It is thought that an increase in the adhesion of circulating reticulocytes to the vascular endothelium may initiate the vascular occlusion underlying the painful crises and organ failures typical of sickle cell disease (SCD). At least 2 receptors, usually present on reticulocytes, seem to be involved in this adhesion process: glycoprotein CD36 (glycoprotein IV) and integrin α4β1 (very late activation antigen–4). Recently, a high frequency of the platelet CD36–deficient phenotype was reported in black Africans. The frequency of this deficiency was similar in subjects with and without SCD. The role of CD36 in vaso-occlusion was then investigated by comparing the clinical course in 2 groups of black Africans homozygous for hemoglobin S, with and without CD36 deficiency, but similar in age, sex, geographical origin, number of α-globin genes, and β-globin gene haplotype. Flow cytometry showed that CD36 was absent from the circulating red blood cells and reticulocytes of platelet CD36–deficient individuals but present on those from patients with normal platelet CD36 expression, and that α4β1 integrin levels were similar on the reticulocytes of the 2 groups. Neither clinical severity, as evaluated by the frequency and characteristics of vaso-occlusive events, nor biological data differed significantly in the 2 groups of patients. Finally, although CD36 has been suggested to play a critical role in the pathogenesis of vaso-occlusion, this study, despite including only a small number of patients, supports the idea that the modulation of expression of a single type of adhesion molecule is insufficient to counteract the pathological process leading to vaso-occlusion in SCD patients.
Introduction
Sickle cell disease (SCD) is a clinical condition involving recurrent vaso-occlusive events leading to acute painful episodes and multiple organ failure. The mechanisms that precipitate vaso-occlusion are unclear. There is evidence to suggest that interactions between cells, particularly between sickle cells and the vascular endothelium, may increase the transit time of sickle cells in the capillary system, thereby initiating vaso-occlusion.1-4 Various experimental systems have demonstrated the greater adhesion of sickle red blood cells (RBCs), especially immature reticulocytes, to the endothelial cells of the microvasculature.5-10 Consistent with this, unusually high levels of adhesion molecules have been reported on immature reticulocytes of SCD patients.3,11 Cell interactions involve at least 2 adhesion molecules: the glycoprotein CD36 (glycoprotein IV) and the integrin α4β1(very late activation antigen–4), both of which are usually present on erythroid progenitors and lost during RBC maturation.12-14
We recently reported15 that the frequency of CD36 deficiency is high in black African individuals (7.7%), as high as in Asians, in whom this deficiency was first described,16 17and significantly higher than in individuals of Caribbean origin and in whites. The frequency of CD36 deficiency appears to be similar in black African individuals with and without SCD. In our previous study, we assessed CD36 expression on platelets and monocytes, but not on reticulocytes and mature RBCs. As we thought it possible that CD36 was involved in the vaso-occlusive process that occurs in SCD, we analyzed CD36 expression on reticulocytes and RBCs and investigated whether CD36 modulated the clinical course of SCD by comparing a group of CD36− patients homozygous for hemoglobin (Hb) S (SS) with a group of CD36+ SS patients.
Patients, materials, and methods
Patients
We studied 14 black African SS patients who presented CD36 deficiency (Table 1). Of these 14 CD36− SS patients, 7 belonged to the group of 18 CD36− individuals previously reported15; that group had included a white woman, an SC-SCD patient of Caribbean origin, and 16 black African subjects, 7 of whom were SS patients. The other 7 patients in our current study were identified later by screening the cohort of SCD patients followed at our center.
A diagnosis of CD36 deficiency was retained if CD36 was absent either from both platelets and monocytes (type I) or from platelets only (type II), as defined by Yamamoto et al.18 We also evaluated (see below) the level of CD36 expression on circulating reticulocytes and mature RBCs.
The clinical history and baseline biological variables of the 14 CD36− SS patients were compared with those of 31 controls, all of whom were SS patients followed at the Sickle Cell Center of Henri Mondor Hospital (Créteil, France) and were known to express CD36 on their blood cells. The 31 controls were selected by matching each of the 14 CD36− SS patients with 2 or 3 controls on the basis of sex, age, geographical origin, number of α-globin genes, and β-globin gene haplotype. The 45 patients studied had been regularly followed up at the Sickle Cell Center for a mean of 5 ± 3 years. As regular blood transfusions, hydroxyurea, and bone marrow transplantation may affect the symptoms of SCD, we considered only clinical and biological data collected before the introduction of any of these treatments. We studied 34 male patients and 11 female patients. Their mean age was 20.6 ± 10.4 years. The patients had lived in France for several years; some, particularly those who were still children, were born in France, but all were of sub-Saharan African origin.
