Abstract
The malignant Hodgkin and Reed-Sternberg (H/RS) cells of Hodgkin disease (HD) express several members of the tumor necrosis factor (TNF) receptor family, including CD30 and CD40, and secrete several cytokines and chemokines. However, little is known about what regulates cytokine and chemokine secretion in H/RS cells. Although H/RS cells are predominantly of B-cell origin, they frequently share phenotypic and functional features with dendritic cells (DCs). Previous studies reported that receptor activator of nuclear factor κB (NF–κB) (RANK), a member of the TNF receptor family, is expressed on DCs, and that RANK ligand (RANKL) enhances DC survival and induces them to secrete cytokines. This study reports that, similar to DCs, cultured H/RS cells expressed RANK. However, unlike DCs, H/RS cells also expressed RANKL. Soluble RANKL activated NF-κB and induced messenger RNA expression of interferon-γ, interleukin-8 (IL-8), IL-13, IL-9, IL-15, and RANTES, in addition to the receptors for IL-9, IL-13, IL-15, and CCR4. RANKL increased IL-8 and IL-13 levels in the supernatants of H/RS cell lines, an effect that was blocked by soluble RANK. Furthermore, soluble RANK decreased the basal level of IL-8 in one cell line, suggesting that IL-8 was induced by an autocrine RANKL/RANK loop. RANKL had no effect on H/RS cell survival in culture, and it did not modulate the expression of bcl-2, bcl-xL, bax, or inhibitors of apoptosis proteins. These data provide evidence of further functional similarities between DCs and H/RS cells. The coexpression of RANK and RANKL in H/RS cells suggests that they may regulate cytokine and chemokine secretion in H/RS cells by an autocrine mechanism.
Introduction
The pathology of Hodgkin disease (HD)1is unique among human cancers. In a lymph node that is involved with HD, the malignant cells known as Hodgkin and Reed-Sternberg (H/RS) cells compose less than 1% of the tumor mass, with the remaining cells being benign infiltrating T and B lymphocytes, monocytes, eosinophils, macrophages, and dendritic cells (DCs).1 The H/RS cells secrete a wide variety of cytokines and chemokines that are believed to contribute to H/RS cell survival and to be involved in the chemotaxis of the infiltrating cells, the immune deficiency associated with the disease, and the symptoms that are frequently observed in patients with HD.2-7 Although molecular studies showed that the majority of H/RS cells are derived from germinal center B lymphocytes, in rare cases T lymphocytes can also be the cells of origin.8,9Additionally, several studies have reported that H/RS cells may share common features with DCs, including the expression of CD21, fascin, and CD83.10-12
Receptor activator of nuclear factor κB (NF–κB) (RANK) ligand (RANKL), also known as TRANCE (tumor necrosis factor [TNF]–related activation-induced cytokine), osteoprotegerin ligand (OPGL), or osteoclast differentiation factor (ODF), is a type II transmembrane protein that belongs to the TNF superfamily.13-16 RANKL is primarily expressed by activated T lymphocytes and osteoblasts, and it is involved in the interaction between T lymphocytes and DCs, osteoclast differentiation from hematopoietic precursor cells, and activation of mature osteoclasts.13,17-19 RANKL can also enhance the survival of DCs, perhaps by up-regulating the antiapoptotic protein Bcl-xL, and can induce DCs to secrete several cytokines, including interleukin-1 (IL-1), IL-6, IL-12, and IL-15.20 21
Two receptors for RANKL have been identified: RANK and osteoprotegrin (OPG).13,22 RANK is a member of the TNF receptor superfamily that shares the highest sequence homology with the extracellular domain of CD40.13 RANK messenger RNA (mRNA) is ubiquitously expressed in human tissues, but RANK protein expression has been detected only in DCs, CD4 and CD8 T lymphocytes, and osteoclast hematopoietic precursor cells, suggesting that expression of the protein is posttranscriptionally regulated.13,23 Like other TNF receptor family members, RANK activates several signaling pathways by interacting with various TNF receptor-associated factors (TRAFs).24-26 The signaling pathways activated by RANK include NF-κB, c-jun amino terminal kinase (JNK), and c-Src.27 The physiologic functions of RANK and RANKL have been demonstrated by knock-out experiments in mice, the results of which showed profound defects in bone resorption, lymph node formation, and B-cell development.17,18 28
Osteoprotegerin is a secreted receptor that binds to both RANKL and TNF–related apoptosis inducing ligand (TRAIL)/Apo-2L.22,29 OPG mRNA is primarily detected in the heart, placenta, lung, and kidney tissues. OPG inhibits RANKL effects on osteoclasts in vitro and in vivo, and OPG−/− mice develop early-onset osteoporosis.30
The expression and function of RANKL, RANK, and OPG in human hematopoietic cancer cells and the function of RANK in malignant cells are unknown. Because of the similarities between H/RS cells and DCs, and because H/RS cells secrete a wide variety of cytokines that have been shown to be induced by RANKL, we examined the expression of RANK in cultured H/RS cells and determined the function of this expression.
