC. Supernatants were harvested and pooled from triplicate wells daily and assayed for cytokines as described.
Effect of tolerance induction on the development of cellular immunity to H-2d–positive cells
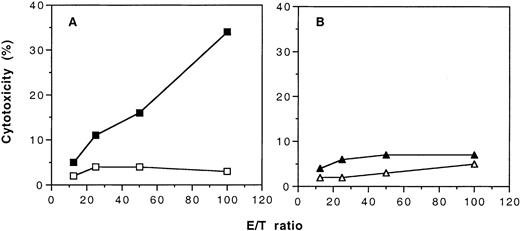
To demonstrate that transfusions of UV-B–irradiated PBMCs not only induced humoral immune tolerance to allogeneic MHC antigens but also induced cellular immune tolerance, we immunized the naive and the tolerant CBA mice with PBMCs from BALB/c mice. When the naive CBA mice were immunized with 2 weekly transfusions of BALB/c PBMCs, these mice developed CTL against H-2d–positive lymphoblasts (Figure5A). In contrast, CTL against H-2d–positive target cells could not be detected in the tolerant CBA mice after immunization with BALB/c PBMCs (Figure 5B). When H-2b–positive lymphoblasts were used as targets, CTL activity was not detected in either the tolerant and the naive CBA mice that had been immunized with BALB/c PBMCs (data not shown).
Development of cytotoxic cell activity in naive and tolerant CBA mice after immunization with 2 weekly transfusions of untreated PBMCs from BALB/c mice.
Target cells were ConA-stimulated lymphoblasts from BALB/c mice and labeled with 3H-thymidine. (A) Results of cytotoxic activity of spleen T cells from naive CBA mice with (▪) and without (■) immunization of BALB/c PBMCs. (B) Results of cytotoxic activity of spleen T cells from tolerant CBA mice with (▴) and without (▵) immunization of BALB/c PBMCs. Each value is a mean of triplicate incubations.
Development of cytotoxic cell activity in naive and tolerant CBA mice after immunization with 2 weekly transfusions of untreated PBMCs from BALB/c mice.
Target cells were ConA-stimulated lymphoblasts from BALB/c mice and labeled with 3H-thymidine. (A) Results of cytotoxic activity of spleen T cells from naive CBA mice with (▪) and without (■) immunization of BALB/c PBMCs. (B) Results of cytotoxic activity of spleen T cells from tolerant CBA mice with (▴) and without (▵) immunization of BALB/c PBMCs. Each value is a mean of triplicate incubations.
To determine whether the CD4+ negative regulatory cells developed in the tolerant CBA mice can suppress the cellular immune responses to H-2d–positive and H-2b–positive target cells, we conducted an adoptive transfer experiment. Results shown in Figure 6 indicated that CD4+ T cells from the tolerant CBA mice could suppress the development of cellular immune response to both H-2d– and H-2b–positive leukocytes.
Development of cytotoxic T-cell activity in CBA mice after adoptive transfer of CD4+ T cells from naive CBA mice or CBA mice tolerant to BALB/c H-2d antigens.
(A) CTL activity to H-2d–positive target cells in the adoptively transferred CBA mice after immunization with BALB/c PBMCs. (B) CTL activity to H-2b–positive target cells in the adoptively transferred CBA mice after immunization with BL/6 PBMCs. (●), CBA mice without adoptive transfer treatment (positive control); (■), CBA mice transferred with 5 × 106 spleen CD4+ T cells of naive CBA mice; (▵), CBA mice transferred with 5 × 106 spleen CD4+ T cells from tolerant CBA mice; (○), naive CBA mice without any treatment (negative control).
Development of cytotoxic T-cell activity in CBA mice after adoptive transfer of CD4+ T cells from naive CBA mice or CBA mice tolerant to BALB/c H-2d antigens.
(A) CTL activity to H-2d–positive target cells in the adoptively transferred CBA mice after immunization with BALB/c PBMCs. (B) CTL activity to H-2b–positive target cells in the adoptively transferred CBA mice after immunization with BL/6 PBMCs. (●), CBA mice without adoptive transfer treatment (positive control); (■), CBA mice transferred with 5 × 106 spleen CD4+ T cells of naive CBA mice; (▵), CBA mice transferred with 5 × 106 spleen CD4+ T cells from tolerant CBA mice; (○), naive CBA mice without any treatment (negative control).
