Anti-CD45 monoclonal antibodies (mAbs) are potentially powerful tools for the depletion of mature leukocytes. As their application for immunotherapy also depends on their effects on bone marrow (BM) progeny, the in vivo effects of an anti-CD45 mAb (anti-RT7a mAb) on BM precursor cells were analyzed in a rat model. Anti-RT7a mAb treatment was performed in LEW.1W (RT1u RT7a) rats with the use of different dosages. In addition, major histocompatibility complex (MHC)–congenic BM transplantation making use of a diallelic polymorphism (RT7a/RT7b) of rat CD45 was applied. Following injection of anti-RT7a mAb into normal LEW.1W rats, T cells were profoundly depleted in blood, lymph nodes, and spleen, whereas B cells were coated only by the antibody. Single injection of anti-RT7a mAb in a high dose induced a lethal aplastic syndrome with severe thrombocytopenia. Rescue of antibody-treated animals with BM from congenic LEW.1W-7B rats (RT1u RT7b) and transplantation of BM from LEW.1W rats pretreated with anti-RT7a mAb into sublethally irradiated LEW.1W-7B recipients revealed a profound effect of the mAb on progeny of myeloid and T-cell lineage. Following repeated antibody treatment of stable mixed chimeras (RT7b/RT7a), very few RT7a-positive B cells were still detectable after 6 months and their number declined during the subsequent year. These observations show that this anti-RT7a mAb effectively depletes mature T cells as well as BM precursor cells of myeloid, T-cell, and thrombocytic lineage after in vivo application. In contrast, mature B cells are not depleted, but precursors also appear to be eliminated. Overall, the findings suggest that the anti-RT7a mAb efficiently depletes early rat hematopoietic stem cells.
Introduction
Monoclonal antibodies (mAbs) can be highly efficient tools for the systemic manipulation of specific cell populations. Antibodies detecting a broad spectrum of hematopoietic lineage cells represent an attractive approach in the therapy of hematopoietic malignancies as well as for tolerance-induction strategies.
CD45, the leukocyte common antigen (LCA) (RT7 in the rat), is a transmembrane tyrosine phosphatase1 expressed on nearly all hematopoietic lineage cells. At least 8 different isoforms of the CD45 antigen are generated by alternative splicing, mainly of exons 4 through 6, of the CD45 gene product. Antibodies against epitopes on several of these isoforms can target subpopulations of mononuclear cells (isoform-specific antibodies). Some of these isoform-specific antibodies have already been shown to be effective in transplantation tolerance induction.2 3
In this study, a rat anti–rat CD45 (RT7) mAb that binds to all leukocytes was examined for its in vivo effects on bone marrow (BM) cells as well as mature leukocytes. In the rat, 2 allomorphic forms of the RT7 antigen (RT7a and RT7b) exist. In animals expressing the RT7aallotype, the antibody (anti-RT7a mAb) binds to all CD45+ cells. Initially, the RT7 system (previously designated ART-1 or Ly-1) had been thought to represent a T cell–specific antigen because rather selective in vitro lysis of T cells by alloantisera against RT7 had been observed.4,5 Subsequently, however, the RT7 antigen was revealed to be a common hematopoietic marker of the LCA family (CD45).6 7
In previous experiments, the anti-RT7a mAb used in this study was shown to cause massive T-cell depletion in vivo and to have an ability to induce tolerance to fully major histocompatibility complex (MHC)–mismatched grafts.8,9 It could also be observed that the anti-RT7a mAb led to BM aplasia when administered in combination with passenger leukocytes.10It was the aim of this study to elucidate in detail the effect of the anti-RT7a monoclonal antibody on BM precursor cells as well as on mature leukocytes. Using congenic rat strains differing in the RT7 allotype, we used MHC-identical BM transplantation as a tool to evaluate the long-term effects of antibody-treatment on BM precursor cells, including rat hematopoietic stem cells.
Materials and methods
Animals
Rats of the LEW.1W, LEW.7B, LEW.1A, LEW.1W-7B, PVG, and PVG.C6−/− strains were used. LEW.7B and LEW.1W-7B rats came from the rat colony of one of the authors (K.W.). PVG.C6−/− rats were a kind gift of Jörg Köhl, Abteilung Mikrobiologie, Medizinische Hochschule Hannover (Germany). Normal PVG rats were obtained commercially (M&B, A/S, Ry, Denmark); LEW.1W and LEW.1A rats were bred at the Central Animal Laboratory of the Medizinische Hochschule Hannover. All animals were reared under conventional conditions at the institute's facility according to federal laws.
Anti-RT7a monoclonal antibody
The anti-RT7a monoclonal antibody is an anti-RT7 alloantibody (rat immunoglobulin G2b [IgG2b] isotype). It has been produced in previous studies of the RT7 system by one of the authors (K.W.) and detects a polymorphic determinant (RT7.1) on membrane proteins encoded by the RT7a allele of the RT7 system (CD45, LCA).
Culture supernatants of the anti-RT7a–producing hybridoma were pooled and purified by affinity chromatography with the use of Protein-G 4 Fast Flow (Pharmacia, Erlangen, Germany) and dissolved in phosphate-buffered saline (PBS) (approximately 1.5 mg antibody protein per milliliter) for intravenous injection (tail vein or penis vein). The mAb concentration of each antibody charge was measured by a fluorescent-activated cell sorting (FACS) titration method. Mean channels of fluorescence of at least 10 different dilutions were compared with a standard curve originating from the standard pool of antibody.
BM transplantation
In the first group (rescue group), LEW.1W animals were treated with a lethal dose of anti-RT7a mAb. On day 2, they received al least 100 × 106 unmodified BM cells of native LEW.1W-7B rats. Peripheral blood was examined regularly for chimeric state by FACS. Lymphoid organs of animals killed on days 100 and 209 were analyzed by a 3-color FACS stain.
In the second group (transfer group), LEW.1W-7B rats were treated with 7 Gy γ-irradiation by a 60Co source in collaboration with the Abteilung für Strahlentherapie, Medizinische Hochschule Hannover, on day −2. On day 0, the rats received the complete BM (at least 100 × 106 cells) of one LEW.1W animal treated with a potentially lethal dose of anti-RT7a mAb on day −1.
