Abstract
To better understand the role of retinoids in myelopoiesis, expression of the retinoid receptor genes (retinoic acid receptors [RARs] and retinoid X receptors [RXRs]) were examined during differentiation of factor-dependent cell-Paterson (FDCP)–mixA4 murine progenitor cells. The major receptor expressed in undifferentiated A4 cells was RARα (primarily the RARα1 isoform). Following induction of myelomonocytic differentiation with granulocyte and granulocyte-macrophage colony-stimulating factors, a dramatic increase in RARα expression (particularly the RARα2 isoform) was seen. In contrast, expression of both RARα isoforms was rapidly extinguished upon induction of erythroid differentiation with erythropoeitin (EPO). A modest induction of RXRα expression was seen, particularly during differentiation in the myelomonocytic lineage. Low expression levels of RARγ2 and RXRβ remained unchanged, irrespective of differentiation pathway. Consistent with the gene expression patterns, RARα agonists and antagonists stimulated myelomonocytic and erythroid differentiation of FDCP-mixA4 cells, respectively. Taken together, these results suggest that erythropoiesis and granulopoiesis require diminished and enhanced RARα activities, respectively, which at physiological all-trans-retinoic acid (RA) concentrations may be accomplished by reciprocal effects of EPO and myelomonocytic growth factors on its expression. This hypothesis is corroborated by data showing that RA, which positively regulates RARα2 expression, can exert inhibitory effects on erythroid differentiation.
Introduction
Retinoids, such as all-trans-retinoic acid (RA), exert a wide range of effects on both normal and malignant hematopoietic cells.1-3 Their actions are mediated through binding to specific nuclear receptors (NRs) that regulate gene transcription.4 To date, 3 different retinoic acid receptor (RAR) and retinoid X (or rexinoid) receptor (RXR) genes have been characterized, each encoding multiple N-terminal protein isoforms.5 RXRs serve as obligatory heterodimerization partners for RARs and for a number of other NRs, including those for thyroid hormone (TR) and vitamin D3, thus integrating different signaling pathways.4,5 For RARs, expression of the RARα2 and β2 isoforms is under the control of highly conserved promoters, which are inducible by RA and possess identical retinoic acid response elements.6
Several lines of evidence support a role of RARα in regulating myeloid development, in particular along the granulocytic pathway. Acute promyelocytic leukemia (APL), which represents a block in granulocytic differentiation, is associated with different reciprocal chromosomal translocations, which consistently involveRARα.7 Resistance of myeloid leukemia cells to differentiating effects of RA is associated with dominant-negative mutations in the RARα gene.8,9 Finally, in analogy to a dominant-negative RARα mutant,10antagonists of RARα11 inhibit granulopoiesis, and RARα-specific agonists12 stimulate this process. Although a role of RA in erythropoiesis has not been as thoroughly investigated, recent studies indicate that inhibition of neutrophilic differentiation by overexpressing a dominant-negative RARα mutant in multipotent progenitor cells enhances their ability to execute erythroid differentiation programs.10 13 Taken together, these observations suggest that granulopoiesis and erythropoesis require activation and repression of RARα transcriptional activity, respectively. However, the mechanisms by which these reciprocal requirements for retinoid signaling could be maintained in the presence of invariant concentrations of RA in the bone marrow are not understood. In this study, we primarily addressed whether these mechanisms, at least in part, could involve opposing effects of myelomonocytic (granulocyte and granulocyte-macrophage colony-stimulating factors [G-CSF and GM-CSF]) and erythroid (erythropoietin [EPO]) growth factors (GFs) on RARαexpression.
