Abstract
Localization of epitopes for platelet-associated (PA) anti–GPIIb-IIIa (αIIbβ3) autoantibodies in chronic immune thrombocytopenic purpura remains elusive. Previous studies suggest that PA antibodies recognize the tertiary structure of intact glycoprotein (GP) IIb-IIIa. To localize their epitopes using antigen-capture enzyme-linked immunosorbent assay (ELISA), the reactivity of 34 PA anti–GPIIb-IIIa antibodies was examined with recombinant GPIIb-IIIa having a defect in ligand-binding sites in either GPIIb or GPIIIa, and no major conformational change was induced: KO variant GPIIb-IIIa was attributed to a 2–amino acid insertion between residues 160 and 161 in the W3 4-1 loop in GPIIb, and CAM variant GPIIb-IIIa was attributed to D119Y in GPIIIa. In one third (11 of 34) of the patients, PA antibodies showed a marked decrease (less than 50%) in reactivity with KO compared with wild-type GPIIb-IIIa. Their reactivity was also impaired against GPIIbD163A-IIIa. In sharp contrast, they reacted normally with CAM GPIIb-IIIa. OP-G2, a ligand-mimetic monoclonal antibody, markedly inhibited their binding to GPIIb-IIIa in patients with impaired binding to KO GPIIb-IIIa, but small GPIIb-IIIa antagonists did not. In addition, a newly developed sensitive ELISA indicated that autoantibodies showing impaired binding to KO are more potent inhibitors for fibrinogen binding. The present data suggest that certain PA anti–GPIIb-IIIa autoantibodies recognize epitopes close to the ligand-binding site in GPIIb, but not in GPIIIa.
Introduction
Chronic immune thrombocytopenic purpura (ITP) is an autoimmune disorder characterized by the early destruction of platelets from antiplatelet autoantibodies.1-3 Autoantibodies from most patients with ITP are mainly directed to the platelet membrane glycoprotein (GP) IIb-IIIa (integrin αIIbβ3) or GPIb-IX.4,5 It has been demonstrated that platelet-associated (PA) autoantibodies, rather than serum antibodies, play a key role in platelet destruction.6,7 Although a few studies demonstrate the localization of autoantigens on GPIIb or GPIIIa for serum antibodies,8-11 characterization of the antigenic epitope(s) for PA autoantibodies remains elusive.12-14Dissociation of the GPIIb-IIIa complex into free GPIIb and GPIIIa by EDTA treatment markedly impaired the reactivity of PA anti–GPIIb-IIIa autoantibodies, suggesting that most PA autoantibodies recognize cation-dependent conformation(s) of GPIIb-IIIa.15,16Characterization by using large recombinant GPIIIa peptides failed to localize the autoantigenic epitopes on GPIIb-IIIa,17 and our recent study indicated that PA anti–GPIIb-IIIa (αIIbβ3) autoantibodies do not react with αVβ3.18 These findings suggest that PA autoantibodies are highly GPIIb-IIIa specific and that they recognize the tertiary structure of intact GPIIb-IIIa. In other words, it is likely that most autoantigenic epitopes are localized in either the GPIIb-IIIa complex or the cation-dependent conformations on GPIIb or GPIIIa.
The GPIIb-IIIa complex (αIIbβ3), a noncovalently associated, divalent cation-dependent heterodimer, is a prototypic integrin and plays a crucial role in normal hemostasis and platelet aggregation as a physiologic receptor for fibrinogen and von Willebrand factor.19,20 The interaction of these ligands with GPIIb-IIIa is mediated at least in part by an RGD sequence. Glanzmann thrombasthenia (GT) is a rare autosomal recessive bleeding disorder characterized by a quantitative or qualitative abnormality in GPIIb-IIIa (αIIbβ3).21Specificity of autoantibodies against GPIIb-IIIa was initially reported by the impaired reactivity of PA autoantibodies with GT platelets lacking GPIIb-IIIa.22 Characterization of molecular defects in GT from dysfunctional GPIIb-IIIa (variant GT) is informative in defining functionally important site(s) in GPIIb-IIIa, and multiple ligand-binding sites have been identified in both GPIIb and GPIIIa.23-25 Loftus et al24 first demonstrated D119 in GPIIIa as one of the critical residues for ligand binding by the characterization of a variant GT, CAM. Recently, we demonstrated that 2–amino acid insertion (R-T) between amino acid residues 160 and 161 in GPIIb is responsible for a ligand-binding defect in a variant GT, KO, and we identified D163 in GPIIb as one of the ligand-binding sites.25 Both KO and CAM variant GPIIb-IIIa showed markedly impaired ligand binding without disturbing its surface expression or inducing major structural change in the receptor.
In the present study, to further characterize PA autoantibodies, we investigated their reactivity against recombinant GPIIb-IIIa expressing these nonfunctional GPIIb-IIIa. Approximately one third of ITP patients with PA anti–GPIIb-IIIa autoantibodies had marked decreases in the reactivity with KO GPIIb-IIIa. Their impaired binding is KO GPIIb-IIIa specific because they reacted normally with CAM variant GPIIb-IIIa. The ligand-mimetic monoclonal antibody (mAb) OP-G2, but not small GPIIb-IIIa antagonists, markedly inhibited their binding to GPIIb-IIIa in patients with impaired binding to KO GPIIb-IIIa. In addition, our sensitive fibrinogen-binding enzyme-linked immunosorbent assay (ELISA) showed that PA autoantibodies have the potential to inhibit fibrinogen binding to GPIIb-IIIa irrespective of their epitope localizations. However, antibodies showing the impaired binding to KO GPIIb-IIIa are more potent inhibitors of fibrinogen-binding than the others. Our data suggest that in one third of patients with ITP, epitopes for PA anti–GPIIb-IIIa autoantibodies locate near the ligand-binding site of GPIIb.
Patients, materials, and methods
Patients
We studied 101 patients with chronic ITP (19 men, 82 women). Diagnoses of chronic ITP were made according to practice guidelines.26 Informed consent was obtained from all patients. Using modified antigen-capture ELISA with platelet GPIIb-IIIa, we detected anti–GPIIb-IIIa (anti-αIIbβ3) autoantibodies in 41 of 101 (41%) platelet eluate samples.18 Among them, 34 eluates containing anti–GPIIb-IIIa antibodies were available and were further characterized in this study (Table 1). Given the limited sensitivity of our assay, it is likely that the remaining 60 patients without detectable anti–GPIIb-IIIa antibodies had autoantibodies directed against antigens other than GPIIb-IIIa, leading to thrombocytopenia.
Antibodies
OP-G2, a murine mAb specific for the GPIIb-IIIa complex, is an activation-independent, ligand-mimetic antibody to GPIIb-IIIa.27 PAC-1, an activation-dependent, ligand-mimetic mAb, was a gift from Dr Sanford Shattil (The Scripps Research Institute, La Jolla, CA).28 OP-G2 and PAC-1 inhibit ligand binding to GPIIb-IIIa, and their binding is abolished by RGD peptides or GPIIb-IIIa–specific antagonists.29,30OP-G2 and PAC-1 have RGD-like RYD sequences in the CDR3 region of the heavy chain and recognize the ligand-binding sites in GPIIb-IIIa.27 AP2 (a mAb specific for the GPIIb-IIIa complex) was a generous gift from Dr Thomas Kunicki (The Scripps Research Institute),31 AP3 (a mAb specific for GPIIIa) was from Dr Peter Newman (The Blood Center of Southeastern Wisconsin, Milwaukee, WI),32 and PT25-2 (an activating mAb specific for GPIIb) was from Drs Makoto Handa and Yasuo Ikeda (Keio University, Tokyo, Japan).33 TP80 (a mAb specific for GPIIb) and MOPC21 (a control immunoglobulin [Ig] G1) were purchased from Nichirei (Tokyo, Japan) and Sigma Chemical (St Louis, MO), respectively. Purification of monoclonal IgG from ascites fluid by affinity chromatography on Protein A-Sepharose CL-4B (Pharmacia, Piscataway, NJ) and biotinylation of the mAbs with NHS-LC Biotin (Pierce Chemical, Rockford, IL) were performed as previously described.34
Anti–HPA-1a (PlA1) alloantibody was purchased from Olympus (Tokyo, Japan), and anti–HPA-3a (Baka) alloantibody was a generous gift from Dr Nobuo Nagao (Osaka Red Cross Blood Center, Japan).