In the third group (elimination group), stable chimeras were generated by lethally irradiating LEW.1A hosts by 11 Gy γ-irradiation on day −1. On the next day, they received a mixture of LEW.7B donor and LEW.1A recipient BM cells (at least 100 × 106 cells).
Donor animals were killed by CO2 or diethylether overdose. All long bones were flushed with TC 199 solution at 4°C. The cell suspension was centrifuged for 10 minutes at 990 rpm and brought to appropriate volumes before injection. No T-cell depletion or erythrocyte lysis was performed.
Flow cytometric analysis
Two- and 3-color antibody staining was carried out by standard protocols. Single cell suspensions of all examined organs (peripheral blood, spleen, thymus, lymph nodes, and BM) were produced by cutting the parenchyma into small pieces and pushing it through steel meshes. In addition, erythrocytes were lysed by NH4Cl. Immunostaining was performed at 4°C on ice with optimal staining concentrations of mAbs.
The following antibodies were used: T cells/T-cell receptor α/β (R73, Pharmingen, San Diego, CA); B cells/CD45RA (Ox33, Pharmingen); granulocytes (HIS 48, Pharmingen); CD45 (OX1, Pharmingen)11; and RT7.2, which detects the RT7b allotype (HIS 41, Pharmingen). The following antibodies were produced from the respective hybridomas (courtesy of Chambers, Pittsburgh and Williams, Oxford, United Kingdom): natural killer (NK) cells/NKR-PIA (3.2.3), CD8a (OX 8), CD4 (W3/25), CD90 (THY1), and CD45RC (OX22).
All samples were analyzed on a FACStar flow cytometer (Becton Dickinson, San Jose, CA). The cell counts (erythrocytes, leukocytes) were performed by standard procedures. Absolute cell numbers for specific subpopulations were calculated on the basis of total leukocyte FACS (open gate) and absolute cell counts.
Immunohistochemistry
Briefly, tissue samples were snap-frozen in liquid nitrogen and 5- to 7-μm kryostat sections were obtained. Samples were dried overnight and fixed in aceton at room temperature for 10 minutes. Normal goat serum in 5% PBS was used for blocking before slides were incubated with 50 μL of their respective mAbs (see above) at optimal staining concentrations. After being washed 3 times with PBS, slides were incubated with goat antimouse immunoglobulins coupled to horseradish peroxidase (Dianova, Hamburg, Germany) and 5% heat-inactivated normal rat serum for 60 minutes. Coloring of antibody-bound cells was achieved by 3-amino-9-ethyl-carbazole (Sigma, Deisenhofen, Germany) as a substrate.
Results
In vitro staining patterns of the anti-RT7a mAb compared with OX1 mAb
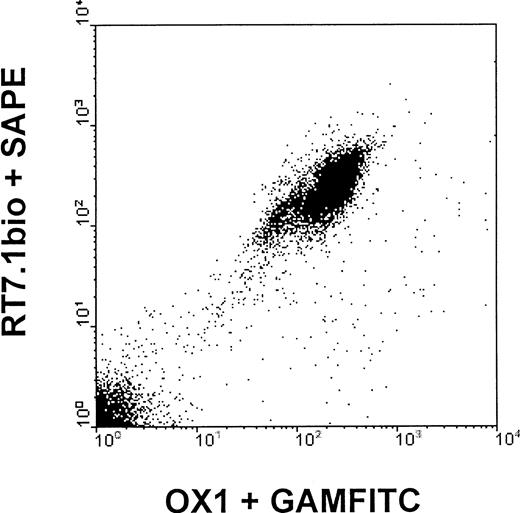
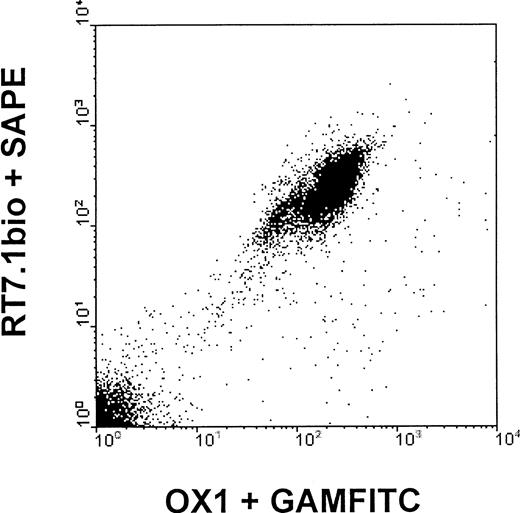
In vitro, all subtypes of peripheral blood mononuclear cells of LEW.1W animals were detected by both anti-RT7a mAb and OX1 mAb.11 For the anti-RT7a mAb, one could observe that binding was slightly more intense to T cells than to B cells, whereas OX1 mAb bound to T cells and B cells (labeled by OX33/CD45RA) with similar intensity. Staining of NK cells was stronger than staining of T cells with OX1, and about equal to that of anti-RT7a mAb. Staining intensity of granulocytes was lower than staining intensity of T cells with both of the mAbs (Figure1).
Staining intensity of different leukocyte populations with OX1 and anti-RT7a mAb.
LEW.1W peripheral leukocytes (obtained from whole EDTA blood samples, erythrocytes lysed by NH4Cl) were double-stained with specific antibodies (R73/T cells, OX33 B cells, 3.2.3/NK cells, and HIS48/granulocytes; indirect staining with phycoerythrin-conjugated goat anti-mouse mAb (GAM) and OX1 or anti-RT7a mAb (RT7.1 fluorescein isothiocyanate–[FITC] or OX1bio plus FITC-conjugated streptavidin, direct staining), respectively. FL1/FL2 dot plots were gated for subpopulations of total leukocytes. Representative histograms for gated cells are shown for OX1 and anti-RT7a mAb staining intensities, and mean channels of fluorescence are indicated.
Staining intensity of different leukocyte populations with OX1 and anti-RT7a mAb.