Study design
Factor-dependent cell-Paterson (FDCP)–mixA4 cells were derived from long-term bone marrow cultures.14These cells are nonleukemic and karyotypically normal, are dependent on interleukin-3 for their survival and self-renewal, and can be induced to differentiate by either stromal layers14 or added exogenous GFs.15 Cultures and differentiation assays of FDCP-mixA4 cells were performed and assessed as described previously.15 Where indicated, erythroid and/or myelomonocytic differentiation was also carried out in the presence of 10−6 M RA (Sigma, St Louis, MO), 10−6 M RARα agonist (Ro40-6055), or 10−5 M RARα antagonist Ro41-5253 (gifts of Michael Klaus, F. Hoffmann–La Roche). Culture conditions for the RA-responsive (HL60 and NB4) and RA-resistant (NB4-R1, NB4-R2, and HL60-R) cells were previously described.16,17 Cells were harvested at specific time points following treatment with indicated agents (or their combinations), and total RNA was isolated following previously published protocols.18 Reverse transcriptase–polymerase chain reaction (RT-PCR) procedures, sequences of gene-specific primers and probes, as well as annealing temperatures, have previously been detailed.18
Results and discussion
Previous studies addressing expression of the retinoid receptor genes in a hematopoietic system have been restricted to leukemic cells and did not discriminate among distinct receptor isoforms.19,20 Using a semiquantitative RT-PCR procedure, we have now examined the RNA levels for RAR isoforms and RXRs in nonleukemic murine myeloid progenitors at distinct stages of their differentiation. For these experiments, undifferentiated FDCP-mixA4 cells (d0) were induced to differentiate with either conditioned medium containing G-CSF/GM-CSF or erythropoietin (EPO). In response to G-CSF/GM-CSF, these cells undergo myelomonocytic differentiation, with the majority of mature cells showing neutrophilic phenotype. In response to EPO, FDCPmix cell lines have the potential to develop into cells of erythroid lineage, including basophilic erythroblasts and more mature hemoglobinized cells. The extent of erythroid maturation of FDCPmix cells can vary from culture to culture and clone to clone, and there is a large spectrum of phenotypes with regard to the proportion of erythroblasts versus more mature hemoglobinized cells. The cells were scored for morphology and harvested at days 2, 4, and 8 after induction (indicated as d2, d4, and d8 in Figure1), and total RNAs were isolated for analysis. By the eighth day after induction of differentiation with G-CSF and GM-CSF or EPO, the majority of cells in culture displayed the appropriate differentiated phenotype of mature cells (Figure 1A). In addition to performing morphological evaluation, at different times after induction of differentiation, we examined the expression of neutrophil- and erythrocyte-specific markers, lysozyme, and GATA-1. As expected, both genes displayed reciprocal patterns of expression, with GATA-1 and lysozyme messenger RNA levels gradually increasing during differentiation of A4 cells to erythrocytes (Figure 1B, lanes 2-4) and neutrophils (Figure 1B, lanes 5-7), respectively. The accuracy of our RT-PCR analysis in reflecting relative expression levels for a given gene in different samples has previously been demonstrated by comparing RT-PCR and Northern blot results obtained for equivalent RNA samples.18 In this set of experiments, RNA derived from either murine P19 (Figure 1B-D, lanes 8-9) or human T2Cl13 (not shown) embryonal carcinoma cells before and after RA treatment served as a control for induction of RARα2 expression. In both P19 and T2Cl13 cell types, induction of RARα2 expression by RA has previously been documented by Northern analysis.18
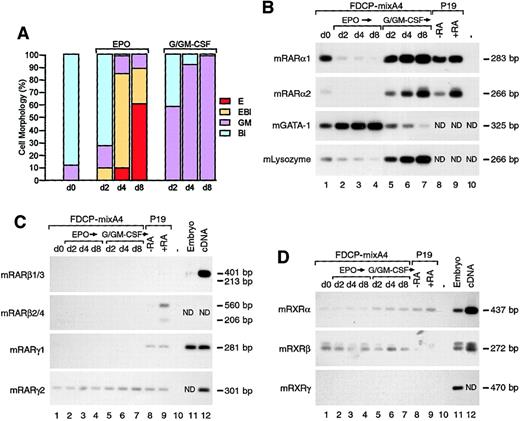
Down-regulation and up-regulation of RARα expression during erythroid and myelomonocytic differentiation.
(A) Morphological analyses of untreated FDCP-mixA4 cells (d0) and cells treated with EPO or conditioned medium containing G-CSF and GM-CSF for d2, d4, and d8, respectively. The percentage of undifferentiated blasts (Bl), erythroblasts (EBl), myelomonocytic cells of the granulocytic and monocytic lineage (GM) and mature erythrocytes (E) are as indicated. (B) Differential expression of the RARα isoforms during multilineage maturation of A4 cells was examined with RT-PCR analysis. Total RNA was derived from untreated A4 cells (d0) and cells treated for different numbers of days (as indicated) with either myelomonocytic GFs or EPO (the same cell populations as used for morphological evaluation in panel A). P19 embryonal carcinoma cells were treated with 10−6 M RA for 24 hours and used as positive controls for RARα2 induction. Changes in the levels of GATA-1 and lysozyme expression reflect the differentiation status of the cells at the level of lineage-specific gene expression. (C) Expression of RARβ and RARγ isoforms in undifferentiated FDCP-mixA4 and cells differentiated along myelomonocytic and erythroid lineages as in panel A. When indicated, total mouse embryo RNA and/or picogram quantities of complementary DNA (cDNA) were used as positive controls. (D) Expression of RXRα, β, and γ in undifferentiated and maturing A4 cells. Positive controls were as in panel C.