Materials and methods
Cell lines and reagents
The human H/RS-derived cell lines KM-H2, HDLM-2, L-428, and HD-MYZ were obtained from the German Collection of Microorganisms and Cell Cultures, Department of Human and Animal Cell Cultures (Braunschweig, Germany). The phenotype and genotype of these cell lines have been previously published.31 All cell lines were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, l-glutamine, and penicillin/streptomycin (Gibco BRL, Gaithersburg, MD) in a humid environment of 5% CO2 at 37°C.
Recombinant human RANKL, CD40 ligand (CD40L, CD154), and activating antibody to human Fas (CH-11), soluble CD30, soluble RANK, and soluble OPG were from Alexis (San Diego, CA). Recombinant human TRAIL trimer (leucine zipper) and activating antibody to human CD30 receptor (M44)32 were kindly provided by Dr Raymond Goodwin (Immunex, Seattle, WA). Antibodies to RANK, RANKL, and OPG were from Imgenex (San Diego, CA); to Bcl-x, Bcl-2, Bax, and cellular inhibitors of apoptosis proteins 2 (cIAP2) were from Santa Cruz Biotechnology (Santa Cruz, CA); to cFLIP and cIAP1, survivin, and NAIP were from R & D Systems (Minneapolis, MN); to XIAP was from Transduction Laboratories (San Diego, CA); and to β-actin was from Sigma Chemicals (St Louis, MO).
Assessment of cell viability
Cells were cultured in 24-well plates in a volume of 500 μL at 5 × 105 cells/mL for all cell lines. Cell viability was assessed with a nonradioactive cell proliferation MTS assay using CellTiter96 Aqueous One Reagent (Promega, Madison, WI), according to the manufacturer's instructions.33 In this assay, formazan absorbance was measured at 490 nm on a μQuant plate reader equipped with KC4 software (Biotek Instruments, Winooski, VT). Each measurement was made in triplicate and the mean value was determined.
Flow cytometry
Cells were stained with fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–conjugated antibodies to CD30, CD40, CD95 (Fas), CD154 (CD40L), B7.1, B7.2, or isotypic-matched control antibodies (all from Pharmingen, San Diego, CA) as previously described.34 35 Data were collected on a Becton Dickinson FACScan flow cytometer and analyzed by WinMDI 2.8 software (Joseph Trotter, Scripps, San Diego, CA).
Western blot analysis
Cellular protein was extracted by incubation in RIPA buffer (Roche Molecular Biochemicals, Indianapolis, IN) for 15 minutes at 4°C and then centrifuged to remove cellular debris. The protein in the resulting supernatant was quantified by the bicinchoninic acid (BCA) method according to the manufacturer's instructions (Pierce, Rockford, IL), diluted 1:1 in protein-loading buffer (0.25 M Tris-HCl, 2% sodium dodecyl sulfate [SDS], 4% β-mercaptoethanol, 1% glycerol, and 0.2 mg/mL bromophenol blue), and boiled for 30 minutes. A total of 30 μg protein was loaded onto 12% Tris-HCl SDS–polyacrylamide gel electrophoresis (SDS-PAGE) Ready Gels (Bio-Rad, Hercules, CA), transferred to a nitrocellulose transfer membrane (Osmonics, Minnetonka, MN), and detected using ECL-Plus (Amersham, Buckinghamshire, United Kingdom).
Electromobility shift assay and antibody supershift assay
Electromobility shift assays (EMSAs) were performed to determine the activation and nuclear translocation of NF-κB as previously described, with minor modifications.36 Briefly, 4 μg nuclear protein extract was incubated with 16 fmol of a32P-labeled 45-mer double-stranded DNA oligonucleotide derived from the human immunodeficiency virus long terminal repeat (5′-TTGTTACAAGGGACTTTCCGCTGGGGACTTTCCAGGGAGGCGTGG-3′) (underlined areas indicate NF-κB binding sites) for 30 minutes at 37°C. The resulting complex was resolved from free oligonucleotide by electrophoresis on 6.6% native polyacrylamide gels. To determine the specificity of NF-κB, antibody supershift assays were performed as previously described.37 Briefly, nuclear protein extract was incubated with antibodies against different NF-κB subunits (p50 and p65), control antibody (cyclin-D1), preimmune serum (PIS), unlabeled oligonucleotide, and mutant oligonucleotide: 5′-TTGTTACAACTCACTTTCCGCTGCTCACTTTCCAGGGAGGCGTGG-3′) for 30 minutes and then assessed for NF-κB by EMSA.