LEW.1W peripheral leukocytes (obtained from whole EDTA blood samples, erythrocytes lysed by NH4Cl) were double-stained with specific antibodies (R73/T cells, OX33 B cells, 3.2.3/NK cells, and HIS48/granulocytes; indirect staining with phycoerythrin-conjugated goat anti-mouse mAb (GAM) and OX1 or anti-RT7a mAb (RT7.1 fluorescein isothiocyanate–[FITC] or OX1bio plus FITC-conjugated streptavidin, direct staining), respectively. FL1/FL2 dot plots were gated for subpopulations of total leukocytes. Representative histograms for gated cells are shown for OX1 and anti-RT7a mAb staining intensities, and mean channels of fluorescence are indicated.
LEW.1W mononuclear cells could also be labeled with both mAbs simultaneously, irrespective of the order of staining (“correlated expression”) (Figure 2). OX22 (CD45RC) and OX33 (CD45RA) mAbs, which detect epitopes present on selective isoforms of CD45 (isoform-specific mAbs) also showed correlated expression on subpopulations of RT7a and OX1-positive cells.
Correlated expression of OX1 and anti-RT7a mAb (equals anti-RT7.1 mAb).
LEW.1W peripheral blood leukocytes (gained as described) were incubated with anti-RT7abio and phycoerythrin-conjugated streptavidin followed by nonlabeled OX1 mAb and FITC-conjugated GAM. Correlated expression is depicted in a representative 2-color FACS stain. Staining the mAbs in a different order gave equal results.
Correlated expression of OX1 and anti-RT7a mAb (equals anti-RT7.1 mAb).
LEW.1W peripheral blood leukocytes (gained as described) were incubated with anti-RT7abio and phycoerythrin-conjugated streptavidin followed by nonlabeled OX1 mAb and FITC-conjugated GAM. Correlated expression is depicted in a representative 2-color FACS stain. Staining the mAbs in a different order gave equal results.
Anti-RT7a mAb and OX1 are anti-CD45 mAbs binding to 2 different epitopes of the constant region of the CD45 molecule. Although basically all mononuclear cells are labeled by both mAbs, staining intensities for specific subpopulations of blood mononuclear cells are somewhat distinct for each of the mAbs (Figure 1).
In vivo depleting capacity of the anti-RT7a mAb in LEW.1W rats, complement-deficient PVG.C6−/− rats, and splenectomized rats
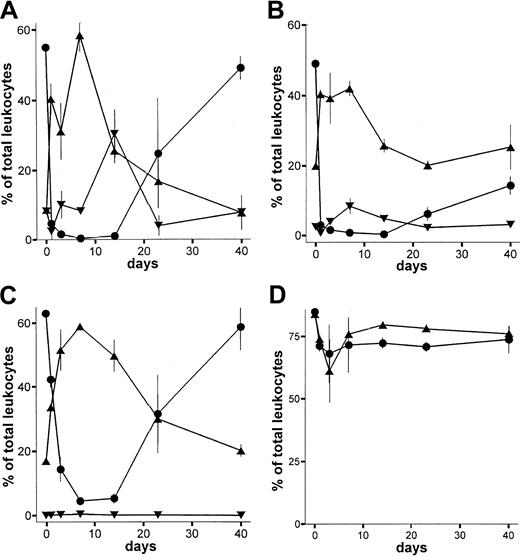
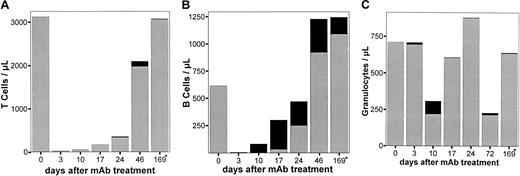
Animals (LEW.1W) treated with a single dose of the monoclonal anti-RT7a mAb showed profound depletion of T cells within blood, spleen, and lymph nodes (Figure3A-C). The maximum depletion in blood and spleen was observed 1 day after injection, whereas lymph node T cells showed the strongest depletion on day 7. The CD4+-to-CD8+ ratio among T cells was shifted slightly toward the CD8+ compartment (not shown). Repopulation of blood, spleen, and lymph nodes was completed by day 20. Thymic T cells were only marginally reduced in number (Figure3D).
Depletion of leukocytes by anti-RT7a mAb.
LEW.1W rats (n = 15) received a low dose (see text; Figure 4) of anti-RT7a mAb on day 0. At each time point indicated, 3 animals were killed. Leukocytes of whole blood and tissue samples (gained as described) were labeled with respective mAbs (compare staining in Figure 1). Blood (panel A), spleen (panel B), and lymph nodes (panel C) were analyzed in FACS. T-cell (-●-), B-cell (-▴-), and granulocyte (-▾-) counts are shown as the fraction of total leukocytes analyzed. In panel D, counts of total thymic T-cell receptor–positive (TCR+) T cells (labeled with R73 mAb) (-●-) are compared with CD8+CD4+ thymocytes (-▴-).
Depletion of leukocytes by anti-RT7a mAb.
LEW.1W rats (n = 15) received a low dose (see text; Figure 4) of anti-RT7a mAb on day 0. At each time point indicated, 3 animals were killed. Leukocytes of whole blood and tissue samples (gained as described) were labeled with respective mAbs (compare staining in Figure 1). Blood (panel A), spleen (panel B), and lymph nodes (panel C) were analyzed in FACS. T-cell (-●-), B-cell (-▴-), and granulocyte (-▾-) counts are shown as the fraction of total leukocytes analyzed. In panel D, counts of total thymic T-cell receptor–positive (TCR+) T cells (labeled with R73 mAb) (-●-) are compared with CD8+CD4+ thymocytes (-▴-).
In contrast to T cells, B cells were not depleted by the anti-RT7a mAb although they were intensively coated with antibody (not shown). In blood, spleen, and lymph nodes, a relative increase of B-cell numbers could be observed (Figure 3A-C). Granulocytes were strongly reduced in number at 1 day after antibody application. Until day 14, their numbers were relatively increased in blood and returned to normal by days 20 to 40. Depletion of NK cells was also a short-term effect. Their number was reduced to below 1% on day 1, but returned to normal by day 3 (not shown).