Synthetic ligands
Platelet isolation and preparation of platelet-associated or serum antibody eluates
Platelets were obtained from blood anticoagulated with Na2-EDTA by differential centrifugation as previously described.34 PA antibodies were eluted from 200 μL washed platelet suspensions at a concentration of 2 × 105/μL by adding 200 μL diethyl ether as previously described.34 Serum auto- or allo-antibodies (1 mL) were incubated with 2 × 109 platelets for 2 hours at room temperature followed by 6 washes with citrate buffer, and then bound antibodies were eluted from 2 × 105 platelets/μL by diethyl ether. A number of control eluates were prepared from platelets from healthy control subjects. Eluates were kept at −80°C until use.
Construction of expression vectors
Wild-type GPIIb and GPIIIa cDNAs cloned into mammalian expression vector pcDNA3 (Invitrogen, San Diego, CA) were generously provided by Dr Peter Newman (Milwaukee, MI) and Dr Gilbert White (University of North Carolina, Chapel Hill), respectively. Expression vectors containing mutant cDNA were generated by polymerase chain reaction–based cartridge mutagenesis or overlap extension polymerase chain reaction.25 Nucleotide sequences of the fragments inserted were confirmed by sequence analysis. Mutant cDNAs used in this study were as follows: 2–amino acid insertion (R-T) between residues 160 and 161 in GPIIb (KO variant),25 D163→A substitution in GPIIb (GPIIbD163A),25 D119→Y substitution in GPIIIa (GPIIIaD119Y, CAM variant),24 and an activated mutant (T562→N substitution in GPIIIa [GPIIIaT562N]).37
Cell transfection
GPIIb and GPIIIa constructs were cotransfected into 293 cells by the calcium phosphate method as previously described.38The 293 cells transiently expressing mutant GPIIb-IIIa were obtained and analyzed 2 days after transfection. In addition, stable transfectants expressing wild-type (WT) or KO-variant GPIIb-IIIa were selected for G418 resistance and cultured in Dulbecco modified Eagle medium with 10% heat-inactivated fetal calf serum (Life Technologies, Gaithersburg, MD).
Flow cytometry
Transfected cells were detached from dishes with 0.02% EDTA and analyzed by flow cytometry.25 Detached cells were washed twice with phosphate-buffered saline and resusupended in Tris-buffered saline containing 2 mM CaCl2 (TBS-CaCl2, pH 7.4). Cells (2000/μL) were incubated with each mAb examined at a final concentration of 10 μg/mL for 30 minutes on ice, washed twice with TBS-CaCl2, and incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti–mouse immunoglobulin (all mAbs except PAC-1: Becton Dickinson, Mountain View, CA) for an additional 30 minutes. PAC-1 binding to cells was assessed in the presence of PT25-2, an activating mAb.37 Cells were incubated with 10 μg/mL PT25-2 and a 1:250 dilution of PAC-1 ascites for 30 minutes on ice, washed twice, and incubated with FITC-conjugated goat anti–mouse IgM (μ-chain specific; Caltag Laboratories, Burlingame, CA) for an additional 30 minutes. Samples were analyzed using a flow cytometer (FACScan; Becton Dickinson).
Antigen-capture ELISA
Antigen-capture ELISA was performed as previously described with slight modification.16 Briefly, 10 × 103/μL washed platelets or 1 × 103/μL detached 293 cells expressing wild-type or mutant GPIIb-IIIa were solubilized into 50 mM TBS containing 2 mM CaCl2, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, and 100 μg/mL leupeptin (Sigma). After insoluble material was removed by centrifugation at 10 000g for 10 minutes, 100 μL lysate was applied to the wells of a microtiter tray, each containing 0.25 μg fixed mAb against GPIIb-IIIa (AP2 or TP80). After incubation, the tray was washed with 50 mM TBS containing 2 mM CaCl2, 0.05% Tween 20, and 0.1% bovine serum albumin (washing buffer). Wells were then incubated with 20 μL eluate for 1 hour at room temperature. After washing, bound IgG was detected with ELISA using biotinylated mouse anti–human IgG (Jackson Immunoresearch Laboratories, West Grove, PA) and avidin-biotin-alkaline phosphatase complex (ABC; Vector, Burlingame, CA). Enzyme-substrate reaction was performed with the ELISA amplification system (Life Technologies) according to the manufacturer's instructions. In each assay, samples were run in duplicate, and 5 control samples (randomly selected from 20 control samples) were examined in the same microtiter tray. Results were expressed as ΔOD [optical density (OD) value obtained from a test sample − (mean + 3 SD) OD value obtained from 5 control samples]. ΔOD above zero was considered positive. We confirmed that there was a linear relation between ΔOD values and anti–GPIIb-IIIa antibody amounts in this assay (data not shown). When we used recombinant GPIIb-IIIa from transient transfectants, we first quantified the amounts of GPIIb-IIIa in the cell lysates by antigen-capture ELISA using biotinylated AP3 and then adjusted them to the same amounts of wild-type GPIIb-IIIa in the stable transfectant lysate.
Fibrinogen-binding assay
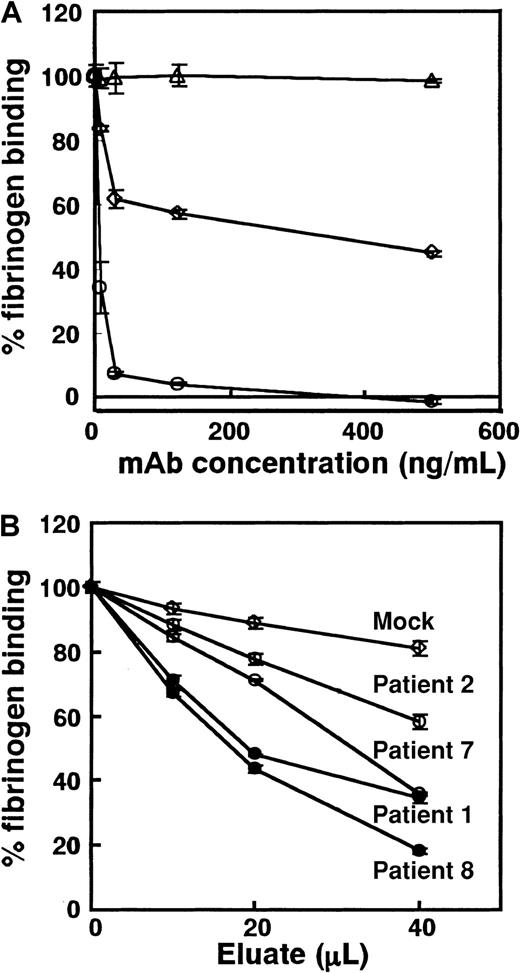
Inhibitory effects of anti–GPIIb-IIIa autoantibodies on fibrinogen binding to GPIIb-IIIa were measured with sensitive ELISA using biotinylated fibrinogen (Calbiochem-Novabiochem, La Jolla, CA) and an activated mutant GPIIb-IIIa (GPIIIaT562N). GPIIb-IIIaT562N is a constitutively active form of GPIIb-IIIa and binds fibrinogen without any activating agent.37 Clottability of the biotinylated fibrinogen was more than 95%.39 Five times 103 μL 293 cells expressing GPIIb-GPIIIaT562N were solubilized into 50 mM TBS containing 1% Triton X-100 and protease inhibitors, and 100 μL lysate was applied to the wells of a microtiter tray containing 0.25 μg fixed TP80. After 1-hour incubation, the wells were washed and incubated with 100 μL diluted eluates or mAbs for 30 minutes at room temperature. Biotinylated fibrinogen was then added to each well (final concentration, 150 ng/mL) and incubated for 1 hour at room temperature. After washing, bound fibrinogen was detected with ABC and the ELISA amplification system (Life Technologies). Data are expressed as percentage fibrinogen binding calculated according to the following formula: % fibrinogen binding = ([OD]x − [OD]i/[OD]m − [OD]i) × 100, where [OD]x is the OD value of fibrinogen binding in the presence of the tested sample, [OD]i is the OD value of fibrinogen binding in the presence of 20 mM EDTA, and [OD]m is the OD value of fibrinogen binding in the absence of 20 mM EDTA.