Cell preparation and flow cytometry
Whole blood was obtained by venopuncture with EDTA used as anticoagulant, then washed in phosphate-buffered saline (PBS). The cells were pelleted by centrifugation; the supernatant was discarded; and a cell suspension was prepared with 10 μL cell pellet and 2 mL PBS containing 0.2% bovine serum albumin (BSA). This cell suspension (100 μL, about 5 × 106 total RBCs) was incubated for 90 minutes at room temperature (RT) with 100 μL diluted anti-CD36 monoclonal antibody (FA6-152) (Immunotech, Marseille, France) in PBS-BSA, at a concentration of 5 μg/mL. The preparation was washed twice in PBS and incubated for 45 minutes at RT with 100 μL of a 1/40 dilution in PBS-BSA of phycoerythrin-conjugated goat F(ab′)2 fragments of anti–mouse immunoglobulin G (IgG) (Immunotech). The cells were washed in PBS and incubated for 30 minutes at RT with 1 mL thiazole orange (TO) dye (Retic-Count) (Becton Dickinson, San Jose, CA), which stains RNA, to distinguish between reticulocytes and RBCs. As a negative control, 100 μL cell suspension was simultaneously incubated with 20 μL of an irrelevant mouse monoclonal antibody (mouse isotypic control) (Becton Dickinson) and treated as described above. Samples were then analyzed on a FACSCalibur machine (Becton Dickinson). RBCs were gated on the basis of forward-scatter versus side-scatter. Preliminary experiments showed that more than 99.7% of gated cells expressed the erythroid-specific glycophorin-A marker. At least 20 000 events were collected in this gate for subsequent analysis. The density of CD36 antigen sites was estimated by means of Dako Qifikit Calibration Beads (Dako, Copenhagen, Denmark), with the primary antibody (anti-CD36) used at saturating concentrations. An equivalent quantity of labeled secondary antibody, also used at a saturating concentration, was simultaneously added to the cell suspension and calibration beads bearing various known numbers of monoclonal antibody (IgG) molecules. The mean fluorescence intensity (MFI) measured for cells and beads was correlated with the number of bound primary antibody molecules and was expressed as the specific antibody-binding capacity (SABC). The data obtained with the beads were then used to establish a calibration curve (SABC vs MFI), and the SABC of the samples studied was calculated by interpolation by means of this calibration curve. Thus, SABC values represent an estimation of the maximum number of antibody molecules bound to the cells (assuming an ideal molar 1:1 ratio of antibody bound per antigen reached in antibody excess). Previous studies have shown a strong correlation between the maximum number of antibody molecules bound to red cells estimated by this procedure and the number of antigen sites, as determined by the Scatchard method.19 Expression of α4β1 was simultaneously determined by the same procedure, but with the use of 100 μL diluted CD49d monoclonal antibody (HP2/1) (Immunotech) in PBS-BSA, at a concentration of 5 μg/mL.
Biological and clinical data
Biological data (hemoglobin concentration, fetal hemoglobin level, blood cell count, creatinine concentration, uric acid concentration, liver enzyme activities, and hemolysis parameters) were obtained for each patient during steady state (ie, at least 1 month after an acute clinical event or 3 months after blood transfusion). The patient's case history was studied retrospectively, and data on complications that occurred during medical follow-up were collected. We analyzed the total number of days spent in the hospital for severe painful crises requiring the administration of morphine, the documented infectious episodes requiring hospital admission, and acute and chronic organ failure related to SCD (ie, stroke, acute chest syndrome, priapism, proliferative retinopathy, aseptic necrosis of the head of long bones, leg ulceration, papillary necrosis, chronic renal or hepatic failure, and chronic respiratory or cardiac failure). These biological and clinical data were compared for the 2 groups and analyzed statistically. We also established a clinical severity scoring system, based on a modified version of the model proposed by Hebbel et al,20 taking into account the complications of SCD classically related to vascular occlusions. We introduced into this scoring system other SCD complications such as papillary necrosis, priapism, and acute chest syndrome, all of which contribute to disease severity (Table 2). We also adapted the score assigned to severe painful crises to our clinical practice, as we used aggressive treatment (hydroxyurea or regular RBC exchange) if patients experienced more than 3 severe acute vaso-occlusive events per year. Indeed, Platt et al21 showed that the number of painful episodes per year is a measure of clinical severity and is correlated with early death, particularly in SCD patients older than 20 years.