Immunohistological staining of lymph nodes and spleen showed profound atrophy of the lymphatic tissues on day 3 with predominant depletion of T cells. B-cell counts (per field) were not reduced as compared with normal tissue (not shown). Therefore, the relative increase in B cells in the FACS analysis was considered to be due mainly to the lack of T cells.
The most striking effect of anti-RT7a mAb therapy on cells in peripheral lymphoid organs was the profound and prolonged depletion of T cells, while mature B cells were not depleted.
To elucidate the mechanism of cell death by anti-RT7a mAb therapy, complement-deficient PVG.C6−/− rats were used. PVG.C6−/− rats lack C6 and therefore lack the ability to form a complement-dependent membrane attack complex. For comparison, normal PVG rats were used. In contrast to LEW.1W rats, leukocyte numbers in PVG rats are 1- to 2-fold lower than in LEW.1W rats (although the CD4+-to-CD8+ ratio is higher), and normal B-cell numbers are 2- to 3-fold higher (K.W., unpublished data, July 2000).
Both native PVG and PVG.C6−/− rats were treated with a low dose of anti-RT7a mAb on day −1. The degree of T-cell depletion and the time point at which cells reached their minimal number were similar to those of normal LEW.1W rats in both PVG groups. The reconstitution to normal T-cell numbers was prolonged in both normal PVG and PVG.C6−/− rats as compared with LEW.1W rats. B-cell counts in both PVG groups were initially high and never decreased to below-normal levels during the time of observation. NK cells and granulocytes were initially depleted and quickly reappeared (not shown).
In another set of experiments, splenectomized LEW.1W rats were treated with a standardized dose of anti-RT7a mAb one day after the operation. Results of FACS analysis were compared with those of normal LEW.1W rats. No significant differences between the 2 groups in the cell numbers of T and B cells could be observed. T cells were strongly depleted, whereas B cells were not affected.
In conclusion, neither a properly functioning membrane attack complex nor the spleen as an organ for the degradation of antibody-coated cells is a necessary prerequisite for the depleting ability of the anti-RT7a mAb.
In vivo titration of anti-RT7amAb effects
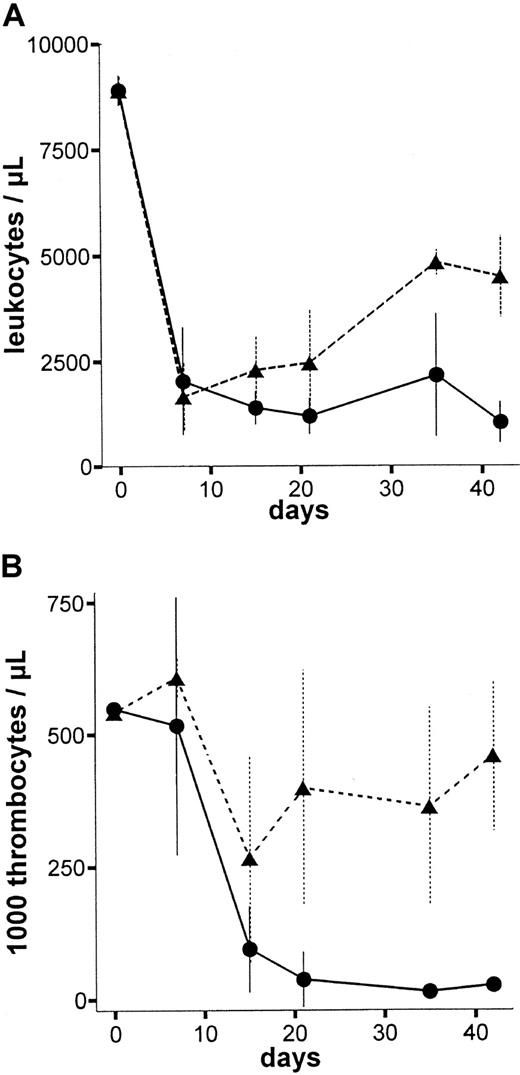
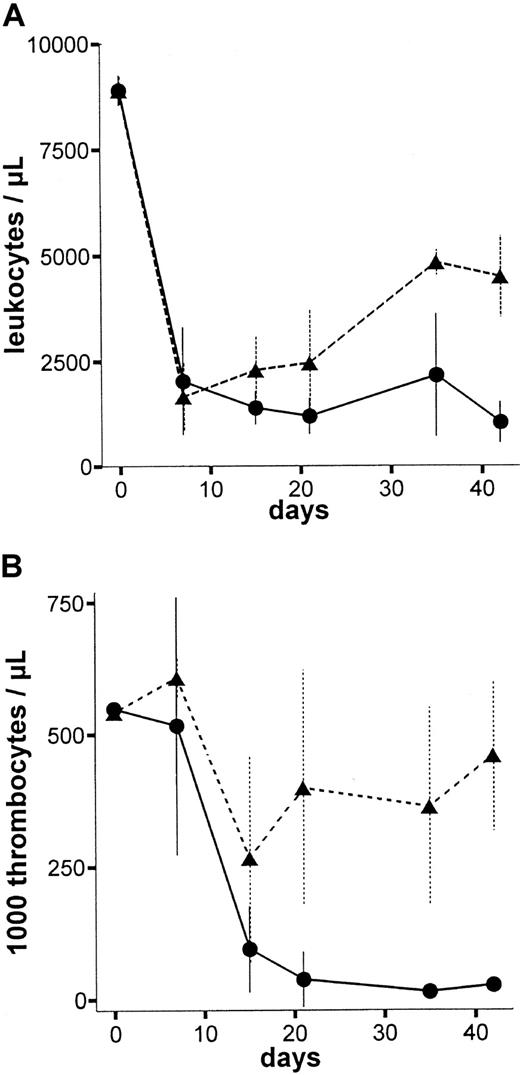
To assess the dose-to-effect ratio of the anti-RT7amAb, in vivo LEW.1W rats were treated with different single doses of the mAb (1.25 mL/0.75 mL/0.4 mL/0.25 mL of standard pool per 100 g body weight [BW]). Figure4 shows leukocyte and thrombocyte counts of animals treated with 1.25 mL per 100 g BW (high dose) and 0.75 mL per 100 g BW (low dose). All animals treated with 1.25 mL per 100 g anti-RT7a mAb died within 6 weeks (high dose = lethal dose), whereas all animals treated with lower doses of the mAb survived indefinitely (low dose is the same as a sublethal dose). In particular, thrombocyte counts turned out to be a sensitive marker for the antibody effect, as numbers fell to values below 100 000/μL approximately 2 to 3 weeks before animal death. Erythrocyte numbers were stable during the time of observation in all animals, except for animals treated with lethal doses of the mAb; these animals showed subsequent thrombocytopenic bleeding in the last days of life.