Down-regulation and up-regulation of RARα expression during erythroid and myelomonocytic differentiation.
(A) Morphological analyses of untreated FDCP-mixA4 cells (d0) and cells treated with EPO or conditioned medium containing G-CSF and GM-CSF for d2, d4, and d8, respectively. The percentage of undifferentiated blasts (Bl), erythroblasts (EBl), myelomonocytic cells of the granulocytic and monocytic lineage (GM) and mature erythrocytes (E) are as indicated. (B) Differential expression of the RARα isoforms during multilineage maturation of A4 cells was examined with RT-PCR analysis. Total RNA was derived from untreated A4 cells (d0) and cells treated for different numbers of days (as indicated) with either myelomonocytic GFs or EPO (the same cell populations as used for morphological evaluation in panel A). P19 embryonal carcinoma cells were treated with 10−6 M RA for 24 hours and used as positive controls for RARα2 induction. Changes in the levels of GATA-1 and lysozyme expression reflect the differentiation status of the cells at the level of lineage-specific gene expression. (C) Expression of RARβ and RARγ isoforms in undifferentiated FDCP-mixA4 and cells differentiated along myelomonocytic and erythroid lineages as in panel A. When indicated, total mouse embryo RNA and/or picogram quantities of complementary DNA (cDNA) were used as positive controls. (D) Expression of RXRα, β, and γ in undifferentiated and maturing A4 cells. Positive controls were as in panel C.
In undifferentiated A4 cells, RARα1 and, to a smaller extent, RARα2 were expressed (Figure 1B, lane 1). The low level of RARα2 expression, which is under the control of RA-inducible promoter, could reflect physiological levels of RA that are present in the serum supplementing the culture media and/or low frequency of spontaneous differentiation in the myelomonocytic lineage (Figure 1B, d0). Without addition of any retinoid, when A4 cells were induced with myelomonocytic GFs, there was a dramatic increase in RARα2 expression (Figure 1B; compare lane 1 with lanes 5-7). Some increase in expression was also observed for the RARα1 isoform (Figure 1B; compare lane 1 with lanes 5-7). These results were consistent with the fact that granulopoiesis is stimulated by RA and inhibited by RARα-specific antagonist or a dominant-negative mutant.10-12 As expected from the above data, myelomonocytic differentiation of A4 cells was also stimulated by RA and RARα agonist (Ro40-6055) (Figure2B, columns 7-8). In addition toRARα, only the RARγ (the RARγ2 isoform) andRXRα and β genes were expressed in FDCP-mixA4 cells (Figure 1C-D, respectively), albeit at considerably lower levels. Expression of RARγ2 (Figure 1C) and RXRβ (Figure 1D) was not influenced by the developmental status of the cells, and expression of RXRα displayed relatively mild increase (relative to d0) during myelomonocytic, but not erythroid, differentiation (Figure 1D).
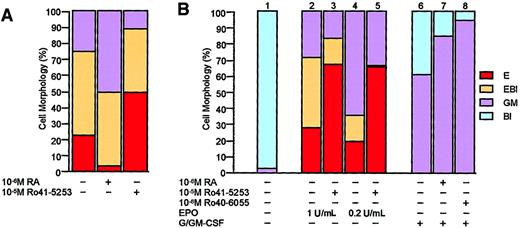
Effect of RA and RARα antagonist on EPO-induced differentiation of FDCPmix.