RNase protection assay
The mRNA expression of cytokines and their receptors plus chemokines and their receptors was determined by RNase protection assay using RiboQuant kits from Pharmingen. Briefly, cell lines (0.5 × 106/mL) were incubated with RPMI or RANKL (1 μg/mL) for 24 hours. Total RNA was isolated from 1 × 107 cells using the guanidium-isothiocyanate method. Ten micrograms of RNA from each sample was hybridized to a32P-labeled antisense probe set (hCR-1, hCR-5, hCK-1, hCK-5, hCR-6, h-APO-3d) and digested with RNase and T1 nuclease. The protected probe fragments were resolved on 5% polyacrylamide gels according to the manufacturer's instructions. Band intensity was quantified by National Institutes of Health image software (version 1.6.1) and normalized to the intensity of GAPDH probe.
Immunohistochemistry
Paraffin-embedded lymph node biopsy sections from patients with nodular sclerosis HD were immunostained using a Techmate 1000 automatic immunostainer (Ventana, Tucson, AZ) as described previously.38 Briefly, sections were deparaffinized in xylene, rehydrated with decreasing concentrations of alcohol and finally phosphate-buffered saline (PBS), and subjected to steam-heat epitope retrieval in 10 mM citrate buffer (pH 6.0) for 30 minutes in a commercially available vegetable steamer. The sections were then rinsed in distilled water, washed in PBS for 5 minutes, and incubated for 15 minutes with DAKO protein block (DAKO, Carpenteria, CA). Next, they were incubated for 2 hours with anti-RANK monoclonal antibody from Alexis, diluted 1:500 in 0.1% bovine serum albumin/PBS. (Results were confirmed using a polyclonal antibody from Santa Cruz diluted at 1:50.) Then sections were washed, and bound antibodies were detected using an LSAB2 peroxidase kit (primary rabbit/mouse; DAKO) with diaminobenzidine as chromogen. Finally, the sections were counterstained with hematoxylin, dehydrated, and mounted. Negative controls were biopsy sections that were immunostained with either nonreactive mouse IgG diluted to the same concentration as the anti-RANK antibody or with no antibody. Results of CD30, CD15, CD20, and LMP-1 expression in primary H/RS cells were available from pre-existing diagnostic reports in 7 patients.
Enzyme-linked immunosorbent assay
Human IL-8 and IL-13 levels were determined in H/RS cell supernatants, after incubation with RANKL (2 μg/mL), soluble RANK (5 μg/mL), or both. Incubations were performed for 24 to 48 hours. Commercially available enzyme-linked immunosorbent assay (ELISA) kits from R & D Systems were used according to the manufacturer's instructions. The lower limit of sensitivity of this assay is 10 pg/mL for IL-8 and 32 pg/mL for IL-13. The results were read at an optical density of 450 nm using a Vmax ELISA reader (Molecular Devices, Menlo Park, CA). Measurements were done in triplicate and results are reported as the mean ± SD.
Results
Functional expression of RANK in H/RS cells
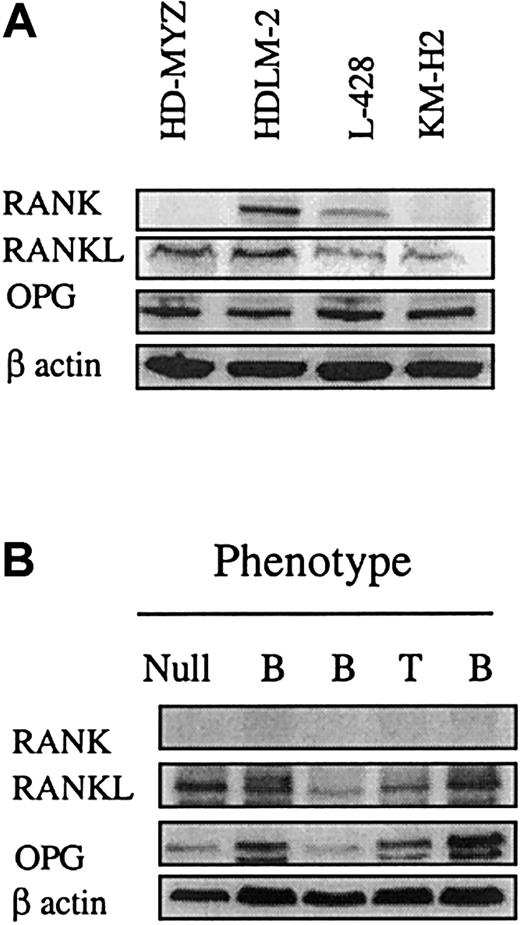
We examined the expression of RANKL and its 2 receptors, RANK and OPG, in 4 well-characterized H/RS cell lines using Western blot analysis. The RANK protein was most prominently expressed in HDLM-2 and L-428 cells, with lower expression observed in HD-MYZ and KM-H2 cell lines (Figure 1A). All cell lines expressed RANK at the mRNA level as determined by real-time polymerase chain reaction (PCR) assay (data not shown). RANKL and OPG were expressed in all 4 HD cell lines (Figure 1A). Results were then were compared with those of other cell lines of hematopoietic origin (Table1). In these cell lines, RANK was most prominently expressed by the multiple myeloma cell line 8226 and was weakly expressed by the T lymphoblastic Jurkat cell line and the anaplastic large cell lymphoma DHL-1 cell line. As shown in Table 1, the phenotype of the cell line (B or T), the expression of CD30, CD40, or Epstein-Barr virus (EBV) did not correlate with RANK expression. All these cell lines expressed OPG, whereas RANKL was expressed in 7 of the 9 cell lines tested (Table 1).