Peripheral blood leukocyte and thrombocyte counts after high-dose and low-dose anti-RT7a mAb treatment.
LEW.1W rats were injected with a high or a low (0.75 mL per 100 g BW) (n = 4) dose of anti-RT7a mAb. Absolute leukocyte (panel A) and thrombocyte (panel B) numbers were defined from whole blood samples and are shown for both groups: -●- with solid line for the high dose group and -▴- with dashed line for the low-dose group. All animals in the high-dose group died within 6 weeks of observation, whereas all animals in the low-dose group survived indefinitely.
Peripheral blood leukocyte and thrombocyte counts after high-dose and low-dose anti-RT7a mAb treatment.
LEW.1W rats were injected with a high or a low (0.75 mL per 100 g BW) (n = 4) dose of anti-RT7a mAb. Absolute leukocyte (panel A) and thrombocyte (panel B) numbers were defined from whole blood samples and are shown for both groups: -●- with solid line for the high dose group and -▴- with dashed line for the low-dose group. All animals in the high-dose group died within 6 weeks of observation, whereas all animals in the low-dose group survived indefinitely.
Effect of the anti-RT7amAb on BM precursor cells (rescue group)
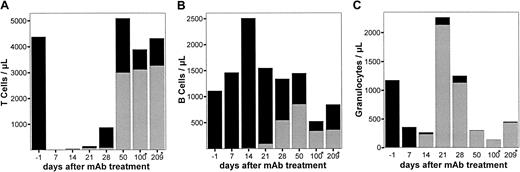
To study the mAb effects on precursor cells, LEW.1W rats (n = 4) received a lethal dose of anti-RT7a mAb on day −1 (see above). On day 0, 100 × 106 BM cells of congenic LEW.1W-7B rats were transplanted by intravenous injection. These cells were MHC-identical to the recipient but carried a different CD45 haplotype (RT7b) not detected by the anti-RT7amAb. As survival of animals in this experiment was guaranteed by the BM transplantation, it was possible to study reconstitution of BM-dependent cell lines of recipient and donor type, respectively.
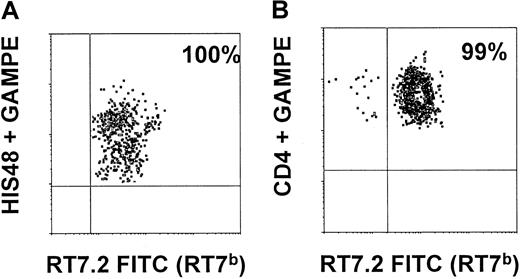
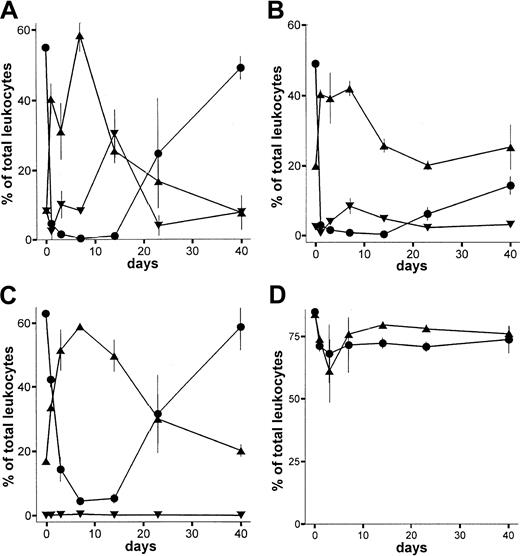
All recipients of BM showed significant allogenic chimerism of leukocytes in peripheral blood. The kinetics of leukocyte subpopulations are shown in Figure 5. Total granulocyte numbers were back to normal after at least 21 days. Recipient granulocytes were almost totally replaced by donor cells after 14 days (Figure 5C). T cells were strongly depleted in the recipient circulation during the first weeks. Reconstitution occurred by donor and recipient cells, resulting in a mixed allogenic chimerism of T cells (Figure 5A). Mature B cells were not depleted in the recipient, and the level of chimerism between recipient and donor B cells reached a maximum of 40%. Analysis of animals killed on days 100 and 209, respectively, showed total replacement of CD90+(THY1+)/HIS48+ BM cells by donor cells (100%) as well as an almost total shift toward donor type within CD4+CD8+ thymic cells (99%) as depicted in Figure 6.
Chimerism in LEW.1W rats treated with a high dose of anti-RT7a mAb and rescue by LEW.1W-7B BM transplantation.
LEW.1W rats (n = 4) received a high dose of anti-RT7a mAb (see above) on day 0. On day 1, 100 × 106 BM cells from LEW.1W-7B donors were transplanted. Leukocytes were obtained from whole blood samples and stained with respective mAbs (Figure 1). Patterns of chimerism are shown for T cells (panel A), B cells (panel B), and granulocytes (panel C). RT7a-positive recipient cells are shown as fractions of total T cells, B cells, and granulocytes as solid black boxes, whereas RT7b-positive donor cells are shown as gray boxes.
Chimerism in LEW.1W rats treated with a high dose of anti-RT7a mAb and rescue by LEW.1W-7B BM transplantation.
LEW.1W rats (n = 4) received a high dose of anti-RT7a mAb (see above) on day 0. On day 1, 100 × 106 BM cells from LEW.1W-7B donors were transplanted. Leukocytes were obtained from whole blood samples and stained with respective mAbs (Figure 1). Patterns of chimerism are shown for T cells (panel A), B cells (panel B), and granulocytes (panel C). RT7a-positive recipient cells are shown as fractions of total T cells, B cells, and granulocytes as solid black boxes, whereas RT7b-positive donor cells are shown as gray boxes.
