In tumor cells, the serine protease granzyme B is the primary mediator of apoptosis induced by cytotoxic T lymphocytes (CTLs)/natural killer (NK) cells. The human intracellular serpin proteinase inhibitor 9 (PI9) is the only known human protein able to inhibit the proteolytic activity of granzyme B. When present in the cytoplasm of T lymphocytes, PI9 is thought to protect CTLs against apoptosis induced by their own misdirected granzyme B. Based on the speculation that tumors may also express PI9 to escape CTL/NK cell surveillance, immunohistochemical studies on the expression of PI9 in various lymphomas were performed. Ninety-two cases of T-cell non-Hodgkin lymphoma (NHL), 75 cases of B-cell NHL, and 57 cases of Hodgkin lymphomas were stained with a PI9-specific monoclonal antibody. In T-cell NHL, highest PI9 expression was found in the extranodal T-cell NHL. In nearly 90% of enteropathy-type T-cell NHLs and 80% of NK/T-cell, nasal-type lymphomas, the majority of the tumor cells expressed PI9. In nodal T-anaplastic large cell lymphomas and peripheral T-cell lymphomas (not otherwise specified), PI9 expression occurred less frequently. In B-cell NHL, PI9 expression was associated with high-grade malignancy; 43% of diffuse large B-cell lymphomas showed PI9+ tumor cells. Finally, PI9 expression was also found in 10% of Hodgkin lymphomas. This is the first report describing the expression of the granzyme B inhibitor PI9 in human neoplastic cells in vivo. Expression of this inhibitor is yet another mechanism used by tumor cells to escape their elimination by cytotoxic lymphocytes.
Introduction
Natural killer (NK) cells and cytotoxic T lymphocytes (CTLs) form an important line of defense against virally infected cells and tumor cells. CTLs and NK cells kill target cells by inducing apoptosis in the latter. This mainly occurs via 2 pathways, one involving the death receptor induced–apoptosis (mainly the Fas ligand/Fas pathway), the other being dependent on the exocytosis of cytotoxic granules from the effector cell. Cytotoxic granules contain perforin, which forms pores in the membrane of the target cell, and several serine proteases, termed granzymes. Studies with purified components,1 transfected cells,2 and knockout mice3 4 have established perforin to be essential for membrane lysis and granzyme B to be necessary for rapid target cell DNA fragmentation and apoptosis.
Currently, strategies used by cells of the immune system and tumor cells to avoid unwarranted apoptosis by CTLs are the focal point of considerable investigation. In this regard, CTLs must protect themselves against their own cytolytic machinery to prevent unwanted lysis and must use several mechanisms to accomplish this. For example, resistance to Fas-mediated apoptosis is induced by the expression of FLICE inhibitory protein (FLIP), which directly inhibits the Fas-mediated signaling pathway.5 A number of reports have indicated that tumor cells use comparable mechanisms to resist lymphocyte-mediated cytotoxicity such as expression of FLIP and Fas ligand.5,6 In addition, they use other mechanisms such as the down-regulation of major histocompatability complex (MHC) class I molecules.7
Recently, a new defense mechanism was reported for T lymphocytes, particularly CTLs, involving a novel human intracellular serine proteinase inhibitor (serpin), proteinase inhibitor 9 (PI9). This serpin efficiently inhibits granzyme B in vitro and in vivo and cells transfected with a PI9 expression vector are protected against granzyme B–mediated apoptosis.8,9 Therefore, it was proposed that PI9 protects CTLs against death induced by their own, misdirected granzyme B.8 Notably, as we showed recently, this inhibitor is also expressed by dendritic cells and at immune-privileged sites (eg, placenta, testis, and the eye). At these sites, PI9 most likely provides protection against degranulating CTLs or NK cells.
We hypothesized that tumor cells also use expression of PI9 as another mechanism to escape immune surveillance by tumor-infiltrating CTLs or NK cells. To verify this hypothesis, we investigated whether lymphomas derived from T or B cells express the granzyme B inhibitor PI9.