Western blot analysis of RANK, OPG, and RANKL expression in cultured H/RS cells and primary lymphoid tumors.
(A) H/RS cell lines show that all cell lines expressed RANKL and OPG. RANK was predominantly expressed in HDLM-2 and L-428. The remaining 2 cell lines also expressed RANKL, but at very low levels. All cell lines expressed RANK and RANKL at the mRNA level (data not shown). (B) Primary lymphoid tumors of different phenotypes (B, T, or null) show that RANK was not expressed in these tumors, whereas all tumors expressed RANKL and OPG.
Western blot analysis of RANK, OPG, and RANKL expression in cultured H/RS cells and primary lymphoid tumors.
(A) H/RS cell lines show that all cell lines expressed RANKL and OPG. RANK was predominantly expressed in HDLM-2 and L-428. The remaining 2 cell lines also expressed RANKL, but at very low levels. All cell lines expressed RANK and RANKL at the mRNA level (data not shown). (B) Primary lymphoid tumors of different phenotypes (B, T, or null) show that RANK was not expressed in these tumors, whereas all tumors expressed RANKL and OPG.
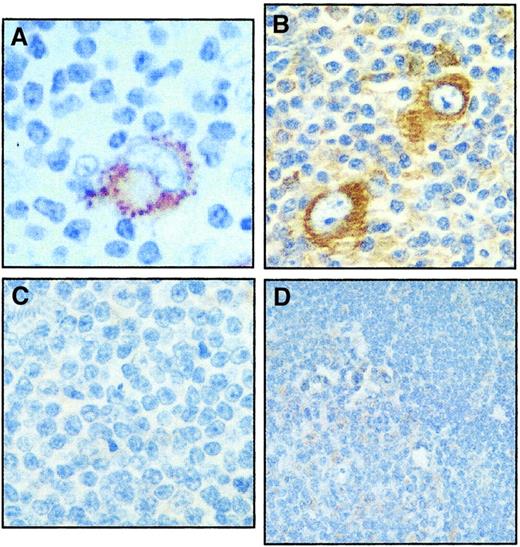
Expression of RANK was also evaluated in primary lymphoid tumors (Figures 1B and 2). Using Western blot analysis, RANK expression could not be detected in 5 primary non-Hodgkin lymphoma specimens, regardless of their phenotype (Figure1B). In contrast, RANKL and OPG were variably expressed in all these specimens. Using immunohistochemestry, RANK was detected in primary H/RS cells in 10 lymph node sections (Figure 2A,B), but was rarely and weakly expressed in sections from small lymphocytic lymphoma (Figure2C) or from a benign hyperplastic lymph node (Figure 2D). Within each lymph node section, an average of 75% of H/RS cells (range 10% to > 75%) expressed RANK (Table 2), which showed a predominantly cytoplasmic staining pattern (Figure 2), but it was rarely, but weakly, expressed in the benign infiltrating cells (Figure 2A,B). There was no difference in the pattern or frequency of RANK expression in cases of nodular sclerosis or mixed cellularity (Table 2). C30, CD15, CD20, or LMP-1 expression data were available on 7 of the 10 primary HD sections (3 nodular sclerosis and 4 mixed cellularity), and no correlation could be found between RANK expression and the expression of these antigens (Table 2).
Immunohistochemical staining of RANK protein in primary lymphoid tumors.
RANK was highly expressed in H/RS cells of a lymph node involved with nodular sclerosis (A) and mixed cellularity (B). The surrounding infiltrating cells rarely, but weakly, expressed RANK. Section from a lymph node involved with B-cell lymphocytic lymphoma (C) and from a benign hyperplastic lymph node (D) showing that RANK was rarely, but weakly expressed in these sections.
Immunohistochemical staining of RANK protein in primary lymphoid tumors.
RANK was highly expressed in H/RS cells of a lymph node involved with nodular sclerosis (A) and mixed cellularity (B). The surrounding infiltrating cells rarely, but weakly, expressed RANK. Section from a lymph node involved with B-cell lymphocytic lymphoma (C) and from a benign hyperplastic lymph node (D) showing that RANK was rarely, but weakly expressed in these sections.