Statistical analysis
The 2 groups were compared by means of the nonparametric Mann-Whitney test for quantitative data (biological data) and Pearson's chi-square test (or Fisher's exact test when necessary) for categorical data. Paired relative risks for each complication were calculated and compared with 1. A P < .05 was considered to be statistically significant.
Results
CD36 expression
The 14 patients selected on the basis of their platelet CD36–deficient phenotype also lacked CD36 on their reticulocytes and RBCs (Table 3), regardless of whether they had type I or type II CD36 deficiency. In contrast, the 31 controls (also SS patients) strongly expressed CD36 on reticulocytes and, to a lesser extent, on mature RBCs. These results were not influenced by reticulocyte count because reticulocytosis did not differ significantly in the 2 groups: 10.22% ± 4.18% (ie, absolute value of 318 × 109/L ± 95 × 109/L) for the CD36− patients, and 10.92% ± 4.03% (259 × 109/L ± 91 × 109/L) for the CD36+ controls.
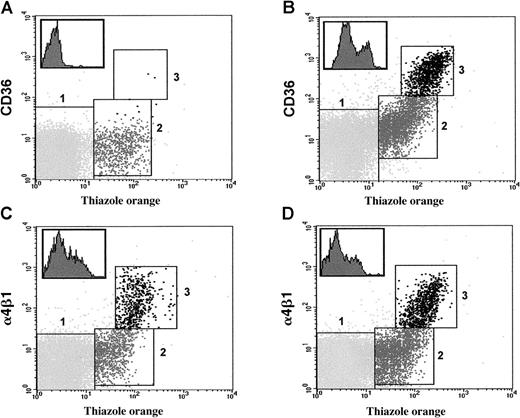
Flow cytometry analysis used to estimate the approximate level of CD36 (see “Patients, materials, and methods”) shows that RBCs (TO-negative) and reticulocytes (TO-positive) could be distinguished by appropriate gating after staining with TO and anti-CD36 antibody (Figure 1). We identified 2 well-defined reticulocyte populations in the control group of 31 CD36+SS patients: a cell population (population 3, Figure 1B) accounting for a mean of 24% ± 8.1% of the total reticulocyte count, with high levels of CD36 (8911 ± 3266 molecules per reticulocyte) and a cell population (population 2, Figure 1B) with lower levels of CD36 (576 ± 381 molecules per cell) (Figure 1). Flow cytometry analysis suggested that reticulocyte population 3, with high levels of CD36, probably corresponded to more immature reticulocytes or stress reticulocytes produced during high levels of bone marrow activity, as they stained strongly with TO (MFI = 125 ± 15) whereas population 2 corresponded to more mature reticulocytes less intensely stained by this reagent (MFI = 38 ± 10). In contrast, in 24 healthy individuals (white origin), reticulocyte population 3 accounted for only 3% ± 1% of the total reticulocyte count (data not shown). The 31 patients of the control group had 150 ± 83 CD36 molecules per RBC.
Flow cytometry analysis of CD36 and α4β1 on RBCs and reticulocytes from SS patients.
Typical profiles showing the simultaneous staining with anti-CD36 (panels A, B) or anti-α4β1 (panels C, D) antibody and thiazole orange of erythroid cell populations (RBCs and reticulocytes) from CD36− (panels A, C) and CD36+ (panels B, D) patients homozygous for Hb S (SS). Mature RBCs, mature reticulocytes, and immature reticulocytes were gated in regions 1, 2, and 3, respectively, according to the intensity of staining with the reagents used. Inserts in the upper left corners show histograms illustrating the heterogeneous expression of CD36 (panel B) and α4β1 (panels C, D) on reticulocytes gated in both regions 2 and 3.