Patients, materials, and methods
Materials
Monoclonal antibody (mAb) PI9-17 (subtype IgG1) was raised against recombinant human PI9 produced in Escherichia coliand purified as described.10 The mAb PI9-K (subtype IgG1) against recombinant human PI9 expressed in Saccharomyces cerevisiae11 was produced in Balb/c mice essentially according to Kohler and Milstein12 and purified from ascites fluid by protein A–Sepharose column chromatography. Both mAb PI9-17 and PI9-K are specific for PI9, but Western blot analysis showed that the antibodies recognize different epitopes10(data not shown). The mAb GB7 (subtype IgG2a), recognizing granzyme B, was produced and purified as described previously.13Biotinylated rabbit–antimouse Fab2 immunoglobulin, streptavidin-biotin-horseradish peroxidase complex (sABC), and biotinylated tyramine were purchased from DAKO (Glostrup, Denmark). Biotinylated goat–antimouse IgG1 and horseradish peroxidase–labeled goat-antimouse IgG2a antibodies were from Southern Biotechnology Associates (Birmingham, AL).
Patient selection
Formalin-fixed, paraffin-embedded tumor biopsies (n = 224) from patients with Hodgkin and non-Hodgkin lymphoma (NHL) were selected from the files of the Comprehensive Cancer Centre Amsterdam (n = 197) and the Department of Pathology, University of Vienna, Austria (n = 17). The lymphomas, classified according to the REAL and World Health Organization classifications,14 15 consisted of 92 cases of T-cell lymphoma, 75 cases of B-cell lymphoma, and 57 cases of Hodgkin lymphoma (Table 1). T-cell NHL cases were divided into nodal anaplastic large cell lymphoma (T-ALCL; n = 57), nodal peripheral T-cell lymphoma not otherwise specified (PTCL NOS; n = 11), enteropathy-type T-cell lymphoma (n = 19), and NK/T-cell, nasal-type lymphoma (n = 5). The B-cell NHL cases were divided into B-cell chronic lymphatic leukemia (B-CLL)/lymphocytic lymphoma (n = 7), mantle cell lymphoma (MCL; n = 3), follicular lymphoma (n = 11), plasma cell myeloma/cytoma (n = 6), diffuse large B-cell lymphoma (n = 40), and Burkitt lymphoma (n = 8). The Hodgkin lymphomas were divided into classical Hodgkin disease, nodular sclerosing subtype (n = 53), and nodular lymphocyte predominant Hodgkin lymphoma (n = 4). In addition, healthy control tissues such as lymph nodes or extranodal lymphoid tissue from regular surgical pathology archival material were used as controls.
Immunohistochemistry and cytochemistry
Sections (3-μm thick) from the paraffin-embedded biopsies were mounted on slides coated with poly-l-lysine. Endogenous peroxidase activity was blocked by incubation for 30 minutes with 0.3% (v/v) H2O2 in methanol. Following antigen retrieval by boiling in 0.01 M sodium citrate, pH 6, for 10 minutes in a microwave oven, tissue sections were incubated with the primary antibody for 1 hour at room temperature. The following antibodies were used: either purified mAb PI9-17 (at 2.5 μg/mL) or mAb PI9-K (at 4.25 μg/mL) in case of staining for PI9 and anti-granzyme B mAb GB7 (1.2 μg/mL). The secondary antibody was biotinylated rabbit-antimouse Fab2 immunoglobulin diluted 1:500. Biotinylated secondary antibodies were detected with sABC. PI9-stained tissue sections were further incubated with biotinylated tyramine followed by a second incubation with sABC. Bound antibodies were visualized by incubation with diaminobenzidine/H2O2 (DAB) or 3-amino-9-ethylcarbazole (AEC). Slides were counterstained with hematoxylin and mounted. Negative control slides were stained with mouse IgG of the appropriate subclass.