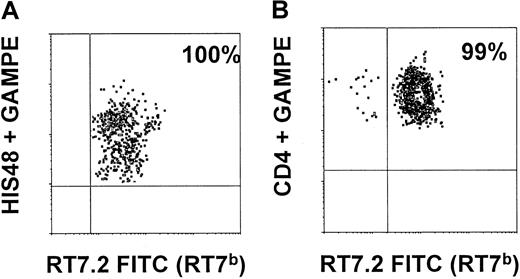
Myeloid BM precursors and thymic T-cell precursors in rescue animals are of donor origin.
On days 100 and 209, a single animal of the rescue group was killed. BM cells prepared from long bones were stained with CD90 (THY1), HIS48, and RT7.2FITC (detecting RT7b-positive donor cells). As shown in panel A, 100% of THY1+HIS48+ BM cells were of donor origin (RT7b-positive). Thymic cells (prepared as described) were stained with CD4 (W3/25), CD8 (OX8), and RT7.2 FITC. As shown in panel B, 99% of CD4+CD8+ thymic cells were of RT7b allotype.
Myeloid BM precursors and thymic T-cell precursors in rescue animals are of donor origin.
On days 100 and 209, a single animal of the rescue group was killed. BM cells prepared from long bones were stained with CD90 (THY1), HIS48, and RT7.2FITC (detecting RT7b-positive donor cells). As shown in panel A, 100% of THY1+HIS48+ BM cells were of donor origin (RT7b-positive). Thymic cells (prepared as described) were stained with CD4 (W3/25), CD8 (OX8), and RT7.2 FITC. As shown in panel B, 99% of CD4+CD8+ thymic cells were of RT7b allotype.
Effect of anti-RT7a mAb donor pretreatment on the engraftment of RT7a-positive BM (transfer group)
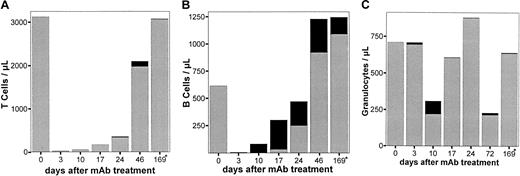
To analyze the effect of anti-RT7a pretreatment on BM engraftment potential, LEW.1W donors (n = 4) received a potentially lethal dose (see above) of the anti-RT7a mAb on day −2. On day 0, their unmodified BM was transplanted into LEW.1W-7B rats sublethally irradiated with 7 Gy γ-irradiation on day −1. Under standard conditions, congenic LEW.1W BM easily engrafts in the irradiated recipient (data not shown). Differences in engraftment and survival of recipients were supposed to be the cause of donor antibody pretreatment.
The most impressive effect was again observed within the granulocytes. No granulocytes of donor origin were found in the recipients, suggesting a lethal effect of the anti-RT7a mAb on cells of myeloid lineage in the donor BM (Figure7). Almost no T cells of donor origin were found in the early phase, whereas donor B cells could already be seen 10 days after BM transplantation. During the next weeks, stable chimerism for B cells developed, whereas mature T cells and granulocytes of donor origin were not found in significant numbers, pointing toward damage of T-cell and granulocyte progeny or of a common progenitor.
Chimerism of LEW.1W-7B rats after 7 Gy γ-irradiation and BM transplantation from LEW.1W rats treated with a high dose of anti-RT7a mAb.
LEW.1W rats (n = 4) were treated with a high anti-RT7amAb dose (see text; Figure 4) on day −1. On day 0, LEW.1W-7B recipients were irradiated with 7 Gy γ-irradiation. Six hours later, one recipient received all BM cells that could be obtained from one donor animal. Leukocytes of whole blood samples from LEW.1W-7B recipients (gained as described) were stained with the respective mAbs (Figure 1). Patterns of chimerism are shown for T cells (panel A), B cells (panel B), and granulocytes (panel C). RT7a-positive donor cells from pretreated animals are shown as fractions of total T cells, B cells, and granulocytes as solid black boxes, whereas RT7b-positive recipient cells of irradiated animals are shown as gray boxes.
Chimerism of LEW.1W-7B rats after 7 Gy γ-irradiation and BM transplantation from LEW.1W rats treated with a high dose of anti-RT7a mAb.
LEW.1W rats (n = 4) were treated with a high anti-RT7amAb dose (see text; Figure 4) on day −1. On day 0, LEW.1W-7B recipients were irradiated with 7 Gy γ-irradiation. Six hours later, one recipient received all BM cells that could be obtained from one donor animal. Leukocytes of whole blood samples from LEW.1W-7B recipients (gained as described) were stained with the respective mAbs (Figure 1). Patterns of chimerism are shown for T cells (panel A), B cells (panel B), and granulocytes (panel C). RT7a-positive donor cells from pretreated animals are shown as fractions of total T cells, B cells, and granulocytes as solid black boxes, whereas RT7b-positive recipient cells of irradiated animals are shown as gray boxes.
Treatment of stable mixed RT7a/RT7b chimeras by anti-RT7a mAb (elimination group)
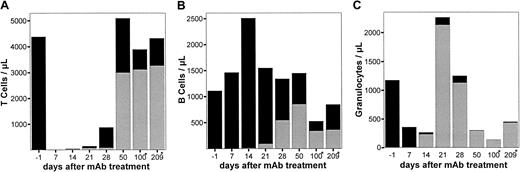
Stable irradiation chimeras were generated with the use of LEW.7B rats as donors and LEW.1A rats as recipients. Recipients were lethally irradiated (11 Gy) on day −1 and transplanted with 100 × 106 donor and recipient cells on day 0 (50% each). The resulting chimeras showed stable multilineage chimerism and long-term survival. These animals (n = 3) were treated with a “lethal” dose of anti-RT7a mAb on days 63, 66, 70, and 122 (Table 1). The RT7a-positive fraction of the chimeric BM was depleted by the anti-RT7a mAb application except for 4% to 8% of total leukocytes. These cells, which were to be seen in FACS until day 300, were revealed to be mature B cells exclusively. Analysis after 520 days, however, could not reveal persisting B cells of RT7a allotype.