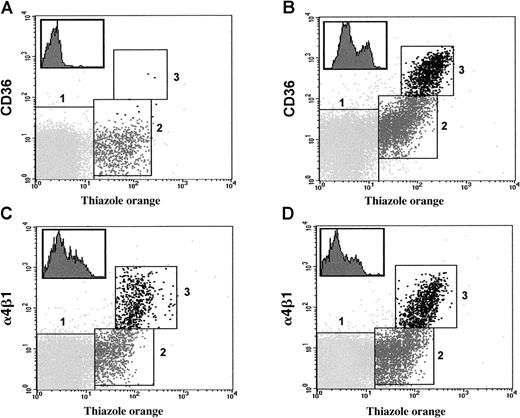
Flow cytometry analysis of CD36 and α4β1 on RBCs and reticulocytes from SS patients.
Typical profiles showing the simultaneous staining with anti-CD36 (panels A, B) or anti-α4β1 (panels C, D) antibody and thiazole orange of erythroid cell populations (RBCs and reticulocytes) from CD36− (panels A, C) and CD36+ (panels B, D) patients homozygous for Hb S (SS). Mature RBCs, mature reticulocytes, and immature reticulocytes were gated in regions 1, 2, and 3, respectively, according to the intensity of staining with the reagents used. Inserts in the upper left corners show histograms illustrating the heterogeneous expression of CD36 (panel B) and α4β1 (panels C, D) on reticulocytes gated in both regions 2 and 3.
Expression of α4β1
Both CD36− and CD36+ SS patients had large numbers of α4β1 molecules on the surface of their reticulocytes, and the number of α4β1 molecules per cell (estimated as determined in “Patients, materials, and methods”) did not differ significantly in the 2 groups (Table 3). It was possible to distinguish 2 reticulocyte populations (populations 2 and 3) in both groups of patients (Figure 1C-D). Population 3, with the highest level of α4β1, accounted for 22% ± 2% of the total reticulocyte count, a proportion very similar to that for reticulocyte population 3, which expressed high levels of CD36 (see above and Figure 1B). In addition, double-labeling studies showed that population 3 overexpressed CD36 (in CD36+ individuals) and α4β1 (data not shown) in both series of patients. In contrast, in 24 healthy individuals (white origin), the reticulocyte population 3 labeled by anti-α4β1 antibody accounted for only 8% ± 1% of the total reticulocyte count (data not shown). Finally, as expected, α4β1 was absent from mature erythroid cells in CD36− and CD36+ SS patients (Table 3).
Clinical data
The 2 groups of patients were similar in terms of the criteria used for matching (Table 4). No significant difference was found between them for the data studied (Table 5). Mean follow-up time was similar for the group of CD36− patients and the controls. No difference was found in the frequencies of the clinical complications of the disease in the 2 groups. The clinical severity score, which took into account all the vaso-occlusive complications that occurred during follow-up, was similar in the 2 groups, as shown in Table 5. The steady-state values of the biological variables studied were also similar for both groups of patients (Table6).
Finally, 10 of the 45 studied patients required, either temporarily or definitively, one of the following treatments: hydroxyurea, iterative blood exchange, or allogeneic bone marrow transplantation. The clinical history of these patients is summarized in Table7. Of these patients, 4 were CD36− whereas the other 6 were CD36+. Clinical severity scores were similar in the 2 groups (5.3 vs 6.0 respectively,P = .74).
Discussion
The symptoms of SCD are dominated by recurrent vaso-occlusive episodes, a high risk of infection, and chronic hemolytic anemia. The severity of these manifestations differs greatly among patients, whereas the genetic basis of the disease is the same in all patients: a single point mutation in the β-globin gene. The initial concept of a single physiopathologic process, whose basis is that polymerization of deoxygenated HbS leads to sickling, is therefore insufficient to explain this clinical polymorphism. This has led to investigation of the role of factors modulating disease severity.2 One of these factors, greater adhesion of RBCs to the microvascular endothelium, has been demonstrated in several experimental systems5-10 and has been suggested as initiating vaso-occlusion.20 These cell-cell interactions involve plasma proteins, such as thrombospondin, von Willebrand factor, and fibronectin,8-10,22 and adhesive molecules, such as CD36 and α4β1.11,23,24 CD36 and α4β1 are normally present on the cell surface of erythroid precursors and other cell lineages,12,22 but have been shown to be present at higher levels on sickle reticulocytes.11 23
The frequency of platelet CD36 deficiency has been reported to be high in Asians16,17 and, more recently, in black Africans with and without SCD.15 CD36 deficiency was diagnosed if CD36 was absent from both platelets and monocytes (type I) or from platelets only (type II). In this study, CD36 was also found to be absent from the erythroid cells of the 14 platelet CD36− patients. These patients therefore displayed 2 types of CD36 deficiency: either all 3 hematopoietic lineages lacked CD36 or only the erythroid and megakaryocytic cells were CD36-deficient.