Results
Reactivity of PA autoantibodies with mutant GPIIb-IIIa lacking ligand-binding function
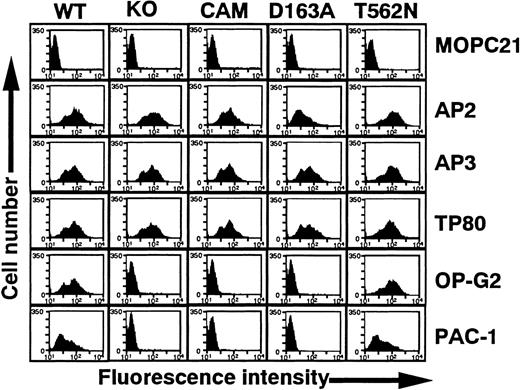
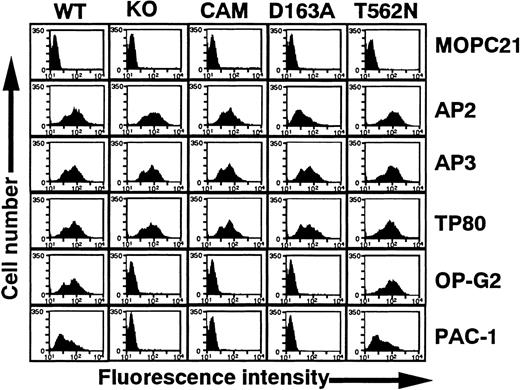
In this study, we characterized the epitopes for 34 platelet eluates containing anti–GPIIb-IIIa autoantibodies. In our previous study, we examined 13 eluates (numbers 1-3, 7, 8, 14, 18, 24-27, 29, and 31) and demonstrated that EDTA treatment of GPIIb-IIIa at 37°C markedly reduced the reactivity of these eluates.16 All eluates except number 16 failed to react with αvβ3 (data not shown).18 These data strongly suggest that the epitopes are conformational and depend on an intact GPIIb-IIIa complex. To further localize them, we used several recombinant GPIIb-IIIa mutants (Figure1). KO and CAM GPIIb-IIIa are well-characterized, naturally occurring mutations originally found in patients with variant GT.24,25 GPIIb-IIIa–specific mAbs AP2 and PT25-2 (not shown), GPIIIa-specific mAb AP3, and GPIIb-specific mAb TP80 reacted equivalently with KO, CAM, and wild-type GPIIb-IIIa expressed on 293 cells. However, neither the activation-independent ligand-mimetic mAb OP-G2 nor the activation-dependent ligand-mimetic mAb PAC-1 in the presence of the activating mAb PT25-2 reacted with these mutant GPIIb-IIIa variants. Taken together with their molecular characterization, these data show that KO and CAM have a ligand-binding defect resulting from the 2–amino acid (R-T) insertion between 160-161 amino acid residues in GPIIb and the D119Y mutation in GPIIIa, respectively, without any major structural changes in the receptor.24 25 In addition, Figure 1 shows that GPIIbD163A induces a similar defect in GPIIb-IIIa to the KO variant.
Flow cytometric analysis of 293 cells transiently expressing mutant GPIIb-IIIa.
WT, wild-type GPIIb-IIIa; KO, GPIIb-IIIa having 2 amino acid insertion between 160-161 of GPIIb; CAM, GPIIb-IIIa having D119→Y in GPIIIa; D163A, GPIIb-IIIa having D163→A in GPIIb; and T562N, GPIIb-IIIa having T562→N in GPIIIa. Cells were incubated with 10 μg/mL following mAbs: AP2, specific for GPIIb-IIIa complex; AP3, specific for GPIIIa; TP80, specific for GPIIb; OP-G2, activation-independent ligand-mimetic mAb to GPIIb-IIIa. Bound antibodies were detected by FITC-labeled goat anti–mouse immunoglobulin. The binding of PAC-1 (activation-dependent ligand mimetic mAb to GPIIb-IIIa) was examined in the presence of 10 μg/mL PT25-2 and detected by FITC-labeled goat anti–mouse IgM. MOPC21 (mouse myeloma IgG1) was used as a control antibody.
Flow cytometric analysis of 293 cells transiently expressing mutant GPIIb-IIIa.
WT, wild-type GPIIb-IIIa; KO, GPIIb-IIIa having 2 amino acid insertion between 160-161 of GPIIb; CAM, GPIIb-IIIa having D119→Y in GPIIIa; D163A, GPIIb-IIIa having D163→A in GPIIb; and T562N, GPIIb-IIIa having T562→N in GPIIIa. Cells were incubated with 10 μg/mL following mAbs: AP2, specific for GPIIb-IIIa complex; AP3, specific for GPIIIa; TP80, specific for GPIIb; OP-G2, activation-independent ligand-mimetic mAb to GPIIb-IIIa. Bound antibodies were detected by FITC-labeled goat anti–mouse immunoglobulin. The binding of PAC-1 (activation-dependent ligand mimetic mAb to GPIIb-IIIa) was examined in the presence of 10 μg/mL PT25-2 and detected by FITC-labeled goat anti–mouse IgM. MOPC21 (mouse myeloma IgG1) was used as a control antibody.
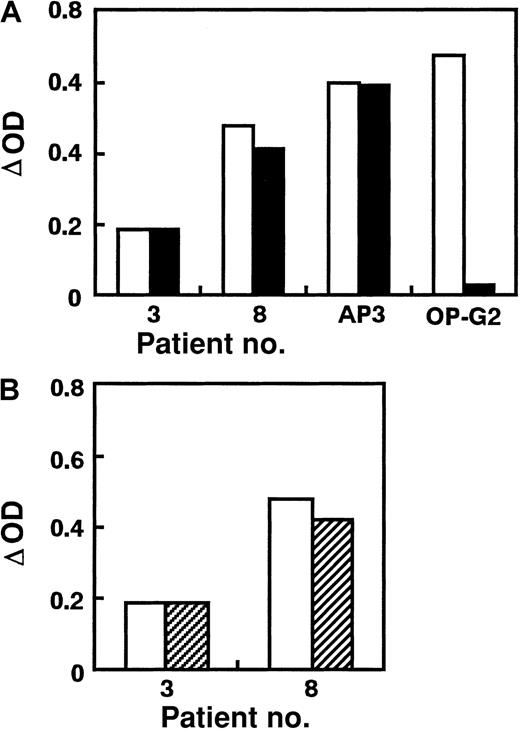
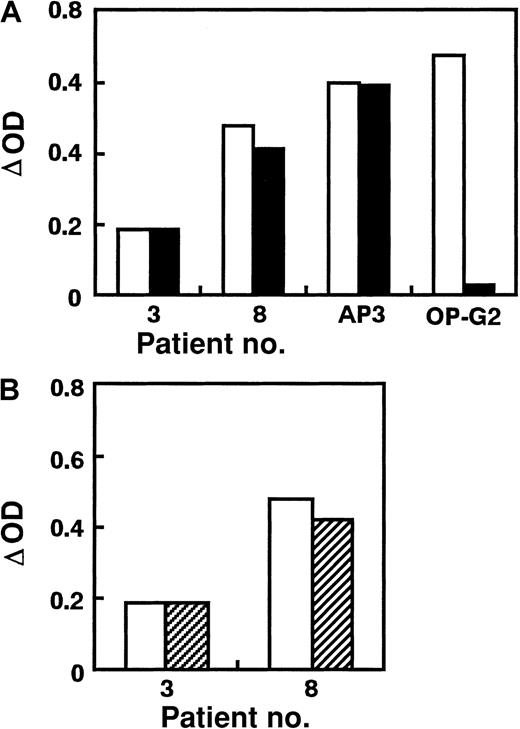
We used AP2 for antigen capture in ELISA because AP2 can equally bind to KO variant and WT GPIIb-IIIa obtained from stable transfectants (Figure 1). In preliminary studies, ΔOD values in antigen-capture ELISA using KO variant GPIIb-IIIa for anti-GPIIb (anti–HPA-3a) or anti-GPIIIa (anti–HPA-1a or biotinylated AP3) antibodies were almost the same as those using WT GPIIb-IIIa, whereas ΔOD for biotinylated OP-G2 was completely negative in antigen-capture ELISA using KO GPIIb-IIIa (Figure 2A). This antigen-capture ELISA showed good reproducibility even in different occasions—eg, ΔOD ratio [(ΔOD in antigen-capture ELISA using KO GPIIb-IIIa/ΔOD in antigen-capture ELISA using WT GPIIb-IIIa) × 100] for AP3, anti–HPA-1a, anti–HPA-3a, and OP-G2 were 97.9% ± 6.1% (n = 9), 95.1% ± 11.3% (n = 3), 95.8% ± 15.4% (n = 3), and 0.1% ± 2.6% (n = 9), respectively. We then examined the reactivity of PA autoantibodies in 34 ITP patients with KO and WT GPIIb-IIIa (Figure 2B, Table 1). Interestingly, 11 (32%) eluates showed marked (more than 50%) decrease in reactivity against the KO variant compared with WT GPIIb-IIIa. When TP80 or AP3 was used as an antigen-capturing mAb, essentially the same data were obtained (data not shown). These data ruled out the possibility that the capturing mAb might inhibit the binding of autoantibodies. We also examined the reactivity of eluates with GPIIbD163A-IIIa. Because AP2 showed less reactivity against GPIIbD163A-IIIa than other anti–GPIIb-IIIa mAbs (Figure 1), we used TP80 for antigen capture. As shown in Figure3, eluates from patients 1, 3, and 8 also showed marked decreases in ΔOD in antigen-capture ELISA using GPIIbD163A-IIIa.