RA inhibits and RARα antagonist stimulates EPO-induced differentiation of FDCP-mixA4 cells in vitro. (A) The RARα antagonist Ro41-5253 was previously described.38 FDCP-mixA4 cells were induced to differentiate in the presence or absence of indicated retinoids, and after 5 days in culture, cells were scored for morphology as in Figure 1A. (B) Effects of retinoids on differentiation of FDCP-mixA4 cells, which were derived from a different passage than those that were used to obtain the results shown in panel A. Cells treated with indicated GF conditions and retinoids were scored for morphology after 5 days in culture. Note that the effects of RARα antagonist on erythroid differentiation of these 2 different passages of FDCP-mixA4 cells are comparable. RARα-specific agonist (Ro40-6055), which as RA stimulates myelomonocytic differentiation of FDCP-mixA4 cells, has previously been described.38
Effect of RA and RARα antagonist on EPO-induced differentiation of FDCPmix.
RA inhibits and RARα antagonist stimulates EPO-induced differentiation of FDCP-mixA4 cells in vitro. (A) The RARα antagonist Ro41-5253 was previously described.38 FDCP-mixA4 cells were induced to differentiate in the presence or absence of indicated retinoids, and after 5 days in culture, cells were scored for morphology as in Figure 1A. (B) Effects of retinoids on differentiation of FDCP-mixA4 cells, which were derived from a different passage than those that were used to obtain the results shown in panel A. Cells treated with indicated GF conditions and retinoids were scored for morphology after 5 days in culture. Note that the effects of RARα antagonist on erythroid differentiation of these 2 different passages of FDCP-mixA4 cells are comparable. RARα-specific agonist (Ro40-6055), which as RA stimulates myelomonocytic differentiation of FDCP-mixA4 cells, has previously been described.38
In sharp contrast to myelomonocytic differentiation, induction of erythroid differentiation with EPO was associated with rapid and complete down-regulation of the RARα gene expression (both RARα1 and α2 isoforms) (Figure 1B; compare lane 1 with lanes 2-4). Similarly to myelomonocytic GFs, EPO had no significant effects on the low levels of expression of RARγ2 and RXRβ (Figure 1C-D). Since down-regulation of RARα expression preceded appearance of differentiated cells in culture, it is not likely that the observed changes in its expression levels reflect general down-regulation of gene expression during erythropoiesis (note absence of any benzidine-positive cells at d2 in Figure 1A). Furthermore, expression levels for a number of other genes, such as transcription factor PU.121 and myeloperoxidase (data not shown), remain unchanged (relative to d0) at d2 and d4 of erythroid differentiation. In line with RARα expression pattern, RA appeared to inhibit EPO-induced differentiation of FDCP-mixA4 cells (Figure 2A), and RARα-specific antagonist (Ro41-5253) stimulated it, regardless of whether optimal or suboptimal concentration of EPO was used (Figure 2A-B, columns 2-5). It is worth noting that although very low levels of spontaneously differentiated cells could be observed in the absence of myelomonocytic GFs, no erythroid cells could be detected without EPO (Figure 2B, column 1). Additionally, without EPO, neither RA nor Ro41-5253 had any effects on cellular morphology (data not shown).
Given that RARα2 expression is directly regulated by RA through a conserved RA response element,6,22 we have investigated whether the opposing effects of RA on myelomonocytic and erythroid differentiation are associated with up-regulation of RARα2 expression levels. Given that changes at the level of gene expression precede overt lineage-specific differentiation,21 we have examined effects of RA on expression of RARα2 isoforms in FDCP-mixA4 cells grown for 2 days (d2) in the absence and presence of myelomonocytic GFs or EPO. On their own, RA, recombinant G-CSF, or recombinant GM-CSF had little stimulatory effect on RARα2 expression (Figure3A; compare lane 7 with lanes 1, 2, and 6). No further induction of RARα2 expression was seen when both G-CSF and GM-CSF were present in the culture medium (Figure 3A, lane 11, and data not shown). However, RA treatment of FDCP-mixA4 cells with either G-CSF or GM-CSF clearly stimulated RARα2 expression (Figure 3A, lanes 3-4), and maximal effect was observed with RA and both G-CSF and GM-CSF together (either recombinant GFs, lane 5, or conditioned medium, lane 12). Under the same RT-PCR conditions, expression of RA-regulated RARβ2 isoform was not detected in FDCP-mixA4 cells cultured with RA or with RA and myelomonocytic GFs (data not shown). Furthermore, RA was able, at least partially, to reverse the negative effects of EPO on expression of RARα2 (Figure 3A; compare lanes 9-10). The effects of RA on RARα2 expression also precede any morphologically identifiable cellular differentiation; at day 2 of the cultures used for RNA isolation, the majority of cells remain as undifferentiated blasts (see Figure 3B). In this respect, it is noteworthy that up-regulation of RARα2 expression by G-CSF/GM-CSF at day 2 of differentiation was considerably higher in an earlier experiment (Figure 1B) in which up to 60% of mature cells were already present after 2 days in culture with myelomonocytic GFs (Figure 1A). These experimental variations in RARα2 expression at earlier stages (d2) of myelomonocytic differentiation disappear later (at day 5 for example; data not shown) and are likely to be due to timing differences for differentiation induction of distinct passages of FDCP-mixA4 cells, which are clearly revealed in morphological analyses (compare Figure 1A and 3B).