The detection of erythroid cells lacking CD36 in SCD patients may have important implications owing to the role of CD36 in RBC adhesion to endothelial cells. Differences among individuals in the expression of this RBC-adhesive molecule could contribute to the clinical variability of SCD. Styles et al25 showed that α4β1 and CD36 levels were lower on the reticulocytes of SCD patients treated with hydroxyurea, a drug known to increase fetal hemoglobin synthesis and to improve the clinical course of SCD patients.26,27 These authors found that hydroxyurea induced a significant decrease in α4β1 expression on reticulocytes and mature RBCs. CD36 levels decreased on reticulocytes, but not to the same extent as α4β1, and often increased on mature RBCs. Styles et al25 then suggested that hydroxyurea-induced changes in the expression of α4β1 and CD36 may account for some of the clinical benefits of this drug. In light of published results suggesting that CD36 is involved in the vaso-occlusive process, the aim of this study was to determine whether CD36 expression on sickle RBCs and reticulocytes could be considered to be a determinant factor of SCD severity in a human model.
Our results suggest that the presence or absence of CD36 on sickle reticulocytes and RBCs has no effect on the severity of sickle cell anemia. Indeed, disease outcome was similar for CD36− SS patients and controls, all of whom had been followed for long periods of time at the same sickle cell center, with the same therapeutic protocol. In particular, the morbidity associated with vaso-occlusive events was not affected by the absence of CD36 from reticulocytes, and the number of day-hospitalizations for severe painful crises and the frequency of organ failure related to vaso-occlusion were similar for the 2 groups of patients. As many of the patients studied were adults or adolescents, we were able to evaluate the outcome of the disease and, in particular, late complications. Furthermore, analysis of the group of 10 patients requiring aggressive treatment (ie, blood transfusion program, hydroxyurea, or allogeneic bone marrow transplantation) showed that the proportion of patients requiring such treatment was similar in CD36− patients (4 of 14) and CD36+ patients (6 of 31) and that the vaso-occlusive lesions observed in these 2 groups of patients were similar. This suggests that the absence of CD36 from reticulocytes and RBCs does not prevent severe vaso-occlusive complications in SCD.
In conclusion, although previous studies have suggested that CD36 plays a key role in the adhesion of sickle RBCs to the endothelium and have provided evidence that this adhesive molecule is involved in the vaso-occlusive process, this study, despite including only a small number of patients, is not consistent with this assertion in in vivo conditions. As in other systems, the adhesive molecules on sickle reticulocytes are probably redundant. Many molecules are presumably involved in the adhesion of sickle cells to the endothelium, and these molecules may interact in initiation of the vaso-occlusive process. Consistent with this, we have shown that both CD36− and CD36+ SS patients express similarly high levels of α4β1 on a subpopulation of reticulocytes accounting for about 22% to 24% of the total reticulocyte count. These conclusions are consistent with the results obtained in vitro by Joneckis et al,28 who demonstrated that CD36-thrombospondin interactions were not inhibited by known antagonists, suggesting that a different mechanism may be responsible for sickle RBC adhesion to the endothelium. We therefore suggest that the modulation of a single type of adhesion molecule (in this case, variation in CD36 expression) is unlikely to be sufficient to counteract the pathologic process leading to vaso-occlusion in SCD patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
P. Bierling, EFS-Ile de France, Laboratoire d'immunologie leuco- plaquettaire, Hôpital Henri Mondor, 51 avenue du Maréchal de Lattre de Tassigny, 94000 Créteil, France; e-mail: philippe.bierling@efs.sante.fr.