Reactivity of PA autoantibodies with KO variant GPIIb-IIIa.
(A) Reaction of mAbs (AP3 and OP-G2) and antiplatelet alloantibodies (anti–HPA-1a and anti–HPA-3a) with KO variant GPIIb-IIIa. (B) PA autoantibodies from 11 patients with chronic ITP, showing a marked decrease (greater than 50%) in the reactivity against KO variant compared with WT GPIIb-IIIa. Results are expressed as ΔOD: [OD value obtained from a test sample − (mean + 3 SD) OD value obtained from 5 control samples]. Open columns represent ΔOD value in antigen-capture ELISA using WT GPIIb-IIIa, and filled columns represent ΔOD value in antigen-capture ELISA using KO mutant. Data are representative of 2 separate experiments.
Reactivity of PA autoantibodies with KO variant GPIIb-IIIa.
(A) Reaction of mAbs (AP3 and OP-G2) and antiplatelet alloantibodies (anti–HPA-1a and anti–HPA-3a) with KO variant GPIIb-IIIa. (B) PA autoantibodies from 11 patients with chronic ITP, showing a marked decrease (greater than 50%) in the reactivity against KO variant compared with WT GPIIb-IIIa. Results are expressed as ΔOD: [OD value obtained from a test sample − (mean + 3 SD) OD value obtained from 5 control samples]. Open columns represent ΔOD value in antigen-capture ELISA using WT GPIIb-IIIa, and filled columns represent ΔOD value in antigen-capture ELISA using KO mutant. Data are representative of 2 separate experiments.
Reactivity of PA autoantibodies with GPIIbD163A-IIIa.
Eluates from patients 1, 3, and 8, with impaired binding to KO variant GPIIb-IIIa, showed the impaired binding to GPIIbD163A-IIIa. Results are expressed as ΔOD: [OD value obtained from a test sample − (mean + 3 SD) OD value obtained from 5 control samples]. Open columns represent ΔOD value in antigen-capture ELISA using WT GPIIb-IIIa, and filled columns represent ΔOD value in antigen-capture ELISA using GPIIbD163A-IIIa.
Reactivity of PA autoantibodies with GPIIbD163A-IIIa.
Eluates from patients 1, 3, and 8, with impaired binding to KO variant GPIIb-IIIa, showed the impaired binding to GPIIbD163A-IIIa. Results are expressed as ΔOD: [OD value obtained from a test sample − (mean + 3 SD) OD value obtained from 5 control samples]. Open columns represent ΔOD value in antigen-capture ELISA using WT GPIIb-IIIa, and filled columns represent ΔOD value in antigen-capture ELISA using GPIIbD163A-IIIa.
We next examined the reactivity of anti–GPIIb-IIIa autoantibodies with CAM variant GPIIb-IIIa, the loss-of-function mutation in GPIIIa. In these experiments, the amount of CAM variant GPIIb-IIIa obtained from transient transfectants was adjusted to that of wild-type GPIIb-IIIa from stable transfectants by monitoring AP3 binding. ΔOD values in antigen-capture ELISA using CAM GPIIb-IIIa showed essentially the same results as KO GPIIb-IIIa for AP3, anti–HPA-1a, anti–HPA-3a, and OP-G2 (Figure 4A)—ΔOD ratios for AP3, anti–HPA-1a, anti–HPA-3a, and OP-G2 were 94.9% ± 3.8% (n = 5), 101.0% ± 13.9% (n = 3), 99.5% ± 11.9% (n = 3), and −0.6 ± 3.4% (n = 5), respectively. In sharp contrast to KO GPIIb-IIIa, none of the patients' eluates showed a decrease in reactivity with CAM GPIIb-IIIa (Table 1, Figure 4B). These findings indicate that PAIgG autoantibodies mainly recognize epitopes disturbed by KO mutant and D163A in GPIIb, but not by CAM mutant in GPIIIa in one third of ITP patients with anti–GPIIb-IIIa autoantibodies.
Reactivity of PA autoantibodies with CAM variant GPIIb-IIIa.
(A) Reactivity of mAbs (AP3 and OP-G2) and antiplatelet allo-antibodies (anti–HPA-1a and anti–HPA-3a) with CAM variant GPIIb-IIIa. (B) Reactivity of PA autoantibodies with CAM variant GPIIb-IIIa. Results are expressed as ΔOD: [OD value obtained from a test sample − (mean + 3 SD) OD value obtained from 5 control samples]. Open columns represent ΔOD value in antigen-capture ELISA using WT GPIIb-IIIa, and filled columns represent ΔOD value in antigen-capture ELISA using CAM variant.
Reactivity of PA autoantibodies with CAM variant GPIIb-IIIa.
(A) Reactivity of mAbs (AP3 and OP-G2) and antiplatelet allo-antibodies (anti–HPA-1a and anti–HPA-3a) with CAM variant GPIIb-IIIa. (B) Reactivity of PA autoantibodies with CAM variant GPIIb-IIIa. Results are expressed as ΔOD: [OD value obtained from a test sample − (mean + 3 SD) OD value obtained from 5 control samples]. Open columns represent ΔOD value in antigen-capture ELISA using WT GPIIb-IIIa, and filled columns represent ΔOD value in antigen-capture ELISA using CAM variant.
We also examined whether there might be any difference in platelet counts between the 11 patients with impaired binding to KO GPIIb-IIIa and the other 23 patients. However, any significant difference in platelet counts was not observed (40.9 ± 27.5 × 103/μL vs 48.8 ± 22.8 × 103/μL; P > .05, Mann-Whitney U test).
Effects of GPIIb-IIIa antagonists or OP-G2 on the binding of PA autoantibodies to GPIIb-IIIa
To further characterize the location of autoantigenic epitopes on GPIIb-IIIa, we examined the effects of small GPIIb-IIIa antagonists or OP-G2 on the binding of autoantibodies to platelet GPIIb-IIIa. As shown in Figure 5A, 1 mM RGDW, 10 μM FK506, 10 μM Ro44-5883, or 10 μg/mL OP-G2 completely inhibited the binding of biotinylated OP-G2 to GPIIb-IIIa. None of these small antagonists inhibited the binding of PA autoantibodies, whereas OP-G2 did markedly inhibit their binding in patients with impaired binding to KO GPIIb-IIIa (Figure 5B). These results indicate that the epitopes for PAIgG autoantibodies are not localized at the ligand-binding site itself but close to it.