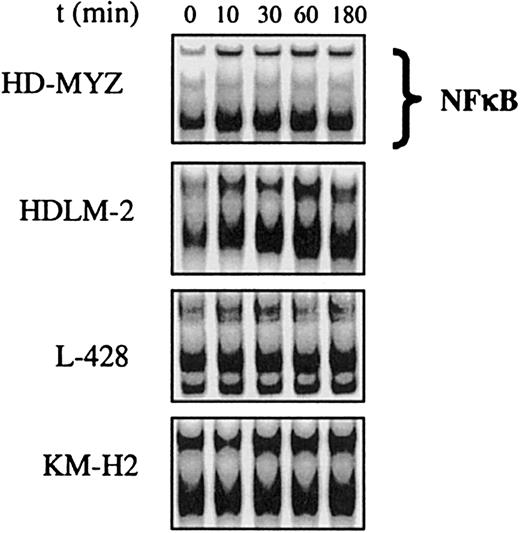
RANK was previously reported to activate NF-κB in different cell systems.13 39 To investigate whether the expression of RANK in the H/RS cell lines was functional, we incubated these cell lines with RANKL (1 μg/mL) and studied NF-κB activation by EMSA. RANKL activation of NF-κB was most prominent in HDLM-2 cells, because it could be detected within 10 minutes of stimulation and peaked at 60 minutes of stimulation. Activation of NF-κB was less prominent in HD-MYZ and L-428 cells and was not observed in the KM-H2 cells (Figure3).
RANKL activates NF-κB in H/RS cell lines.
Incubation of these H/RS cell lines with RANKL (1 μg/mL) activated NF-κB, as shown by the EMSA method. NF-κB activation was most prominent in the HDLM-2 cell line. Activation was detected as early as 10 minutes after incubation with RANKL and lasted for up to 3 hours.
RANKL activates NF-κB in H/RS cell lines.
Incubation of these H/RS cell lines with RANKL (1 μg/mL) activated NF-κB, as shown by the EMSA method. NF-κB activation was most prominent in the HDLM-2 cell line. Activation was detected as early as 10 minutes after incubation with RANKL and lasted for up to 3 hours.
Effect of RANKL on cultured H/RS cell survival in vitro
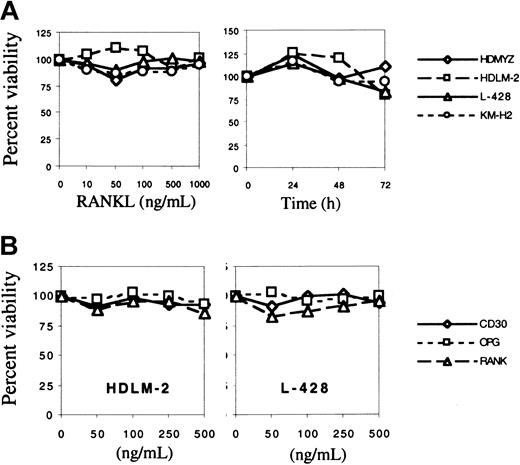
RANKL has been reported to be a survival factor for DCs.23 To investigate whether RANKL plays a similar role in H/RS cells, we incubated these 4 cell lines with increasing concentrations of RANKL (0-1000 ng/mL) for 24 to 72 hours. Cell viability and proliferation were determined using the MTS assay. RANKL had no significant effect on the survival or proliferation of any of the H/RS cell lines in vitro (Figure4A).
Effect of RANKL on the survival of H/RS cell lines.
(A) H/RS cells were incubated with increasing concentrations of RANKL for 24 hours (left panel), or they were treated with 1 μg/mL RANKL for 24, 48, or 72 hours (right panel). RANKL had no significant effect on cell survival or proliferation. (B) Effect of endogenous RANKL on the survival of H/RS cell lines. H/RS cell lines were incubated with increasing concentrations of soluble RANK, OPG, or CD30 receptors for 24 or 48 hours. The 2 cell lines that expressed high levels of RANK were examined, HDLM-2 (left panel) and L-428 (right panel). Blocking the interaction between endogenous RANKL and its receptor did not have a significant effect on the survival of these cell lines. Data shown represent average results of 3 independent experiments. No differences were observed when cells were incubated with soluble receptors for 24 or 48 hours. Soluble CD30 was used as the control because these cell lines do not express CD30L.
Effect of RANKL on the survival of H/RS cell lines.
(A) H/RS cells were incubated with increasing concentrations of RANKL for 24 hours (left panel), or they were treated with 1 μg/mL RANKL for 24, 48, or 72 hours (right panel). RANKL had no significant effect on cell survival or proliferation. (B) Effect of endogenous RANKL on the survival of H/RS cell lines. H/RS cell lines were incubated with increasing concentrations of soluble RANK, OPG, or CD30 receptors for 24 or 48 hours. The 2 cell lines that expressed high levels of RANK were examined, HDLM-2 (left panel) and L-428 (right panel). Blocking the interaction between endogenous RANKL and its receptor did not have a significant effect on the survival of these cell lines. Data shown represent average results of 3 independent experiments. No differences were observed when cells were incubated with soluble receptors for 24 or 48 hours. Soluble CD30 was used as the control because these cell lines do not express CD30L.
Because these cell lines coexpressed RANKL and RANK, we hypothesized that these cells may have been maximally stimulated with endogenous RANKL through an autocrine survival loop. Therefore, we reasoned that if we interrupted this loop, we might decrease cell survival. To test this hypothesis, we incubated H/RS cell lines with increasing concentrations of soluble RANK and soluble OPG. H/RS cells incubated with soluble CD30 were used as a control. At concentrations ranging from 5 to 500 ng/mL neither soluble RANK nor OPG had an effect on H/RS cell survival in vitro (Figure 4B).