Double immunofluorescence staining for PI9 and granzyme B was performed on sections of T-ALCL as follows. After antigen retrieval sections were preincubated for 10 minutes with normal goat serum, diluted 1:50. Subsequently, sections were stained with mAb PI9-17 at 2.5 μg/mL as well as with GB7 at 6 μg/mL for 1 hour. The secondary antibodies used were biotinylated goat-antimouse IgG1, diluted 1:100, and horseradish peroxidase–labeled goat-antimouse IgG2a, diluted 1:100. Granzyme B was detected by incubation with DAB. After blocking the remaining peroxidase activity with 0.3% (v/v) H2O2 in methanol, sections were subsequently incubated with sABC and biotinylated tyramine. Following a second incubation with sABC, PI9 was detected by incubation with AEC. Sections were counterstained with hematoxylin and mounted.
Cases were scored independently by 2 pathologists. A case was considered positive for PI9 when cytoplasmic staining of tumor cells for PI9 was observed unequivocally. According to the number of tumor cells staining positively, these cases were divided into 5 categories: less than 5%, 5% to 25%, 25% to 50%, 50% to 75%, and 75% to 100% of the tumor cell population staining positive for PI9. Staining of reactive lymphocytes and dendritic cells within the tumor cell population acted as a positive, internal control. Quantification of relative numbers of lymphocytes positive for granzyme B present in tumor sections was performed using a commercially available interactive video overlay-based measuring system (Q-Prodit, Leica, Cambridge, United Kingdom), as described previously.16 17
Statistical methods
The relationship between PI9 expression and the presence of granzyme B+ CTLs was analyzed by Pearson χ2test. P values, based on 2-tailed statistical analysis, below .05 were considered significant. All analyses were performed using the SPSS statistical software (SPSS, Chicago, IL).
Results
PI9 is expressed in neoplastic cells in Hodgkin lymphomas and NHLs
Neoplastic cells expressing PI9 were detected in 36 of 92 cases (39%) of T-cell lymphoma, 20 of 75 cases (27%) of B-cell lymphoma, and 6 of 57 cases (10%) of Hodgkin lymphoma (Table 1). The percentage of PI9+ neoplastic cells in a given type of tumor varied from less than 5% to 100%. Figure 1A shows a case in which most neoplastic cells were PI9+; Figures 1C and 2B show an example in which tumor cells were completely negative for PI9. In these cases, dendritic cells present in the tumor area served as an internal positive control for PI9 detection (arrowhead). In PI9+cases, a strong cytoplasmic staining of the tumor cells was observed (Figures 1A and 2A), consistent with the reported intracellular localization of PI9.
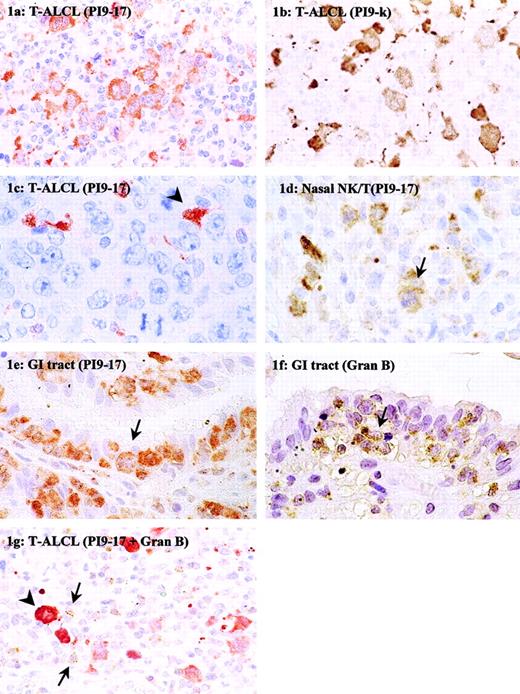
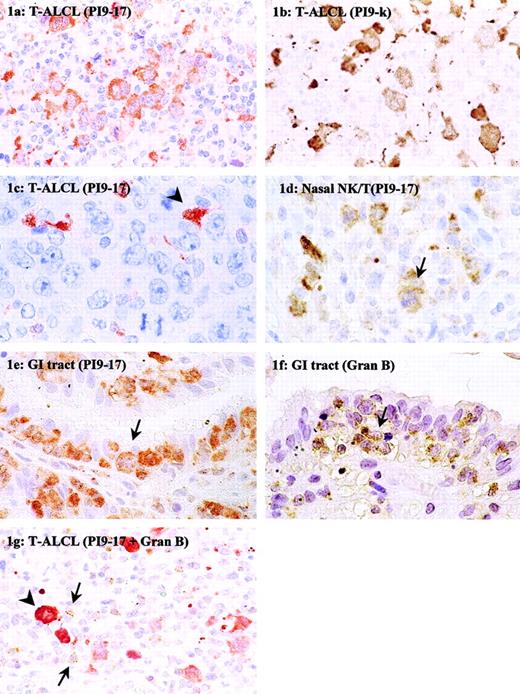
Immunohistochemical detection of PI9 in nodal and extranodal T-cell NHLs.