Effects of GPIIb-IIIa antagonists and nonlabeled OP-G2 on the binding of mAbs and PA autoantibodies against GPIIb-IIIa.
(A) Effects on the binding of biotinylated mAbs AP3 and OP-G2. (B) Effects on the binding of PA autoantibodies from 6 patients with ITP. PA autoantibodies from patients 1, 3, and 8 showed the impaired binding to KO variant GPIIb-IIIa, whereas those from patients 2, 7, and 12 equally reacted with KO variant and WT GPIIb-IIIa. The binding of mAbs or PA autoantibodies in the presence of 1 mM RGDW (sparsely dotted columns), 10 μM FK506 (densely dotted columns), 10 μM Ro44-5883 (checkered columns), or 10 μg/mL nonlabeled OPG2 (solid columns) were examined in antigen-capture ELISA. Open columns represent ΔOD values in the absence of the inhibitors. ΔOD is [OD value obtained from a test sample − (mean + 3 SD) OD value obtained from 5 control samples].
Effects of GPIIb-IIIa antagonists and nonlabeled OP-G2 on the binding of mAbs and PA autoantibodies against GPIIb-IIIa.
(A) Effects on the binding of biotinylated mAbs AP3 and OP-G2. (B) Effects on the binding of PA autoantibodies from 6 patients with ITP. PA autoantibodies from patients 1, 3, and 8 showed the impaired binding to KO variant GPIIb-IIIa, whereas those from patients 2, 7, and 12 equally reacted with KO variant and WT GPIIb-IIIa. The binding of mAbs or PA autoantibodies in the presence of 1 mM RGDW (sparsely dotted columns), 10 μM FK506 (densely dotted columns), 10 μM Ro44-5883 (checkered columns), or 10 μg/mL nonlabeled OPG2 (solid columns) were examined in antigen-capture ELISA. Open columns represent ΔOD values in the absence of the inhibitors. ΔOD is [OD value obtained from a test sample − (mean + 3 SD) OD value obtained from 5 control samples].
Reactivity of serum autoantibodies against KO mutant
We then examined the reactivity of serum antibodies in ITP patients whose PA autoantibodies showed marked reduction in the reactivity against KO GPIIb-IIIa. Antigen-capture ELISA is not sensitive enough to detect serum autoantibodies against GPIIb-IIIa because of high background4,40; therefore, serum samples from only 2 patients (patients 3 and 8) were available for this analysis. Serum antibodies were affinity purified with platelets and eluted by diethyl ether. In contrast to the PA antibodies, serum antibodies equally reacted with KO variant and WT GPIIb-IIIa, and OP-G2 did not inhibit their binding to GPIIb-IIIa (Figure6). These results confirm previous findings that the GPIIb-IIIa autoantigenic target for serum antibodies may be different from that for PA autoantibodies.16 41
Reactivity and binding of serum autoantibodies to GPIIb-IIIa.
(A) Reactivity of serum autoantibodies from patients 3 and 8 with KO variant GPIIb-IIIa. (B) Effects of 10 μg/mL nonlabeled OP-G2 on the binding of the serum autoantibodies to WT GPIIb-IIIa. Serum antibodies from patients 3 and 8, whose PA autoantibodies showed the impaired binding to KO variant GPIIb-IIIa, were affinity purified using platelets. Open columns represent ΔOD value in antigen-capture ELISA using WT GPIIb-IIIa. Solid columns represent ΔOD value in antigen-capture ELISA using KO variant GPIIb-IIIa. Hatched columns are ΔOD value in antigen-capture ELISA using WT GPIIb-IIIa in the presence of 10 μg/mL nonlabeled OP-G2.
Reactivity and binding of serum autoantibodies to GPIIb-IIIa.
(A) Reactivity of serum autoantibodies from patients 3 and 8 with KO variant GPIIb-IIIa. (B) Effects of 10 μg/mL nonlabeled OP-G2 on the binding of the serum autoantibodies to WT GPIIb-IIIa. Serum antibodies from patients 3 and 8, whose PA autoantibodies showed the impaired binding to KO variant GPIIb-IIIa, were affinity purified using platelets. Open columns represent ΔOD value in antigen-capture ELISA using WT GPIIb-IIIa. Solid columns represent ΔOD value in antigen-capture ELISA using KO variant GPIIb-IIIa. Hatched columns are ΔOD value in antigen-capture ELISA using WT GPIIb-IIIa in the presence of 10 μg/mL nonlabeled OP-G2.
Inhibitory effect of PA autoantibodies against fibrinogen binding
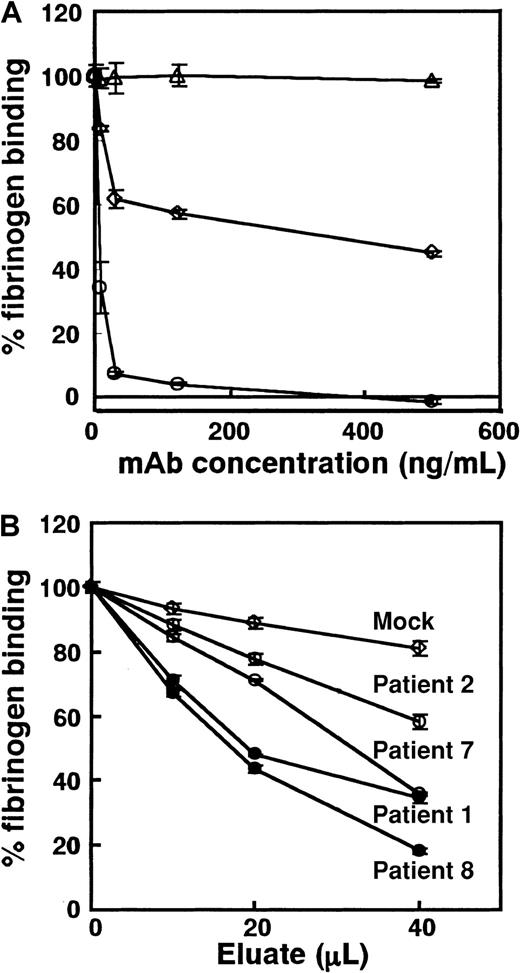
We examined whether PA autoantibodies might inhibit ligand binding. Because eluates contain only small amounts of antibodies, conventional fibrinogen binding assay using washed platelets is not suitable for this purpose. To overcome this problem, we developed sensitive ELISA using mutant GPIIb-IIIa (GPIIb-IIIaT562N), a constitutively activated form of the receptor that can bind to its ligand without any activating agent (Figure 1). TP80 was used as a capturing mAb because it did not inhibit fibrinogen binding to GPIIb-IIIaT562N (data not shown). Figure7A shows inhibitory effects of mAbs on fibrinogen binding to GPIIb-IIIaT562N in this ELISA. OP-G2 at a concentration of 125 ng/mL completely inhibited fibrinogen-binding, and IC50 of OP-G2 was approximately 3.4 ng/mL. AP2 inhibited approximately 55% of the fibrinogen binding at a saturating concentration, which is compatible with the data reported previously.31 IC50 values for FK633 and Ro44-9883 were 0.36 nM and 0.06 nM, respectively, whereas cyclo RGDfV specific for αvβ3 even at 20 nM did not inhibit the fibrinogen binding (data not shown). Compared with IC50 obtained by conventional methods (39.3 nM for FK633, 4.4 nM for Ro44-988335),35 our ELISA is approximately 100 times more sensitive. Using this system, we examined the effects of eluates on fibrinogen binding. PA autoantibodies from patients 1, 2, 7, and 8 equally bound to GPIIb-IIIaT562N and WT GPIIb-IIIa (data not shown). As shown in Figure 7B, all eluates examined inhibited fibrinogen binding to GPIIb-IIIa dose dependently. Although ΔOD values in antigen-capture ELISA using WT for the tested eluates were similar (0.900-1.100), PA autoantibodies showing the impaired binding to KO GPIIb-IIIa more strongly inhibited fibrinogen binding than those showing the same reactivity with KO and WT GPIIb-IIIa.