The effect of RANKL on intracellular proteins that influence cell survival was subsequently investigated in the H/RS cell lines. Cells were incubated with RANKL (1 μg/mL) or medium for 24 or 48 hours and the levels of intracellular proteins were measured by Western blot analysis. RANKL had no effect on the expression of Bcl-xL, Bax, or Bcl-2 proteins (data not shown). Furthermore, RANKL had no effect on the antiapoptotic protein FLICE-inhibiting protein (cFLIP) or on any of the inhibitors of apoptosis proteins (IAPs) (data not shown).
Although RANKL had no significant effect on the survival of cultured H/RS cells in vitro, we examined whether RANKL can modulate the apoptotic effect induced by chemotherapy, TRAIL, or Fas ligand. Cells were incubated with doxorubicin (0.5 μg/mL), RANK (1 μg/mL), or both for 24 or 48 hours and the viable cell number was determined using the MTS assay. Doxorubicin was effective in killing L-428, HDLM-2, and KM-H2 cells (data not shown). The combination of doxorubicin plus RANKL was not different from doxorubicin alone, indicating that RANKL could not inhibit doxorubicin-induced cell death in these H/RS cell lines. Similarly, RANKL had no effect on FasL- or TRAIL-induced cell death in the H/RS-sensitive cell lines (data not shown).
Effect of RANKL on cell surface protein expression
The H/RS cells frequently express TNF receptor family members, including CD30, CD40, and CD95.40 In addition, H/RS cells frequently express the costimulatory molecules B7.1 and B7.2. To determine whether RANKL is involved in regulating the expression of these proteins, cultured H/RS cell lines were incubated with recombinant RANKL (1 μg/mL) for 24 or 48 hours. Cell surface expression of these proteins was determined using FACS analysis. Among these 4 cell lines, only HD-MYZ cells did not express CD30 or CD40 (data not shown). The remaining cells expressed CD30, CD40, and CD95 (Fas) (data not shown). None of these cell lines expressed CD40 ligand (CD40L, CD154). When these cell lines were incubated with RANKL, no significant effect was observed on the expression of CD30, CD40, CD95, or CD154 (data not shown). Similarly, RANKL had no effect on B7.1, B7.2, or HLA-DR expression (data not shown).
Because CD154 (CD40L) was reported to up-regulate RANK expression in human DCs,13 we examined the effect of CD154 and CD30 ligand (CD30L, CD153) on RANK expression in H/RS cell lines. H/RS cells were incubated with agonistic antibody to CD30 (M44, 10 μg/mL)32 or recombinant CD154 (1 μg/mL) for 24 or 48 hours. The expressions of RANKL and RANK were determined using Western blot analysis. Neither the activation of CD30 nor of CD40 had a significant effect on the expression of RANK or RANKL in these cell lines (data not shown).
Effect of RANKL on cytokine and chemokine secretion
One of the major features of H/RS cells is their ability to secrete a wide array of cytokines and chemokines.2-7 RANKL has been shown to enhance the secretion of several cytokines in DCs.21 To investigate whether RANKL can regulate the secretion of cytokines and chemokines in H/RS cell lines, we incubated these cell lines with RANKL (1 μg/mL) for 6 to 24 hours. The level of mRNA expression of different panels of cytokines, chemokines, and their receptors was determined using the RNase protection assay. The most prominent effect was observed on IL-8 expression in the HD-MYZ cell line (Figure 5A). After incubation with 1 μg/mL RANKL for 24 hours, IL-8 mRNA expression increased by 7-fold. A 2- to 3-fold increase in the mRNA expression of IL-15, IL-9, IL-13, and IL-13 receptor was observed in different H/RS cell lines (Figure 5B and Table 1). After 6 hours of incubation with RANKL, the mRNA expression of interferon-γ (IFN-γ), IL-9, IL-15, IL-15Rα, RANTES, CCR4, and IL-8 were increased in different cell lines (Table3). However, after 6 hours of incubation with RANKL, none of the HD cell lines showed any significant changes in the mRNA levels for FasL or Fas, TRAIL or its receptors, FLICE, TRADD, or FLIP (data not shown). After 24 hours of incubation with RANKL, none of these cell lines showed a significant change in the mRNA expression of the receptors for IL-7, IL-9, IL-15, IL-4, or IL-2; CCR-1, CCR-3, CCR-4, CCR-5, CCR-8, or CCR-2 (partial data are shown in Figure 5B). Furthermore, RANKL had no effect on the mRNA expression of IP-10, macrophage inflammatory protein 1α (MIP-1α), MIP-1β, monocyte chemotactic protein 1 (MCP-1), thymus- and activation-regulated chemokine (TARC), IL-5, IL-4, IL-10 (human), or IL-14 in any of these cell lines (data not shown).