Effect of RA on RARα2 during myelomonocytic differentiation and on the expression of RARα2 by EPO.
RA potentiates induction of RARα2 during myelomonocytic differentiation and reverses inhibition of its expression by EPO. (A) Semiquantitative RT-PCR analysis of RARα1 and α2 expression in FDCP-mixA4 cells after a 2-day treatment with the indicated GFs and/or retinoids. The recombinant GFs were used at 1000 U/mL (G-CSF) and 50 U/mL (GM-CSF). Concentration of RA was 10−6 M. G-CSF/GM-CSF indicates standard FDCP-mixA4 myelomonocytic differentiation with the use of conditioned medium.15 39Erythroid differentiation was induced by EPO (1000 U/L [1 U/mL]) and resulted in a decrease of RARα1 and RARα2 expression (lane 9) versus untreated control (lane 8). These data are consistent with the results shown in Figure 1B, which were derived from a different passage of FDCP-mixA4 cells. Addition of RA partially reversed this effect (lane 10). Glyceraldehyde phosphate dehydrogenase (GAPDH) levels were used as a control. (B) Frequency of hematopoietic cells representing different lineages and maturation stages in cultures from which RNA was isolated and used in the above RT-PCR analysis. Note that at day 2 the majority of cells in each culture retain the blast cell–line morphology characteristic of undifferentiated FDCP-mixA4 cells. Therefore, RA-mediated enhancement of the RARα2 expression precedes any morphological changes.
Effect of RA on RARα2 during myelomonocytic differentiation and on the expression of RARα2 by EPO.
RA potentiates induction of RARα2 during myelomonocytic differentiation and reverses inhibition of its expression by EPO. (A) Semiquantitative RT-PCR analysis of RARα1 and α2 expression in FDCP-mixA4 cells after a 2-day treatment with the indicated GFs and/or retinoids. The recombinant GFs were used at 1000 U/mL (G-CSF) and 50 U/mL (GM-CSF). Concentration of RA was 10−6 M. G-CSF/GM-CSF indicates standard FDCP-mixA4 myelomonocytic differentiation with the use of conditioned medium.15 39Erythroid differentiation was induced by EPO (1000 U/L [1 U/mL]) and resulted in a decrease of RARα1 and RARα2 expression (lane 9) versus untreated control (lane 8). These data are consistent with the results shown in Figure 1B, which were derived from a different passage of FDCP-mixA4 cells. Addition of RA partially reversed this effect (lane 10). Glyceraldehyde phosphate dehydrogenase (GAPDH) levels were used as a control. (B) Frequency of hematopoietic cells representing different lineages and maturation stages in cultures from which RNA was isolated and used in the above RT-PCR analysis. Note that at day 2 the majority of cells in each culture retain the blast cell–line morphology characteristic of undifferentiated FDCP-mixA4 cells. Therefore, RA-mediated enhancement of the RARα2 expression precedes any morphological changes.