Inhibitory effects of mAbs and PA autoantibodies from patients with chronic ITP on fibrinogen-binding to activated GPIIb-IIIa (GPIIb-IIIaT562N).
Biotinylated fibrinogen was added to each well containing immobilized GPIIb-IIIaT562N in the presence of various concentrations of (A) mAbs or (B) PA autoantibodies. Bound fibrinogen was measured with ELISA, and percentage fibrinogen binding was calculated as described in “Patients, materials, and methods.” (A) Open circles, OP-G2; open squares, AP2; and open triangles, MOPC21 (control IgG1). Horizontal axis represents final concentration of mAbs incubated with fibrinogen. (B) Closed circles, eluates from patients 1 and 8 showing the impaired binding to KO variant GPIIb-IIIa; open circles, eluates from patients 2 and 7 that reacted equally with KO variant and WT GPIIb-IIIa; and open squares, mean data of 3 mock control eluates. Horizontal axis represents the amount of eluate added. Experiments were run in triplicate, and data are expressed as mean ± SD of the results.
Inhibitory effects of mAbs and PA autoantibodies from patients with chronic ITP on fibrinogen-binding to activated GPIIb-IIIa (GPIIb-IIIaT562N).
Biotinylated fibrinogen was added to each well containing immobilized GPIIb-IIIaT562N in the presence of various concentrations of (A) mAbs or (B) PA autoantibodies. Bound fibrinogen was measured with ELISA, and percentage fibrinogen binding was calculated as described in “Patients, materials, and methods.” (A) Open circles, OP-G2; open squares, AP2; and open triangles, MOPC21 (control IgG1). Horizontal axis represents final concentration of mAbs incubated with fibrinogen. (B) Closed circles, eluates from patients 1 and 8 showing the impaired binding to KO variant GPIIb-IIIa; open circles, eluates from patients 2 and 7 that reacted equally with KO variant and WT GPIIb-IIIa; and open squares, mean data of 3 mock control eluates. Horizontal axis represents the amount of eluate added. Experiments were run in triplicate, and data are expressed as mean ± SD of the results.
Discussion
In this study, we have demonstrated that in one third of patients with chronic ITP who had PA anti–GPIIb-IIIa autoantibodies (11 of 34 patients), the reactivity of autoantibodies with KO variant GPIIb-IIIa was markedly impaired (less than 50%). OP-G2, but not small GPIIb-IIIa antagonists, markedly inhibited their binding to GPIIb-IIIa only in patients with impaired binding to KO GPIIb-IIIa, and the degree of the inhibition by OP-G2 was almost the same as that observed in KO GPIIb-IIIa. In addition, we developed a new sensitive ELISA to examine fibrinogen binding to the activated GPIIb-IIIa and demonstrated that autoantibodies showing the impaired binding to KO GPIIb-IIIa are more potent inhibitors of fibrinogen binding. In sharp contrast, none of autoantibodies showed impaired binding to CAM variant GPIIb-IIIa. Our findings strongly suggest that their major epitopes locate close to the ligand-binding site in GPIIb, but not in GPIIIa, in one third of patients with chronic ITP.
Localization of epitopes for PA anti–GPIIb-IIIa autoantibodies in chronic ITP remains obscure. Varon and Karpatkin12 first demonstrated impaired reactivity of anti-GPIIb mAb (3B2) with ITP platelets, probably because of the presence of PA autoantibodies, which suggested that the autoantigens may locate close to the 3B2-binding site. Recent studies suggested that ITP autoantibodies mostly recognize the tertiary structure of intact GPIIb-IIIa,15-18 and flexibility of its conformation make it difficult to further localize autoantigens. To overcome these difficulties, we used recombinant GPIIb-IIIa with a mutation in the ligand-binding site in either GPIIb or GPIIIa without any major conformational change. With regard to ligand-binding sites, multiple sites have been identified in N-terminal regions of both α (GPIIb) and β (GPIIIa) subunits.42In several integrin α subunits such as α2 and αL, an inserted domain critically involved in ligand binding is present in the N-terminal region.43,44 Although αIIb does not have the I domain, β3 has an I-like domain that contains an invariant DXSXS sequence (X represents any amino acid).45,46 Molecular analysis of CAM variant GT first revealed the importance of D119, which is the first residue of the DXSXS sequence, and this region of β3 appears to be directly involved in ligand binding.24 With regard to non-I domain integrin α subunits including αIIb(GPIIb), Springer47,48 recently proposed that the 7 N-terminal sequence repeats (W1-W7) are folded into a β-propeller domain in which W3 4-1 loop and W3 2-3 loop are probably involved in ligand binding.47,48 Molecular analysis of KO variant GT first revealed the importance of D163 within the loop between W2 and W3 (W3 4-1 loop).25 These data support the concept that both GPIIb and GPIIIa constitute a ligand-binding pocket. Indeed, the binding of ligand-mimetic antibodies, OP-G2 and PAC-1, was completely abolished in KO and CAM GPIIb-IIIa.24,25,49 In contrast, Puzon-McLaughlin et al49 recently demonstrated that several nonligand-mimetic antibodies recognized a single epitope in either subunit, even if they recognized the tertiary structure of intact GPIIb-IIIa. The binding of a number of PA autoantibodies was only impaired to KO GPIIb-IIIa, but not to CAM GPIIb-IIIa. OP-G2 inhibited the binding of the PA autoantibodies, whereas small GPIIb-IIIa antagonists did not. Thus, these autoantigens were not localized at the ligand-binding site but were localized close to it in GPIIb—that is, the W3 4-1 loop and its surrounding regions in GPIIb may directly or indirectly participate in the formation of autoantigens. Although platelet eluates were not affinity purified for GPIIb-IIIa in this study, it is unlikely that contaminated autoantibodies directed against antigens other than GPIIb-IIIa interfered with the binding of anti–GPIIb-IIIa autoantibodies because we used GPIIb-IIIa purified by the immobilized anti–GPIIb-IIIa mAb and because only the binding to KO GPIIb-IIIa was impaired in our assay. KO and CAM GPIIb-IIIa similarly showed aberrant expression of some ligand-induced binding sites (LIBS), such as the PMI-1 epitope, probably because of a disturbance of divalent cation-binding to GPIIb-IIIa by these mutations.24,25 However, it is unlikely that PA autoantibodies recognized the epitopes, which are located far from ligand-binding sites and are disturbed by the divalent cation-binding defect, because their binding was inhibited by OP-G2 and only impaired to KO GPIIb-IIIa. Recently, using αIIb-αVβ3 chimeras, McMillan et al50 suggested that 8 of 14 PA anti–GPIIb-IIIa autoantibodies bound to epitopes existing between F223 and Q459 in GPIIb, which correspond to W4-W7 in the β-propeller model. Because W3 and W4-W7 repeats locate close to each other in the predicted tertiary structure of the β-propeller, it would be possible that the swapping of αIIb for αV might affect the conformation of W3 4-1 loop and its surrounding regions. Alternatively, they defined epitopes different from ours. Further studies are needed to resolve this apparent discrepancy.