RANKL induces the expression of several cytokines in H/RS cell lines.
Cell lines were incubated with RANKL (1 μg/mL) for 24 hours. Total RNA was extracted and subjected to RNase protection assay. (A) RANKL up-regulated IL-8 mRNA expression in the HD-MYZ cell line. (B) RANKL up-regulated the mRNA expression of IL-15, IL-9, IL-13, and IL-13 receptors in several cell lines. Table 1 shows the quantitative relative expression of cytokine mRNA by using the GAPDH signal as a control for the amount of input RNA.
RANKL induces the expression of several cytokines in H/RS cell lines.
Cell lines were incubated with RANKL (1 μg/mL) for 24 hours. Total RNA was extracted and subjected to RNase protection assay. (A) RANKL up-regulated IL-8 mRNA expression in the HD-MYZ cell line. (B) RANKL up-regulated the mRNA expression of IL-15, IL-9, IL-13, and IL-13 receptors in several cell lines. Table 1 shows the quantitative relative expression of cytokine mRNA by using the GAPDH signal as a control for the amount of input RNA.
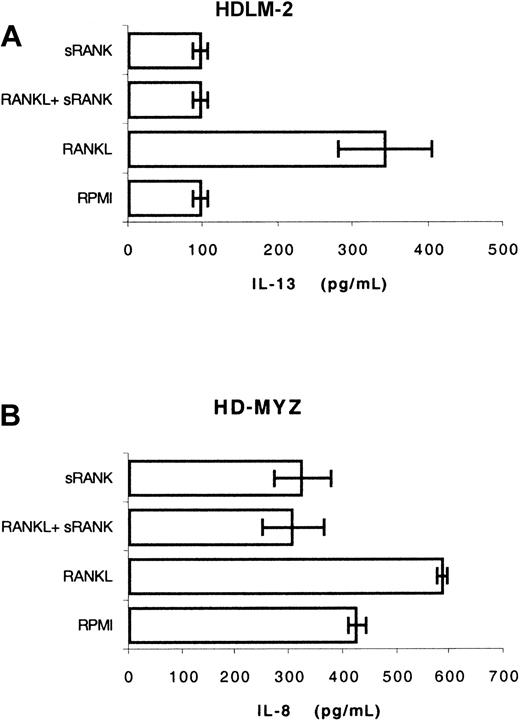
Three H/RS cell lines produced detectable levels of IL-13 in the culture supernatants. After 48 hours in culture, HDLM-2 produced a basal level of 95 pg/mL, L-428 produced 55 pg/mL, and KMH-2 produced 150 pg/mL. In HDLM-2 cells, RANKL (2 μg/mL) increased the IL-13 level from 96 ± 10 pg/mL to 340 ± 63 pg/mL, an effect that was completely blocked by adding soluble RANK (5 μg/mL) to the culture (Figure 6A). However, soluble RANK alone had no effect on the basal level of IL-13.
RANKL induces IL-13 and IL-8 secretion in H/RS cells.
(A) HDLM-2 cells were incubated with RPMI, RANKL, soluble RANK receptor, or both RANKL and RANK for 48 hours. Supernatants were collected and assayed (without dilution) for IL-13 by ELISA. RANKL increased IL-13 from 95 pg/mL to 340 pg/mL. (B) A similar experiment was performed using the HD-MYZ cell line. The level of IL-8 was measured after 24 hours in culture (supernatants were diluted at 1:20). In this cell line, soluble RANK decreased the basal level of IL-8 and blocked the effect of exogenous RANKL.
RANKL induces IL-13 and IL-8 secretion in H/RS cells.
(A) HDLM-2 cells were incubated with RPMI, RANKL, soluble RANK receptor, or both RANKL and RANK for 48 hours. Supernatants were collected and assayed (without dilution) for IL-13 by ELISA. RANKL increased IL-13 from 95 pg/mL to 340 pg/mL. (B) A similar experiment was performed using the HD-MYZ cell line. The level of IL-8 was measured after 24 hours in culture (supernatants were diluted at 1:20). In this cell line, soluble RANK decreased the basal level of IL-8 and blocked the effect of exogenous RANKL.
The HD-MYZ cell line secreted high levels of IL-8 requiring 1:20 dilutions of the supernatants to perform the ELISA. In this cell line, soluble RANK decreased the basal level of IL-8 suggesting that IL-8 was induced by a RANKL/RANK autocrine loop (Figure 6B). Accounting for the 1:20 dilution factor, exogenous RANKL increased IL-8 from a basal value of 8500 ± 312 pg/mL to 11 720 ± 192 pg/mL within 24 hours, an effect that was blocked by soluble RANK.