Role of IL-12 in tolerance induction
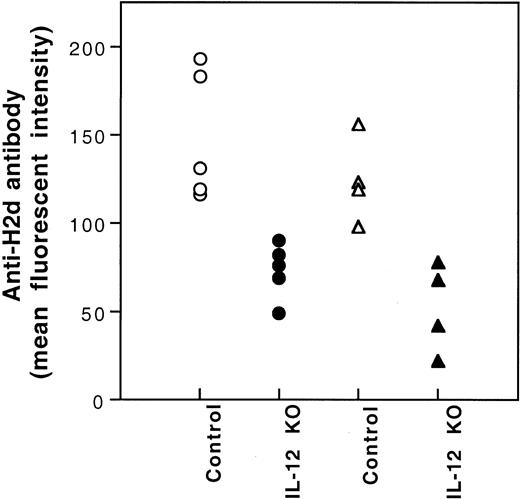
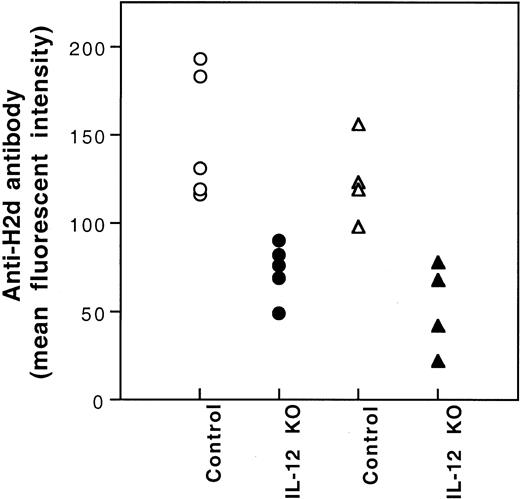
It has been reported that the production of IL-12 by antigen-presenting cells (APCs) plays an important role in the development of type 1 T cells.11-13 Because immunization of CBA mice with BALB/c PBMCs is associated with the polarization to type 1 T-cell cytokine response (Figure 4) and the production of IL-12 by BALB/c spleen stimulatory leukocytes in mLC is inhibited after UV-B–irradiation (data not shown), the inhibition of IL-12 production by UV-B may play a role in the induction of tolerance by UV-B–irradiated PBMCs. To investigate this possibility, we studied the effect of IL-12 administration on the induction of tolerance by UV-B–irradiated PBMCs. Five hundred nanograms IL-12 dissolved in 100 μL PBS was injected intraperitoneally immediately after each transfusion of UV-B–irradiated PBMCs. None of the treated CBA mice developed antibody to BALB/c donor leukocytes after 4 weekly transfusions of UV-B–irradiated BALB/c PBMCs plus IL-12 treatment. Thereafter, the CBA mice were challenged with 2 fully immunogenic doses of BALB/c PBMCs. All the CBA mice treated with UV-B–PBMCs and IL-12 became immunized (Table 2). However, the mean antibody activity of this group of CBA mice was lower than that of the naive control group (Table 2). To further investigate the role of IL-12 in the induction of tolerance by UV-B–irradiated leukocytes, we studied the alloantigenicity of PBMCs from BALB/c mice with knockout of the IL-12 β chain gene. Results showed that PBMCs from the knockout mice had reduced alloantigenicity (Figure7).
Immunogenicity of PBMCs of BALB/c mice with knockout of IL-12β chain gene.
CBA (○,●) and BL/6 (▵,▴) mice were transfused with PBMCs (2.5 × 104) from the wild-type (Control) or the IL-12β chain knockout (IL-12 KO) BALB/c mice weekly for 2 weeks. The development of antibody to BALB/c leukocytes in the serum collected 1 week after the second transfusion was measured by immunofluorescence flow cytometry. MFIs for preimmune CBA and BL/6 mice were 14 and 9, respectively.
Immunogenicity of PBMCs of BALB/c mice with knockout of IL-12β chain gene.
CBA (○,●) and BL/6 (▵,▴) mice were transfused with PBMCs (2.5 × 104) from the wild-type (Control) or the IL-12β chain knockout (IL-12 KO) BALB/c mice weekly for 2 weeks. The development of antibody to BALB/c leukocytes in the serum collected 1 week after the second transfusion was measured by immunofluorescence flow cytometry. MFIs for preimmune CBA and BL/6 mice were 14 and 9, respectively.