Our sensitive ELISA showed that PA autoantibodies inhibited the fibrinogen binding irrespective of epitope location, though autoantibodies showing impaired binding to KO GPIIb-IIIa were more potent inhibitors for fibrinogen binding. Our data suggest that PA anti–GPIIb-IIIa autoantibodies mostly recognize epitopes localized in N-terminal regions of intact GPIIb-IIIa even in the remaining two thirds of patients with chronic ITP whose PA autoantibodies equally react with KO and WT GPIIb-IIIa. Using platelet aggregation studies, platelet functional defects such as aspirinlike or storage pool disease–like defects have been reported in some patients with chronic ITP.51,52 On the other hand, GT-like functional defects caused by anti–GPIIb-IIIa autoantibodies without thrombocytopenia have been reported in some patients with bleeding tendency.53-58 Although analysis of platelet function in chronic ITP has been limited by concomitant thrombocytopenia, our sensitive ELISA suggested that in most patients, PA anti–GPIIb-IIIa autoantibodies have the potential to inhibit fibrinogen binding and may contribute to functional defects in this disorder. In addition, it has recently been suggested that antibodies directed against conformational epitopes on GPIIb-IIIa are more pathogenic than other platelet antibodies and act through an Fc-dependent mechanism in an animal model.59 Because most PA anti–GPIIb-IIIa antibodies examined in this study were directed against conformational epitopes, it is possible that these antibodies may be potent in platelet destruction through an Fc-dependent mechanism. On the other hand, epitopes for serum anti–GPIIb-IIIa autoantibodies have not been localized in the N-terminal region of GPIIb-IIIa (the cytoplasmic domain of GPIIIa, a 50-kd chymotryptic fragment of GPIIIa containing the cysteine-rich repeat, and a C-terminal 65-kd chymotryptic fragment of GPIIb heavy chain).9-11 Previous studies suggested that PA anti–GPIIb-IIIa autoantibodies may differ in specificity from serum antibodies even in the same patient.16 41 We clearly demonstrated that affinity-purified serum antibodies from patients whose PA antibodies showed the impaired binding to KO GPIIb-IIIa equally reacted with KO and WT GPIIb-IIIa. Our findings further confirm the difference in the specificity between PA and serum antibodies.
In this study, we have revealed important aspects of autoantigenic epitopes in GPIIb-IIIa in chronic ITP. In one third of patients with chronic ITP, PA anti–GPIIb-IIIa autoantibodies mostly recognize epitope(s) disturbed by the single amino acid substitution (D163A) and the 2–amino acid insertion in the W3 4-1 loop in GPIIb. Thus, the W3 4-1 loop and its surrounding regions in GPIIb may be one of the hot spots for autoantigenic epitopes. Our data also provide a new aspect regarding the effect of PA anti–GPIIb-IIIa autoantibodies on platelet function in chronic ITP. Further analysis of autoantigenic epitopes would provide new insight into the pathophysiology and the treatment of this disorder.
We thank Dr S. J. Shattil (Scripps Research Institute) for mAb PAC-1, Dr T. J. Kunicki (Scripps Research Institute) for mAb AP2, Dr P. J. Newman (Blood Center of Southeastern Wisconsin) for mAb AP3, Drs M. Handa and Y. Ikeda (Keio University) for mAb PT25-2, and Dr N. Nagao (Osaka Red Cross Blood Center) for anti–HPA-3a. We also thank N. Iwamoto for her skillful technical assistance.
Supported by grants from the Ministry of Education, Science and Culture, Tokyo; the Japan Society for the Promotion of Science, Tokyo; and the Welfide Medical Research Foundation, Osaka, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yoshiaki Tomiyama, Department of Internal Medicine and Molecular Science, Graduate School of Medicine B5, Osaka University, 2-2, Yamadaoka, Suita, Osaka 565-0871, Japan; e-mail:yoshi@hp-blood.med.osaka-u.ac.jp.

![Fig. 2. Reactivity of PA autoantibodies with KO variant GPIIb-IIIa. / (A) Reaction of mAbs (AP3 and OP-G2) and antiplatelet alloantibodies (anti–HPA-1a and anti–HPA-3a) with KO variant GPIIb-IIIa. (B) PA autoantibodies from 11 patients with chronic ITP, showing a marked decrease (greater than 50%) in the reactivity against KO variant compared with WT GPIIb-IIIa. Results are expressed as ΔOD: [OD value obtained from a test sample − (mean + 3 SD) OD value obtained from 5 control samples]. Open columns represent ΔOD value in antigen-capture ELISA using WT GPIIb-IIIa, and filled columns represent ΔOD value in antigen-capture ELISA using KO mutant. Data are representative of 2 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1819/7/m_h81811514002.jpeg?Expires=1769141531&Signature=MQwW7x-NbppUYbwfLEjlY7fqJi6Pmv9sXmLZVoj7r96FC4Vg-pLTF66N03NZcKnk5b5ztvPirDZxvIjueJ90vJI3CCQ9uMHNLN4-d70e2m3jkEFkwfHyzP3aCyaj23U5yA8ckjBlCua3CHZDeBNBIuYmuKi33JMfixcUvXOiY9MSfRDbZUpOWVfr12HOOOxkB4wzYaqUJfg3xgGKAuixmWjT5ry5UHXXDf2OiOko5DEzN2fCRPLWpLb87IcV6EkDkS5wh6QG3TcZ0S0eNUSM6YSW-8j9MenIS6dCroZi-HN7p3KtBAhcQY-UV4K~zKzRI15kNl4pLJRVLLyr-GpdMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Reactivity of PA autoantibodies with GPIIbD163A-IIIa. / Eluates from patients 1, 3, and 8, with impaired binding to KO variant GPIIb-IIIa, showed the impaired binding to GPIIbD163A-IIIa. Results are expressed as ΔOD: [OD value obtained from a test sample − (mean + 3 SD) OD value obtained from 5 control samples]. Open columns represent ΔOD value in antigen-capture ELISA using WT GPIIb-IIIa, and filled columns represent ΔOD value in antigen-capture ELISA using GPIIbD163A-IIIa.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1819/7/m_h81811514003.jpeg?Expires=1769141531&Signature=rFJ52HeXkvLEiRzAmdVi8OVOQLMI4w-B9W9KppXaVYeWmE0FuQSuG8kFdpenJ7XFkvM~1B650uOKb5A~4xCeB16Rc8Rb9Dv3epUDRciafhf2GqGxhP-cD27sBBCIAfEBt5u72b02EGuz2OtQvhGaSDxXmP6r8rv-AvVWqVOjoSRVSrJ34Dobhvnaz~YYP1DErupe-2CjKNSkc4ESRVh838~FJlbt3iyB1P1qnP07krEfW40AkKFnWdrLlsfHAbM3dSCOiMdzGZ0td7EQPCatYmRmUKjAO9ewW5pNR2ZI0AN7toVaB~yrm4cUo7~wwoDjCwUgRo1rpeaIpMi1S-Y0jA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Reactivity of PA autoantibodies with CAM variant GPIIb-IIIa. / (A) Reactivity of mAbs (AP3 and OP-G2) and antiplatelet allo-antibodies (anti–HPA-1a and anti–HPA-3a) with CAM variant GPIIb-IIIa. (B) Reactivity of PA autoantibodies with CAM variant GPIIb-IIIa. Results are expressed as ΔOD: [OD value obtained from a test sample − (mean + 3 SD) OD value obtained from 5 control samples]. Open columns represent ΔOD value in antigen-capture ELISA using WT GPIIb-IIIa, and filled columns represent