Discussion
In this study, an anti-rat CD45 mAb (anti-RT7a mAb) was used, and its effects on mature leukocytes as well as on BM precursor cells were analyzed. In vitro, the antibody was shown to bind to all types of leukocytes,12-14 although staining intensity varied slightly among different leukocyte populations. This result was basically in keeping with the known expression pattern of the LCA as detected by OX1 antibody.11 The epitopes detected by OX1 and anti-RT7a are distantly located on the CD45 molecule, as antibodies did not block simultaneous binding of one another. The minor differences in binding patterns of OX1 and anti-RT7a mAb to leukocyte subpopulations may be due to steric interactions of the mAbs with sites of heavy glycosylation or by capping of epitopes.
In vivo, profound T-cell depletion in peripheral blood, spleen, and lymph nodes was the most obvious effect observed in animals after a single injection of the anti-RT7a mAb. This is in contrast to the in vivo effects described for OX1, which does not lead to relevant leukocyte depletion. This functional difference is most likely due to the different species origin as well as the different isotype of the antibodies (rat IgG2b for anti-RT7a versus mouse IgG2c for OX1). Many previous studies have shown that the nonvariable parts of antibodies play an essential role in mediating their functional effects (opsonization, complement activation, triggering of killer cells, cross-linking of target structures). Since leukocyte depletion showed an identical pattern in normal and in complement-deficient rats, complement lysis is probably not important for the depleting effect of the anti-RT7a mAb. Also, splenectomy of the animals could not prevent leukocyte depletion, arguing against a relevant contribution of the reticuloendothelial system of spleen for elimination of the cells. Nevertheless, cell-mediated effects by Fc-receptor–mediated interactions are probably essential for destruction of the leukocytes since other anti-RT7aantibodies having different isotypes that had been tested in preliminary studies were markedly less effective (data not shown).
Interestingly, mature B cells were not depleted by anti-RT7a mAb in vivo, although their in vitro staining was high (but lower than that of T cells; Figure 1). A similar difference between T and B cells in susceptibility to lysis by the antibody had already been shown by previously studying complement-mediated lysis by anti-RT7 antisera in vitro; T cells but not B cells were destroyed in the presence of the antisera.5 Since T and B cells show different patterns of CD45 isoform expression,12-14 it may be that morphological distinctions of the CD45 molecule are responsible for the different sensitivity toward antibody-mediated cell lysis. Another possible explanation might be a different role of CD45 for activation of T and B cells.15 In an in vitro model, CD45-deficient T-cell lines could not respond to stimulation except for one cell line in which one of the SYK protein kinases mediated signaling in absence of CD45.16 As the same kinase (p72syk) led to partial activation of CD45− B cells,17 one could imagine that distinct equipment with kinases could contribute to a difference in the role of CD45 for T and B cells and result in lysis of T cells, but not B cells. Additionally, it has been reported that T cells are absent in genetically CD45-deficient humans18,19 and mice,20 21 whereas B cells are vivid but inactive.
Overall, our observations suggest that either certain morphological features of CD45 isoform molecules or signal transduction via the CD45 pathway (or both) play a role in the depletion mechanism of the anti-RT7a mAb. These aspects are currently being studied in more detail.
In addition to the depletion of T cells from the circulation as well as from lymph nodes and spleen, a lethal outcome was frequently observed in animals treated with a high dose of the anti-RT7a mAb, generally following a transient recovery of the leukocyte numbers in the periphery. In these cases, injection of anti-RT7 mAb obviously led to BM failure22 associated with fatal bleeding disorders or lethal opportunistic infections. Histologically, the cellularity of the BM was strongly reduced. Particularly impressive was a progressive loss of megakaryocytes from the BM. While several noxas causing aplastic anemia are known,23 24 this is, to our knowledge, the first description of an antibody that leads to BM aplasia after a single injection.
To analyze the effect of anti-RT7 mAb treatment on hematopoietic progenitors in more detail, congenic BM transplanatation models were used; the rationale for this was based on the diallelic polymorphism of CD45 (RT7a/RT7b) in the rat. In a rescue model, recipient survival after a generally lethal anti-RT7a mAb dose was secured by MHC-identical, but RT7-mismatched, BM transplantation (RT7b → RT7a), so that the progeny of the transplanted BM could not be damaged by the antibody. Quite obviously, granulocytes were progressively and completely replaced by donor-type cells within several weeks, indicating that the progenitors of early myeloid lineage had been destroyed by antibody treatment. In the lymphatic compartment, a mixed chimerism was observed in the later course with donor as well as recipient T and B cells (approximately 50:50 for B cells and 75:25 for T cells), suggesting ongoing recipient lymphopoiesis. Long-term analysis of T-cell progeny in the thymus, however, revealed almost no CD4+CD8+ cells of recipient type. This finding strongly suggests that antibody treatment may also have destroyed very early T-cell precursors so that de novo lymphopoiesis is exclusively due to transplanted RT7b-expressing cells. The recovering and persisting T cells of recipient type may have been derived from the few surviving mature T cells, which proliferate after the antibody disappears.
These observations were confirmed by experiments in a transfer model, in which BM transplantion was carried out shortly after lethal antibody treatment of the BM donor (RT7a → RT7b). In this model, neither myeloid cells nor T cells of donor origin could be found in significant numbers (fewer than 1% of total leukocytes), while B cells survived in relevant numbers within the recipient, leading to mixed allogenic chimerism for B cells. Since few—but detectable—numbers of mature T and B cells can be found in unmodifed BM grafts, it is possible that the donor B cells found in the recipients were derived from mature B cells that were not depleted by the antibody treatment. The long-term persistence of RT7a-positive B cells in both the rescue as well as the “transfer” model may therefore indicate insensitivity of B-cell progeny to the antibody treatment. This view was apparently supported by observations in stable, mixed multilineage chimeras (RT7a + RT7b → RT7a) repeatedly treated with potentially lethal doses of anti-RT7a mAb. As expected, these animals lost their RT7a-positive cells of all lineages, apart from a few B cells that could still be detected 100 days after the antibody treatment. After 2 years of observation, however, no RT7a-positive cells could be detected, indicating that finally B cells had also been eliminated. These findings could be explained by a long life span of mature B cells that are insensitive to RT7a mAb treatment. The final elimination of these cells, however, could indicate depletion of progeny of very early B cells by the antibody.