Up-regulation of RARα expression during myelomonocytic differentiation is interesting in light of findings demonstrating degradation of RARα following its activation by RA.23 Up-regulation of RARα transcription may be critical for maintenance of RA signaling, which is required for optimal granulopoiesis, and loss of the ability to up-regulateRARα expression could potentially impair granulopoiesis. In this respect, it is worth noting that the level of RARα2 induction correlated with the degree of differentiation achieved in NB4, or HL60, cells after treatment with RA alone (Figure4A) or with RA and superinducers of differentiation,24 such as hexamethylene bisacetamide (not shown). Since RARα2 was not up-regulated in APL cell lines resistant to RA-induced differentiation, expression of this isoform may serve as an accurate indicator of the overall integrity of the RA-signaling pathway. Whether the inability to induce RARα2 expression has any functional role in RA resistance of APL cells remains to be determined. Nevertheless, it is noteworthy that induced expression levels of RARα2 appeared lower in myeloid leukemia cells (HL60, NB4, and KG-1) than in nonleukemic progenitors (Figures 4A and data not shown), suggesting that deregulation of RA signaling may have a more general role in myeloid malignancies rather than just a role in APL. This hypothesis is corroborated by recently published data showing apparent down-regulation of RA signaling in a number of primary leukemic cell samples, which were derived from patients with various types of acute myelogenous leukemia.25 The above theme would be reminiscent of the loss of expression and RA-inducibility of RARβ2 (controlled by a promoter highly related to that of RARα2) in a number of solid tumors.26 27
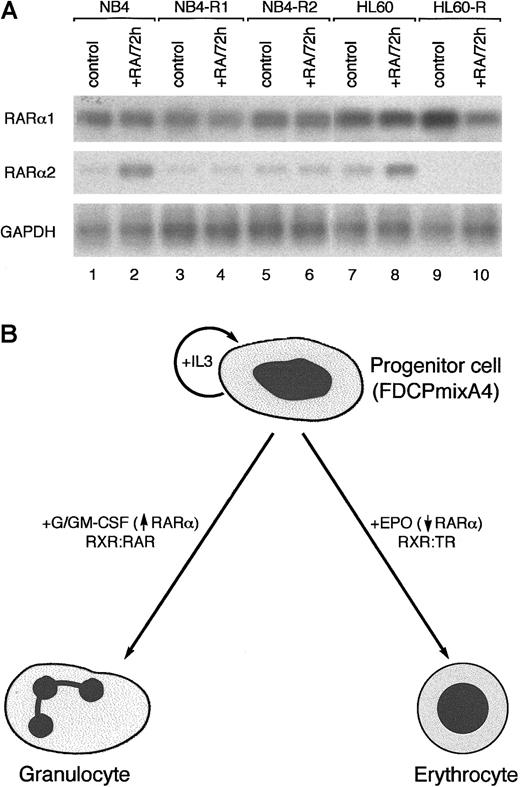
Induction of RARα2 by RA in RA-responsive, but not RA-resistant, leukemic cell lines.
(A) RT-PCR analysis of RARα1 and α2 expression in NB4 and HL60 cells that are sensitive (lanes 1-2 and 7-8) and resistant (lanes 3-6 and 9-10) to differentiation induction with RA. (B) Schematic diagram illustrating differential and lineage-specific effects of hematopoietic GFs on RARα expression. Given limiting RXR levels and the role of RXR as an obligatory heterodimerization partner for granulopoiesis- and erythropoisis-associated RAR and TR, respectively, the effects of hematopietic GFs on progenitor cell differentiation may, at least in part, be mediated by facilitating the formation of appropriate NR heterodimers.
Induction of RARα2 by RA in RA-responsive, but not RA-resistant, leukemic cell lines.
(A) RT-PCR analysis of RARα1 and α2 expression in NB4 and HL60 cells that are sensitive (lanes 1-2 and 7-8) and resistant (lanes 3-6 and 9-10) to differentiation induction with RA. (B) Schematic diagram illustrating differential and lineage-specific effects of hematopoietic GFs on RARα expression. Given limiting RXR levels and the role of RXR as an obligatory heterodimerization partner for granulopoiesis- and erythropoisis-associated RAR and TR, respectively, the effects of hematopietic GFs on progenitor cell differentiation may, at least in part, be mediated by facilitating the formation of appropriate NR heterodimers.
The results described above, which suggest a role for RARα in granulopoiesis, are consistent with the findings that disruption of theRARα gene in the mouse leads to abnormal granulocytic differentiation.28 Despite the low levels of RARγ2 expression in the FDCP-mixA4 cells, deletion of the RARγgene has no apparent effect on hematopoiesis.28Nevertheless, at present, one cannot exclude that under some specific physiological conditions, RARγ2 may prove to be required for proper hematopoietic differentiation to occur. The effects of theRARα knock-out on commitment and differentiation along the erythroid lineage also remain to be thoroughly addressed. The absence of any phenotype resulting from disruption of just the RARα1 isoform,29 in contrast to testes degeneration and high postnatal lethality in mice lacking expression of the entireRARα gene,30 suggests that the RARα2 isoform may also be more important in the hematopoietic processes. The RARα1 and α2 isoforms differ in their N-terminal A-region sequences, which have been shown to possess transcription-activating function (AF-1), which is both promoter and cell-context specific.31-33 Given that AF-1 can be positively regulated by phosphorylation,34-36 it is tempting to speculate that up-regulation of RARα2 expression during myelomonocytic differentiation results from phosphorylation of RARα1 (the predominant receptor expressed in undifferentiated cells) and/or α2 AF-1 in response to activation of cytokine receptor signaling. The mechanism by which both myelomonocytic GFs and EPO regulate theRARα gene expression is currently under investigation.