ΔOD value in antigen-capture ELISA using CAM variant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1819/7/m_h81811514004.jpeg?Expires=1769141531&Signature=wKZ7WXuyGGD1-M42E06A2Tb4c8HjQ9Rnnt~v4yKODxLsfVwxv1jzYhkpMIQegMuHqsHZJDnHZFIHYDzc94r6W2Ska14t5PHBKruGZgr9SmG-yoqLlMxZCDRk58ASMwPfDthioNSlpLUrFyXIh9EF5alLHFhP5Ost0GvYsfkuKP352dwIhJo64T1gQGZVainQ~FdWbKwRu3nJvme0Vb6blyxJP-hRINhX2upLYEMBIH4xgG3Pi7TRpTJUF1wFp8NZCZVLGKxhUIe2Oemjo9sRghVVymZfLTzKRH2SnGI03MY0XUo2EuuGD-GMm5muh-Hrs30RvOW38SVV0FXW7ELzEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Effects of GPIIb-IIIa antagonists and nonlabeled OP-G2 on the binding of mAbs and PA autoantibodies against GPIIb-IIIa. / (A) Effects on the binding of biotinylated mAbs AP3 and OP-G2. (B) Effects on the binding of PA autoantibodies from 6 patients with ITP. PA autoantibodies from patients 1, 3, and 8 showed the impaired binding to KO variant GPIIb-IIIa, whereas those from patients 2, 7, and 12 equally reacted with KO variant and WT GPIIb-IIIa. The binding of mAbs or PA autoantibodies in the presence of 1 mM RGDW (sparsely dotted columns), 10 μM FK506 (densely dotted columns), 10 μM Ro44-5883 (checkered columns), or 10 μg/mL nonlabeled OPG2 (solid columns) were examined in antigen-capture ELISA. Open columns represent ΔOD values in the absence of the inhibitors. ΔOD is [OD value obtained from a test sample − (mean + 3 SD) OD value obtained from 5 control samples].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1819/7/m_h81811514005.jpeg?Expires=1769141531&Signature=R0B8aH1gEMeN5sgQ5tEUb7wcR88MzNSc2MsX~qCeIMwwb9wmBA4iWQQoFl~B7uW5FGIxxmcsotoTvIE7pINzXg1UwTq23Jdhzwjr6jkdsFIGf0rrcdpRQHLH0zSj4Qn-USoV8U-bRZdFITvWq79x-fF~0NTfPj26mcBIEcpzneLek6JBc9UK~SmMHHpDssXeKgw4NxQ48jKlHxJlFsoOGbg6jr2Qql4A8R4oNu-P0vSIFGN6R4XWC5I9AJhaGE4~VxhEp3YqO~l9KO3xC8cVHFQrlkzUPk~k5Q43sfpN5FlvalW5cCb6MvEY58QnoR0rIY0HTe7L8tJFXy7RRtlSyw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 2. Reactivity of PA autoantibodies with KO variant GPIIb-IIIa. / (A) Reaction of mAbs (AP3 and OP-G2) and antiplatelet alloantibodies (anti–HPA-1a and anti–HPA-3a) with KO variant GPIIb-IIIa. (B) PA autoantibodies from 11 patients with chronic ITP, showing a marked decrease (greater than 50%) in the reactivity against KO variant compared with WT GPIIb-IIIa. Results are expressed as ΔOD: [OD value obtained from a test sample − (mean + 3 SD) OD value obtained from 5 control samples]. Open columns represent ΔOD value in antigen-capture ELISA using WT GPIIb-IIIa, and filled columns represent ΔOD value in antigen-capture ELISA using KO mutant. Data are representative of 2 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1819/7/m_h81811514002.jpeg?Expires=1769141532&Signature=xJvh1KEKiY3VzMWvMzY3q4hL7Ab-GsyaHk78yypnABrMDzbk4CrEWwj5hMyfcu0Ta--4lNsuh4qN1nNMy5RLh7GYeXh2KKHqrtaIEE3jrrY11Fx-JitON6CsSSMK4wAVenIEhs3b7jU5bIZ0J5QpGjAv9yE13jWZVCj5wei5KyyVnGcZrAX8VYXoRNKpDf~PeH255p8R0ENJc17I8axRKLIE3bC~BkhkynS~ivoksK2IQoI4PZfPW9Noa5szLvpoaDFnmFHMFQ-EtoO5ZjCReCUkXjNDn3fBemiPZC-BZTBvvAcZB0FTYMeT4WrQ0HWCMeEKSdv3jsp5DI2GNILCXQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Reactivity of PA autoantibodies with GPIIbD163A-IIIa. / Eluates from patients 1, 3, and 8, with impaired binding to KO variant GPIIb-IIIa, showed the impaired binding to GPIIbD163A-IIIa. Results are expressed as ΔOD: [OD value obtained from a test sample − (mean + 3 SD) OD value obtained from 5 control samples]. Open columns represent ΔOD value in antigen-capture ELISA using WT GPIIb-IIIa, and filled columns represent ΔOD value in antigen-capture ELISA using GPIIbD163A-IIIa.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1819/7/m_h81811514003.jpeg?Expires=1769141532&Signature=MO8Ka9942Z4WSwSxlEFHtTeyk7L6VpLfhw4dEOp4xNVnONX2FlDXB6jcNXI68qjWzPTyGcgSKCXCtvTPtwnQUh2qcX3tQcqZ4NjM8M-gUkqnIk310DZt2hDdkKPZh-GLG0J7Xdeivzzlk~9j1TXz0uH0omsmNVv~extUyfO0vn63E0Qf5rxmcEfd3YS1X-77XMUSkV7OPE~1kITyG47A0gezwZ~Oo-j5krSbFgGhwi41jz4I4nBXscSFQUAHnxY-Z6ojzydrBx9MBHg5tQViB-DoiNA6Km6yYH0XPLneDkD4sFyeUxu4eifqVikDNfifFeg~Jpqf1SvCc1u5F1AuCQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Reactivity of PA autoantibodies with CAM variant GPIIb-IIIa. / (A) Reactivity of mAbs (AP3 and OP-G2) and antiplatelet allo-antibodies (anti–HPA-1a and anti–HPA-3a) with CAM variant GPIIb-IIIa. (B) Reactivity of PA autoantibodies with CAM variant GPIIb-IIIa. Results are expressed as ΔOD: [OD value obtained from a test sample − (mean + 3 SD) OD value obtained from 5 control samples]. Open columns represent ΔOD value in antigen-capture ELISA using WT GPIIb-IIIa, and filled columns represent ΔOD value in antigen-capture ELISA using CAM variant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1819/7/m_h81811514004.jpeg?Expires=1769141532&Signature=C~ZFuzz4ghZONeI30MGE5PHImmfQH8ObIoarwio4JaTpA3lFe6wub4y1FJ8WAq9nYYHL1GElezP~pEh965lFdrzO4ex~vSNtSN7ezy-O597MLdJKyWEMcog6Le-~cOMvZGpF1xHdG55gyZc3QbSTRkjgdGZ7tr3xGtcnD5SSKD5OT23Xrh5L2-YxuWKlh6wripvCZ1eIdEngKWt9LTne1tQV49MRYvNsO~Q1HMYwHO50WMcMjIhZm70TBfWyYenErgYVYI5pLej3hxH7-aHpQikJJBeicPmpPrY-s7aPV06GYIbRB4nQW7a7vHqKyUuN~2MIDooZGgZGqRq7aJPVVw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Effects of GPIIb-IIIa antagonists and nonlabeled OP-G2 on the binding of mAbs and PA autoantibodies against GPIIb-IIIa. / (A) Effects on the binding of biotinylated mAbs AP3 and OP-G2. (B) Effects on the binding of PA autoantibodies from 6 patients with ITP. PA autoantibodies from patients 1, 3, and 8 showed the impaired binding to KO variant GPIIb-IIIa, whereas those from patients 2, 7, and 12 equally reacted with KO variant and WT GPIIb-IIIa. The binding of mAbs or PA autoantibodies in the presence of 1 mM RGDW (sparsely dotted columns), 10 μM FK506 (densely dotted columns), 10 μM Ro44-5883 (checkered columns), or 10 μg/mL nonlabeled OPG2 (solid columns) were examined in antigen-capture ELISA. Open columns represent ΔOD values in the absence of the inhibitors. ΔOD is [OD value obtained from a test sample − (mean + 3 SD) OD value obtained from 5 control samples].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/6/10.1182_blood.v98.6.1819/7/m_h81811514005.jpeg?Expires=1769141532&Signature=W0T~I3LxTikJUa4zyNhY6L~xPgn2qkH-TembJD2r~gKh1N6AcJeFGHf8pDs9GWdADF~5I5qUot8jQZgOEkfGjTVhwWsN3XB4oAcRJr6MlokbDdhbCfjoOWcs8DePl9ZBPVEkZruTfj72A8f~yDqQqNU8x-TmtzJnsNAo18iEVNw7P98E1w~FjdGuIrivMPP4MfdfW31A4GMDCCSOlPzIx~FehXvHnnGc06WiCVI4ho1xCVwjTNkJ-y-LW7P9DZImeKzd5urDpyFAFUQ2DGc2BnLcr6CTXzgHIEWohU8G-Fu~XBc8PtvWhk07EXOSqi9LPDDodhRUWXvG-O2v4uaRbg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)