Effect of sublethal gamma irradiation on induced immune tolerance
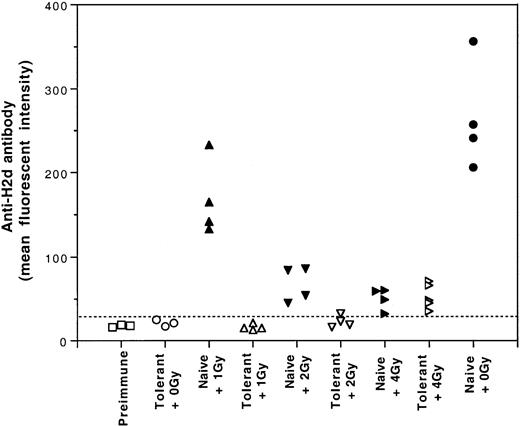
One potential application of induced tolerance was to establish hematopoietic mixed chimerism under sublethal conditioning in the tolerized recipients. Therefore, it was important to investigate the effect of sublethal gamma irradiation on the tolerance induced by UV-B–irradiated leukocytes. For this study, tolerant and naive CBA mice were treated with 0, 1, 2, or 4 Gy gamma irradiation. After gamma irradiation, each mouse was challenged with 2 weekly intravenous transfusions of a full immunogenic dose (2 × 105) of BALB/c PBMCs. The first challenge transfusion was given 6 hours after gamma irradiation. Results showed that the immune tolerance induced by UV-B–PBMCs was susceptible to 4 Gy gamma irradiation (Figure8). Nevertheless, immune tolerance was preserved when the tolerant mice were treated with no more than 2 Gy gamma irradiation (Figure 8).
Effect of gamma irradiation on the immune tolerance induced by transfusions of UV-B–irradiated leukocytes.
Tolerant and naive CBA mice were irradiated with 0, 1, 2, or 4 Gy gamma irradiation and challenged with 2 weekly intravenous infusions of a full immunogenic dose (2 × 105) of BALB/c PBMCs. The development of anti–H-2d antibody activities was measured by immunofluorescence flow cytometry. Broken line indicates the cutoff value for positive antibody detection.
Effect of gamma irradiation on the immune tolerance induced by transfusions of UV-B–irradiated leukocytes.
Tolerant and naive CBA mice were irradiated with 0, 1, 2, or 4 Gy gamma irradiation and challenged with 2 weekly intravenous infusions of a full immunogenic dose (2 × 105) of BALB/c PBMCs. The development of anti–H-2d antibody activities was measured by immunofluorescence flow cytometry. Broken line indicates the cutoff value for positive antibody detection.
Discussion
The primary goals of this study were to characterize the immune tolerance induced by transfusions of UV-B–irradiated PBMCs and to obtain necessary information for potential clinical application of the induced tolerance to transplantation of allografts. Experiments were conducted to determine the identity of negative regulatory cells developed during the induction of the immune tolerance and to investigate the effect of the tolerance induction on the cytokine production profile of T cells. Recent success using UV-B to inactivate leukocytes in platelet concentrates for the prevention of HLA alloimmunization in patients with acute non-lymphocytic leukemia14 supports the feasibility and the safety of such an approach.
Results of our earlier adoptive transfer study demonstrated that the induction of humoral immune tolerance by UV-B–irradiated leukocytes is associated with the development of negative regulatory MNLs.4 To determine the identity of the negative regulatory MNLs, the same adoptive transfer approach was used to test different subsets of MNLs for suppression of the humoral immune response to H-2d–positive leukocytes. Our results show that only CD4+ T cells could transfer the suppressive activity. Further study indicated that the transfer of suppressive activity was compromised after the depletion of CD25+ T cells (Figure 3). These findings suggest that CD4+/CD25+ T cells are the major players in the suppression of the humoral immune response to allogeneic leukocytes. They are consistent with recent reports that CD4+regulatory T cells can be induced ex vivo to inhibit T-cell response to alloantigens15 and that CD4+CD25+T cells are the unique immunoregulatory cells for the maintenance of peripheral tolerance.8-10 Nevertheless, how the transfusion of UV-B–irradiated PBMCs induces the development of negative regulatory helper T cells and how the immune response is suppressed by CD4+/CD25+ T cells remain to be determined.
In addition, the results of our adoptive transfer study (Figure 3B) showed that the negative regulatory CD4+ T cells not only suppressed the humoral immune response to BALB/c H-2dantigens but also inhibited the humoral immune response to BL/6 H-2b antigens. The lack of absolute specificity to H-2d antigens is consistent with our earlier finding that CBA mice tolerant to BALB/c H-2d antigens also became tolerant to BL/6 H-2b antigens.4 It is possible that CD4+CD25+-negative regulatory cells from the tolerant CBA mice may respond to different H-2 antigens and suppress the humoral immune response to H-2b antigens.
Earlier, it was reported that the induction of tolerance by neonatal priming of allogeneic cells resulted in the polarized production of type 2 cytokines by T cells.16,17 Because we observed the development of negative regulatory T-helper cells after tolerance induction by transfusions of UV-B–inactivated PBMCs, it was of interest to investigate the production of cytokines by T cells from the tolerant CBA mice. The results of our study revealed enhanced production of type 2 T-cell cytokines, such as IL-4, IL-5, and IL-10 (Figure 4, Table 1). In contrast, immunization of CBA mice to allogeneic BALB/c leukocytes led to the diminished production of type 2 T-cell cytokines and the enhanced type 1 T-cell cytokine response (Figure 4). These findings indicate that the tolerance induction by UV-B–PBMCs is associated with polarization to type 2 T-cell cytokine response. However, the possibility that CD4+ Tr1 regulatory cells may be responsible for the increased production of IL-10 should also be considered.10 Despite uncertain cellular sources of IL-4, IL-5, and IL-10, the ex vivo T-cell cytokine study could be useful for monitoring tolerance induction by UV-B–PBMCs in patients.