Tissue sections of T-ALCL (A-C,G), NK/T-cell, nasal type (D), and enteropathy-type T-cell NHL (E,F) were stained with mAb PI9-17 (A,C-E), mAb PI9-K (B), or antigranzyme B mAb GB7 (F). The section in panel G was double-stained with mAb PI9-17 (red) and GB7 (brown). The arrowhead in panel C denotes an example of a PI9+ dendritic cell. The arrows in panels D to F point to tumor cells positive for PI9 (D,E) and granzyme B (F). In panel G the arrows point to tumor-infiltrating CTLs positive for granzyme B and the arrowhead denotes an example of a PI9+ neoplastic cell. Original magnification × 630.
Immunohistochemical detection of PI9 in nodal and extranodal T-cell NHLs.
Tissue sections of T-ALCL (A-C,G), NK/T-cell, nasal type (D), and enteropathy-type T-cell NHL (E,F) were stained with mAb PI9-17 (A,C-E), mAb PI9-K (B), or antigranzyme B mAb GB7 (F). The section in panel G was double-stained with mAb PI9-17 (red) and GB7 (brown). The arrowhead in panel C denotes an example of a PI9+ dendritic cell. The arrows in panels D to F point to tumor cells positive for PI9 (D,E) and granzyme B (F). In panel G the arrows point to tumor-infiltrating CTLs positive for granzyme B and the arrowhead denotes an example of a PI9+ neoplastic cell. Original magnification × 630.
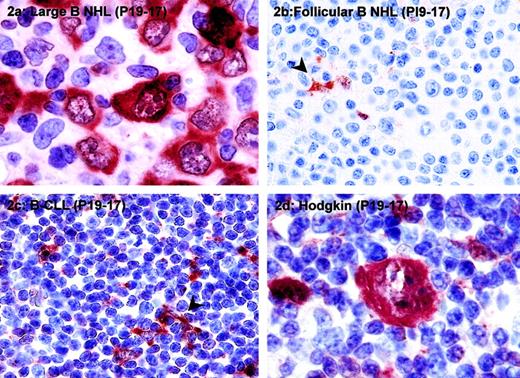
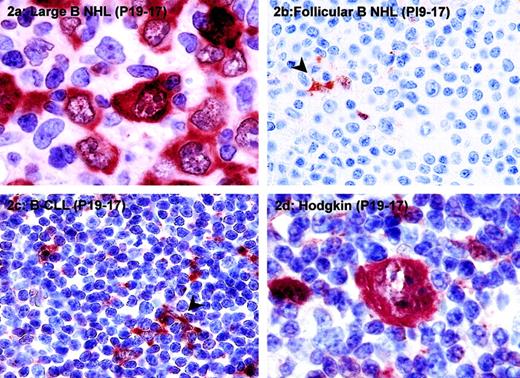
PI9 is expressed in high-grade B-cell NHLs and some cases of Hodgkin lymphoma.
The following tumors were stained for PI9: diffuse large B-cell lymphoma (A), follicular B-cell NHL (B), B-CLL (C), and classical Hodgkin disease, nodular sclerosing subtype (D). Tissue sections were stained with mAb PI9-17 as described in “Patients, materials, and methods” and counterstained with hematoxylin. The arrowheads in panels B and C denote PI9+ dendritic cells, which served as a positive internal control for staining. Original magnification × 1000 for panels A and D; × 400 for panels B and C.