Discussion
In this paper we report that cultured H/RS cells express RANKL and its 2 receptors, RANK and OPG. Our data add to the complex biologic features of H/RS cells because these cells express several other TNF family receptors including CD30, CD40, Fas, and TNFR-1.40 In normal tissue, RANK mRNA is expressed in skeletal muscles, colon and intestines, adrenal glands, and thymus, whereas RANK protein is predominantly expressed by DCs, activated T cells, and osteoclasts.23 Except for activated T cells that can express RANKL and RANK, H/RS cells are now added to multiple myeloma cells to be the only malignant cells that coexpress RANK and RANKL. It is possible that the weak expression of RANK and RANKL in Jurkat and DHL-1 cells may reflect their T-cell origin. It is interesting to note that the majority of H/RS cells are derived from germinal center B cells, with rare cases originating from T lymphocytes.8,9 In this study we observed weak expression of RANK in the benign hyperplastic germinal center cells, but the physiologic role of this expression is unclear. However, this finding may be related to the observation that RANK−/− and RANK−/− mice lacked lymph node formation.17 18
RANK was expressed by primary H/RS cells, but its expression was rare among the benign infiltrating cells, perhaps reflecting their inactivated status. In this study, RANK induced NF-κB activation in H/RS cells and up-regulated the expression of several cytokines and chemokines. Although NF-κB activation was not observed in all cell lines, the up-regulation of cytokines and chemokines was observed in all cell lines, perhaps by activating other signaling pathways that can be mediated by RANK such as JNK.24,41 In DCs, RANKL induced the expression of IL-1, IL-6, IL-12, and IL-15.21In our study, different H/RS cell lines responded differently to RANK activation, reflecting the heterogeneity of these cell lines. In the HD-MYZ cell line, RANK activation up-regulated IL-8 and the receptors to IL-9, IL-13, and IL-15. RANK activation in the HDLM-2 cell line predominantly induced the expression of IL-9 and CCR4, and to a lesser extent IFN-γ, IL-15, IL-13, and RANTES. RANK activation in the L-428 cell line induced IFN-γ, IL-13, and RANTES expression, whereas RANKL up-regulated IL-15 expression in the KMH-2 cell line. It is possible that RANK activation is involved in regulating other cytokines and chemokines that were not tested in this study. Complementary DNA microarray experiments will better define the outcome of RANK activation in these cell lines.
The expressions of IL-13 and IL-13 receptor have been recently reported in cultured and primary H/RS cells and play a role in the survival of H/RS cells.3,42 Therefore, the ability of RANK to up-regulate both IL-13 and IL-13 receptor suggests that RANK may indirectly have a role in the growth regulation of H/RS cells. RANK may also play an important role in regulating the cellular infiltrate surrounding H/RS cells by regulating the expression of critical chemokines such as IL-8.43
In addition to the reported similarities between H/RS cells and DCs, we found new common features between these 2 cell types. Both cell types express RANK, and RANK activation induces the expression of several cytokines and chemokines. However, several important differences between H/RS cells and DCs were also observed in this study. First, DCs do not express RANKL. Second, unlike DCs, whereas RANK activation provides survival signals by up-regulating Bcl-xL,14 we found no role for RANK in regulating the survival or proliferation of the tested H/RS cell lines. RANK activation in H/RS cells did not regulate the expression of several intracellular proteins that are known to be involved in regulating cell life and death, including Bcl-xL, Bax, Bcl-2, cFLIP, and IAPs. Whether RANKL may provide survival signals to primary H/RS cells is currently unknown. In B cells, IL-13 and CD40L can inhibit apoptosis by up-regulating Bcl-xL.44 It is unknown whether RANKL may act synergistically with other survival factors such as IL-13, CD40L, or CD30L. Third, CD40L was reported to up-regulate RANK in DCs,13 but failed to do so in H/RS cells.
In normal tissues, RANKL mRNA is detected in the thymus, lymph nodes, and resting CD4+ and CD8+ T lymphocytes. RANKL protein is expressed by osteoblasts, bone stromal cells, and activated T lymphocytes. In this study, all the H/RS cell lines expressed RANKL regardless of their phenotype (B, T, or monocytoid), in addition to 2 T-cell lines (Jurkat and SUP-M2) and a multiple myeloma B-cell line (8226). Interestingly, RANKL was also expressed by primary B and T lymphomas. The role of RANKL in these B- and T-cell primary lymphoma tumors remains unclear.
The expression of RANK and RANKL in H/RS cells suggests that they may regulate cytokine and chemokine expression by an autocrine loop mechanism. In short-term culture, interrupting this autocrine loop by soluble RANK had no effect on H/RS cell survival. It is not known whether interrupting this autocrine loop may decrease the cellular infiltrate around primary H/RS cells. Although RANKL did not enhance HD cell survival, down-regulating certain cytokines and chemokines may indirectly influence H/RS cell survival by decreasing the cellular infiltrate that may provide survival signals.
In conclusion, our study shows for the first time that RANK and RANKL are functionally expressed in H/RS cell lines and that RANK is expressed in primary H/RS cells. The expression of RANK and RANKL is likely to be involved in regulating the cellular infiltrate and cytokine and chemokine secretion in HD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Anas Younes, Department of Lymphoma and Myeloma, M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: ayounes@mdanderson.org.