Production of IL-12 by APCs during the initial phase of the immune response is critical for the development of Th0 cells to Th1 cells.11-13 Absence of IL-12 steers the development of Th0 cells to Th2 cells.12 13 Because tolerance induction by UV-B–irradiated leukocytes polarizes T cells to the type 2 cytokine response and UV-B–irradiation inhibits IL-12 production by BALB/c spleen cells (K.J.K., unpublished observation, July 1998), the inhibition of IL-12 production by UV-B irradiation of BALB/c APCs may play a critical role in the tolerance induction by UV-B–PBMCs. Our finding that the administration of 500 ng recombinant IL-12 interfered with the induction of complete immune tolerance by UV-B–irradiated BALB/c PBMCs supports this possibility. To further demonstrate that the production of IL-12 by donor leukocytes is important for transfusion-associated alloimmunization to MHC antigens, we investigated the alloantigenicity of peripheral PBMCs from BALB/c mice with knockout of the IL-12β chain. We found that leukocytes from the IL-12β chain-deficient mice indeed had reduced alloantigenicity (Figure 7). However, the leukocytes from these knockout mice were not tolerogenic. These results suggest that the inhibition of IL-12 production is necessary but not sufficient for the induction of tolerance by UV-B– irradiated PBMCs.
Although our previous study showed that transfusions of allogeneic leukocytes inactivated with UV-B can induce complete humoral immune tolerance,4 it is unknown whether cellular immune tolerance can be induced at the same time. If the tolerance induced by UV-B–inactivated leukocytes is to be applied for allogeneic bone marrow and solid organ transplantation, it is important to learn whether cellular immune tolerance can be induced by the same approach. Our recent study using bone marrow and spleen cells from the tolerized CBA mice for hematopoietic reconstitution and prevention of graft-versus-host disease in lethally irradiated BALB/c mice5 suggested possible induction of the cellular immune tolerance by UV-B–irradiated allogeneic leukocytes. To confirm the development of cellular immune tolerance, we challenged the tolerized CBA mice with fully immunogenic doses of BALB/c PBMCs and found no development of significant T-cell–mediated cytotoxicity to H-2d–positive targets (Figure 5).
Further adoptive transfer study (Figure 6) showed that the CD4+-negative regulatory T cells from the tolerant CBA mice could also suppress the development of CTL activities to both H-2d– and H-2b–positive target cells. These results are consistent with the concurrent induction of both humoral and cellular immune tolerance by transfusions of UV-B–irradiated allogeneic PBMCs, and they support the idea that the induced cellular immune tolerance is mediated by the development of negative regulatory CD4+ T cells. However, it is unknown whether the same negative regulatory CD4+ T cells are responsible for the suppression of humoral and cellular immune responses.
If the tolerance induction by UV-B–irradiated PBMCs is to be applied to allogeneic hematopoietic stem cell transplantation, it is important to know how pretransplantation conditioning regimens, such as gamma irradiation, affect the induced immune tolerance. Therefore, we investigated the effect of sublethal doses of gamma rays on induced tolerance and found that 4 Gy gamma irradiation annulled the tolerance induced by UV-B leukocytes despite the immunosuppressive effect of 4 Gy gamma irradiation (Figure 8). Nevertheless, tolerance was preserved when the dose was reduced to 2 Gy or less. Susceptibility of the tolerance to gamma irradiation suggests that the induced negative regulatory or anergized immune cells are sensitive to gamma irradiation. This finding indicates that the dosage of pretransplantation gamma irradiation is important and should be carefully selected.
In summary, transfusions of UV-B–inactivated allogeneic leukocytes can induce humoral and cellular immune tolerance. Tolerance induction by UV-B leukocytes leads to the enhanced production of type 2 T-cell cytokines in mLC and to the development of CD4+CD25+-negative regulatory T cells. Induced tolerance is susceptible to 4 Gy or more sublethal gamma irradiation. These findings have provided useful information for potential clinical application of this approach to patients.
Supported by grant R01 HL-58809 from the National Heart, Lung and Blood Institute, National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
K. J. Kao, Department of Pathology, Immunology and Laboratory Medicine, Box 100275, University of Florida, Gainesville, FL 32610; e-mail: kjkao@ufl.edu.