PI9 is expressed in high-grade B-cell NHLs and some cases of Hodgkin lymphoma.
The following tumors were stained for PI9: diffuse large B-cell lymphoma (A), follicular B-cell NHL (B), B-CLL (C), and classical Hodgkin disease, nodular sclerosing subtype (D). Tissue sections were stained with mAb PI9-17 as described in “Patients, materials, and methods” and counterstained with hematoxylin. The arrowheads in panels B and C denote PI9+ dendritic cells, which served as a positive internal control for staining. Original magnification × 1000 for panels A and D; × 400 for panels B and C.
Enteropathy-type T-cell lymphomas show highest PI9 expression
Of all T-cell NHLs, the highest PI9 expression was observed in extranodal enteropathy-type T-cell NHLs, regarding not only the number of positive cases but also the percentage of positive neoplastic cells (Table 1). In 17 of 19 (89%) of the enteropathy-type T-cell NHLs, PI9-expressing neoplastic cells were present. Figure 1E shows PI9+ tumor cells infiltrating in the epithelial layer. In addition, PI9 protein-expressing tumor cells were found in 4 of 5 (80%) NK/T-cell, nasal-type lymphomas. Figure 1D shows PI9+ tumor cells in the mitotic phase (arrow). Of the nodal T-cell NHLs, 12 of 57 (21%) cases of T-ALCL and 3 of 11 (27%) cases of PTCL NOS contained PI9-expressing cells. A PI9+ and PI9− case of ALCL is presented in Figure 1, panels A and C, respectively. Whereas in most cases of the enteropathy-type lymphomas nearly all tumor cells expressed the PI9 protein, the percentage of PI9+ tumor cells in the T-ALCL and PTCL NOS groups varied widely.
Although the mAb PI9-17 used in this study has been extensively tested for its specificity,10 the immunohistochemical staining was confirmed with a second mAb against PI9, mAb PI9-K, which recognizes a different epitope than mAb PI9-17. Five cases of PI9− and 5 cases of PI9+ T-cell NHL were stained with mAb PI9-K. Figure 1, panels A and B, represent the staining results with mAb PI9-17 and PI9-K, respectively, on sections of the same ALCL. It is apparent from the pictures that the cytoplasmic staining with mAb PI9-K resembled the staining with mAb PI9-17. Not only the staining pattern, but also the number of PI9+cells detected with mAb PI9-17 and PI9-K were similar. Cases that were detected as PI9− with mAb PI9-17 were also scored negative when PI9-K was used. These results confirmed the data obtained with mAb PI9-17.
PI9 is expressed in B-cell NHLs and some cases of Hodgkin lymphoma
Expression of PI9 was not restricted to T-cell lymphomas because it was also found in B-cell NHLs and Hodgkin lymphomas (Table 1). PI9 expression was observed specifically in B-cell NHLs of intermediate to high grade. In 43% of diffuse large B-cell lymphoma and 25% of Burkitt lymphoma tumor cells, PI9 was expressed, whereas only 1 of 27 (4%) low-grade B-cell NHLs was positive for PI9 staining. An example of PI9 expression in diffuse large B-cell lymphoma is shown in Figure2A. In most cases of diffuse large B-cell lymphoma, 75% to 100% of the tumor cells expressed PI9 (Table 1 and Figure 2A). In PI9− low-grade B-cell NHLs, such as follicular lymphomas (Figure 2B) and B-CLLs (Figure 2C), dendritic cells served as a positive internal control for PI9 staining (arrowhead).
Expression of PI9 in tumor cells was also found in 5 of 53 (9%) cases of nodular sclerosing Hodgkin lymphoma and in 1 of 4 (25%) cases of nodular lymphocyte predominant Hodgkin lymphoma. A PI9-expressing Reed-Sternberg cell, the characteristic neoplastic cell in Hodgkin disease, is shown in Figure 2D. In addition to staining with mAb PI9-17, several cases of diffuse large B-cell lymphoma and Hodgkin lymphoma were stained with mAb PI9-K as well. In all cases, the staining patterns obtained with the 2 antibodies were completely identical (data not shown).