Down-regulation of RARα expression during erythroid differentiation may be required to allow RXR, present in limiting concentrations, to interact with other NRs, such as TR, that require its association to function and play important roles in erythropoiesis.37 On the basis of the above results, we suggest that erythropoiesis and granulopoiesis require diminished and enhanced RARα activities, respectively, which at physiological RA concentrations are accomplished by reciprocal effects of myelomonocytic and erythroid GFs on its expression (see model in Figure 4B). The respective negative and positive effects of RA on hematopoietic differentiation in response to EPO and GM-CSF/G-CSF may, at least in part, be explained by the levels of RA-inducible expression of the RARα2 isoform.
We are grateful to Alex Chen and Stella Pearson for technical assistance, and Michael Klaus for a gift of the RARα antagonist.
Supported by the Specialist Programme Grants (A.Z. and T.E.) from the Leukaemia Research Fund of Great Britain; in part by the Samuel Waxman Cancer Research Foundation (J.Z.); and by a studentship from the Institute of Cancer Research and a Human Potential–Research Training Networks Programme grant from the European Commission (K.P. and A.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Arthur Zelent, Leukaemia Research Fund Centre at the Institute of Cancer Research, Chester Beatty Laboratories, 237 Fulham Rd, London SW3 6JB, UK; e-mail: a.zelent@icr.ac.uk.

![Fig. 3. Effect of RA on RARα2 during myelomonocytic differentiation and on the expression of RARα2 by EPO. / RA potentiates induction of RARα2 during myelomonocytic differentiation and reverses inhibition of its expression by EPO. (A) Semiquantitative RT-PCR analysis of RARα1 and α2 expression in FDCP-mixA4 cells after a 2-day treatment with the indicated GFs and/or retinoids. The recombinant GFs were used at 1000 U/mL (G-CSF) and 50 U/mL (GM-CSF). Concentration of RA was 10−6 M. G-CSF/GM-CSF indicates standard FDCP-mixA4 myelomonocytic differentiation with the use of conditioned medium.1539Erythroid differentiation was induced by EPO (1000 U/L [1 U/mL]) and resulted in a decrease of RARα1 and RARα2 expression (lane 9) versus untreated control (lane 8). These data are consistent with the results shown in Figure 1B, which were derived from a different passage of FDCP-mixA4 cells. Addition of RA partially reversed this effect (lane 10). Glyceraldehyde phosphate dehydrogenase (GAPDH) levels were used as a control. (B) Frequency of hematopoietic cells representing different lineages and maturation stages in cultures from which RNA was isolated and used in the above RT-PCR analysis. Note that at day 2 the majority of cells in each culture retain the blast cell–line morphology characteristic of undifferentiated FDCP-mixA4 cells. Therefore, RA-mediated enhancement of the RARα2 expression precedes any morphological changes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/8/10.1182_blood.v98.8.2563/6/m_h82011647003.jpeg?Expires=1769258708&Signature=opA9fIh4n3nI4zZ4IW75BHsjjSwHoQYef2vD6-cNyyv~wzVosKr2rCIHX~sL9r7JnHzCTTw2iY9okXZenT9xaDYAcNivhBfr6RYl4yHx-ESWG3cfA745elbR8bOCc7t4GmuqQOIs2b9G4-BHD6fPewIGmElLJYTnlK~7u1coZJRZBnI0pYfdC~wPrUcQwxAJGwhAv6MvkQSLwbZpP2oymKk~uKoWx8a0FQbM7fsVhcSSPrCGxoa4khcwZw9kgstK8pcYMFAY0fYsBfJIs8-rKnEPU3W2LNZJe3WTqALjuwod0MnxRC-I-o1tCVEcB-FP8~VdIGhfoLWdt0pxK3aejA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)