PI9 expression is not always related to the cytotoxic phenotype of neoplastic cells in T-cell NHLs
In many cases of T-cell NHL, particularly in extranodal lymphoma, the neoplastic cells co-expressed the serine proteinase target of PI9, granzyme B (Table 2 and Figure 1F). In T-cell NHLs of the gastrointestinal tract and the nose, all cases were positive for granzyme B, with the exception of one NK/T-cell, nasal-type lymphoma. In most of these cases, high levels of PI9 were observed, but in 3 cases no PI9 expression was detected. Furthermore, comparison between the expression of PI9 and granzyme B revealed that in T-ALCL and PTCL NOS the expression of these proteins was also not related. Only about half of the PI9+ T-ALCLs were also positive for granzyme B. On the other hand, 23 of 28 granzyme B+ T-ALCLs were negative for PI9. Nearly all cases of PTCL NOS were negative for granzyme B, but 3 of 10 cases did express PI9. Overall, these results showed that PI9 and granzyme B expression are not always related in T-cell NHL.
PI9 is expressed mainly in lymphomas harboring many activated CTLs
Expression of PI9 in T-ALCLs tended to be related to a high percentage (ie, ≥ 15%) of activated CTLs (Table3). Nine of 12 PI9+ cases showed 15% or more granzyme B+ CTLs, but this was just not significant (P = .08). Figure 1G shows an example of a T-ALCL that was positive for PI9 and had a high number of granzyme B–expressing CTLs surrounding the tumor cells. Most Hodgkin lymphoma contained less than 5% granzyme B+ CTLs. However, 3 of 4 PI9+ cases of Hodgkin lymphoma analyzed for CTL infiltrates contained a relative high percentage of 10% or greater granzyme B+ CTLs (data not shown).
Discussion
We have described, for the first time, that PI9 is expressed in vivo by human tumors, more specifically by various types of lymphomas, that is, a substantial number of T-cell and B-cell NHLs as well as some cases of Hodgkin lymphoma. In B-cell NHL, PI9 is particularly expressed by high-grade lymphomas. These results implicate a novel mechanism by which tumor cells may evade the attack of the immune system.
Tumor cells use various defense mechanisms against CTL-induced apoptosis, such as down-regulation of MHC class I molecules, resulting in less efficient tumor cell recognition by CTLs, and expression of proteins that confer resistance to Fas-mediated apoptosis.6 Examples of the latter mechanism are expression of Fas ligand, which kills attacking cytotoxic lymphocytes, and FLIP, which directly inhibits the Fas-mediated signal transduction pathway.5 Expression of PI9 would be an important additional defense mechanism, because this protein confers resistance to the other main pathway for induction of apoptosis, that is, granzyme B-induced apoptosis.
Except for the viral serpin CrmA, PI9 is the only protein known that efficiently inhibits granzyme B in vitro and in vivo.8,9It was initially described to be expressed by T- and B-cell lines. The function of PI9 in these cells was presumed to provide protection against their own, misdirected granzyme B.8 PI9 may play a protective role against granzyme B-induced apoptosis not only in CTLs, but also in other cell types as was suggested by its specific expression in dendritic cells and at immune-privileged sites.10 Thus, the present study points to a novel mechanism for tumor cells to escape from CTL-induced apoptosis. This is particularly important because granzyme B is the predominant mediator of early induction of DNA fragmentation and CTL-induced apoptosis of tumor cells.2 3
Several other serpins than PI9, especially those belonging to the subfamily of so-called ovalbumin serpins, have been implicated in processes associated with tumorigenesis and apoptosis. But, in contrast to PI9, these ovalbumin serpins cause resistance to death receptor rather than granzyme B-mediated apoptosis. Both human PAI-218 and PI1019 can protect cells from apoptosis induced by tumor necrosis factor α. Loss of the serpin maspin correlates with increased malignancy and metastasis of breast carcinomas.20,21 In addition, high tumoral expression of maspin was found to be associated with improved overall survival of patients with oral squamous cell carcinoma.22Squamous cell carcinomas also exhibit increased levels and release of SCCA,23 a serpin that is able to inhibit apoptosis in human tumor cells.24 The viral serpin CrmA is an efficient inhibitor of caspases. Although somewhat less efficiently, it can also inhibit granzyme B activity.25 However, expression of CrmA in tumor cells preferentially renders them resistant to the Fas-mediated component of CTL-induced apoptosis.26
Cytolytic molecules like granzyme B and perforin are reportedly expressed not only in reactive lymphocytes, but also in neoplastic cells in Hodgkin lymphomas and NHLs as well.27,28 Granzyme B+ tumor cells were mainly found in extranodal T-cell lymphomas and NK cell lymphomas, localized in mucosal lymphoid tissue.29 It was suggested that these lymphomas are the neoplastic counterparts of activated CTLs or NK cells.29Although the function of granzyme B in these tumor cells is still unknown, its presence provides suggestive evidence that these tumors could defend themselves against an immune response by release of granzyme B, and in that way kill tumor-infiltrating CTLs. In such a scenario, the tumor cells would need to protect themselves against endogenous granzyme B and one would anticipate concomitant expression of granzyme B and PI9 in these cells. In agreement herewith, a large number of PI9+ tumor cells was present in enteropathy-type T-cell lymphoma and NK/T-cell, nasal-type lymphoma, that is, tumors that also contain high levels of granzyme B. However, from Table 2 it is clear that there is no correlation between PI9 and granzyme B expression in the nodal T-ALCL. Thus, PI9 expression in tumor cells was not strictly associated to granzyme B expression in these cells.
Although its regulation is unclear, PI9 expression in tumors can be postulated to reflect selection due to immunologic pressure. It can be speculated that in these cases, the tumors may have become resistant to CTL-mediated apoptosis by expressing high levels of PI9. Indeed, PI9 expression in T-ALCL and Hodgkin lymphoma tended to be related to a high percentage of tumor-infiltrating, granzyme B+ CTLs (Table 3). Notably, neoplastic cells may use different defense mechanisms against CTL-induced apoptosis. This may explain why PI9 expression and the presence of a high percentage of granzyme B+ CTLs are not absolutely correlated. PI9 not only inhibits granzyme B but also some caspases,30 31 which may render these cells less susceptible to therapy. This may explain why tumors expressing high levels of PI9, such as gastrointestinal tract T-NHLs, NK/T cell nasal types, and high-grade B-cell NHLs, have a particularly poor clinical outcome.
It can be postulated that PI9 expression is an intrinsic feature of normal T and B cells and that tumors derived from these cells, therefore, also express PI9. However, we showed that in normal human tissues PI9 was expressed mainly by T cells but hardly by B cells.10 Therefore, we expected to find PI9 preferentially in neoplastic equivalents of CTLs and NK cells, that is, T-cell NHL in the gastrointestinal tract and NK/T-cell, nasal type. Surprisingly, a high percentage of B-cell NHL was also found to be positive for PI9. Even more, PI9 was also detected in epithelial tumor cell lines (B.A.B., unpublished results, February 1999). These results indicate that PI9 expression is not exclusively used by tumors originating from T lymphocytes, but may be a common mechanism used by tumor cells of different origin. It might be argued that the lack of staining of low-grade B-cell NHL might be due to the lack of cytoplasm of small neoplastic B cells. However, this is very unlikely because these tumors are diagnosed by staining for their marker bcl-2, which like PI9 is a cytoplasmic protein.
In conclusion, PI9 is expressed by a significant proportion of tumors derived from both T and B cells. Because of the ability of PI9 to efficiently inhibit granzyme B activity, PI9 expression in tumors may be an important new mechanism for tumor cells to escape from apoptosis induced by tumor-infiltrating CTLs.
We thank Angelique Verlaan for her help with the immunohistochemical stainings.
Supported by the Dutch Cancer Foundation (grant VU-98-1718).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
J. Alain Kummer, Dept of Pathology, Free University Hospital, PO Box 7057, 1007 MB Amsterdam, The Netherlands; e-mail: ja.kummer@vumc.nl.