Owing to the lack of a suitable marker for rat hematopoietic stem cells (HSCs), it is not yet clear whether very early rat HSCs are CD45+. Wickenhauser et al25 described HSCs in humans as weakly CD45+. In early human myeloid precursors, CD45RA expression is altered during maturation,26 and recently analysis of LSM-1 expression, a possible physiological substrate for CD45, revealed low expression for CD34+CD33−(CD45low) and CD34+CD33+(CD45interm) human hematopoietic progenitor cells.27 Recent studies using the Hoechst 33342 dye, however, have suggested a high CD45 expression for so-called side population cells in human, rhesus monkey, and mouse.28 These cells show all characteristics of HSCs when extracted from BM. Overall, the observations from our study would be compatible with the possibility that very early progenitor cells strongly expressing CD45 give rise to myeloid, megakaryocyte, T-lineage, and B-lineage cells. In this case, the CD45-associated signal transduction pathway should function similarly to that in T cells and allow for cell destruction following antibody binding. However, it cannot be completely excluded that early precursors are either CD45− or only weakly positive, and that their death is brought about by indirect mechanisms such as interleukin withdrawal,29,30 action of cytotoxic cytokines,31 or breakdown of the specific micromilieu following depletion of more mature cell populations.
In conclusion, the study shows that an antibody against the rat CD45 homolog RT7 is highly effective in depleting not only peripheral leukocytes, but also BM precursor cells. The mechanism of cell depletion by the antibody is not clear since mature B cells are obviously protected from depletion. This suggests that either CD45 isoform expression or differential signal transduction triggered by antibody binding to the CD45 molecule contributes to the functional effects of the anti-RT7a mAb. The findings further suggest that early hematopoietic precursors strongly expressing CD45 give rise to granulocytes, thrombocytes, and T cells, and probably also B cells. For the future, there are 3 major fields in which depleting anti-CD45 mAbs might be of great use. At first, antibodies capable of depleting hematopoietic stem cells can be introduced into the therapy of hematopoietic malignancies or for conditioning for BM transplantation for other indications; studies on this issue are currently underway. For this purpose, native antibodies may be more specific than the respective isotope conjugates,32 which always have a nonspecific bystander effect on neighboring cells. Second, such antibodies might be useful for tolerance induction in the field of transplantation through elimination of the mature T-cell repertoire as has been demonstrated previously.9 Third, depleting anti-CD45 mAbs could be used as a tool in models studying differentiation (ortrans-differentiation) of HSCs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hans J. Schlitt, Klinik für Viszeral- und Transplantationschirurgie, Medizinische Hochschule Hannover, D-30623 Hannover, Germany; e-mail:schlitt.hans@mh-hannover.de.

![Fig. 1. Staining intensity of different leukocyte populations with OX1 and anti-RT7a mAb. / LEW.1W peripheral leukocytes (obtained from whole EDTA blood samples, erythrocytes lysed by NH4Cl) were double-stained with specific antibodies (R73/T cells, OX33 B cells, 3.2.3/NK cells, and HIS48/granulocytes; indirect staining with phycoerythrin-conjugated goat anti-mouse mAb (GAM) and OX1 or anti-RT7a mAb (RT7.1 fluorescein isothiocyanate–[FITC] or OX1bio plus FITC-conjugated streptavidin, direct staining), respectively. FL1/FL2 dot plots were gated for subpopulations of total leukocytes. Representative histograms for gated cells are shown for OX1 and anti-RT7a mAb staining intensities, and mean channels of fluorescence are indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/10/10.1182_blood.v99.10.3566/6/m_h81022544001.jpeg?Expires=1769548061&Signature=GVAOlNqncs1y5Nn2ZqBywzTaEQ0GyZY1HB6t~Akpq-eUeyHE-i4sEgeUVl9~beUQV4sJHGFJ64kHBdyhUuNq3CIS0-szLBKKkXCr8ox1FCE77KVEZGrnIHMfTDD0M-XH3B6JTEyrOsjPa0UMnDcvJTtwkEmsvW6tQBLV8CFSPfxmL9rTL3GtBtkc3uxPydfJUxmTnOY0a7PEknyZykbM1xm--VLSJRSH5BZ2uTv4aggtpdSS0pnxinIS6ZDSWqVmexvrY1HpcP8l3qWIOpV4KiMGXmNkrIBCh3k~y3u2meqORLd1GlL9H0mrOCyiP3Hr3aujoQARzUBCORQKFn~MuQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 1. Staining intensity of different leukocyte populations with OX1 and anti-RT7a mAb. / LEW.1W peripheral leukocytes (obtained from whole EDTA blood samples, erythrocytes lysed by NH4Cl) were double-stained with specific antibodies (R73/T cells, OX33 B cells, 3.2.3/NK cells, and HIS48/granulocytes; indirect staining with phycoerythrin-conjugated goat anti-mouse mAb (GAM) and OX1 or anti-RT7a mAb (RT7.1 fluorescein isothiocyanate–[FITC] or OX1bio plus FITC-conjugated streptavidin, direct staining), respectively. FL1/FL2 dot plots were gated for subpopulations of total leukocytes. Representative histograms for gated cells are shown for OX1 and anti-RT7a mAb staining intensities, and mean channels of fluorescence are indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/10/10.1182_blood.v99.10.3566/6/m_h81022544001.jpeg?Expires=1769679693&Signature=kSw~fep6dk9A8Wa0P4Ue~Ub7C2PXLpsr3UQZNyqxtloOpHvPT-iwDLBVEyHVYqJG3ElmBauRXTisCt5A3gEwIMYdf3Zb1S8dPc4MrsUSYeztLO9rMTUlt2j0dr3bGpjnLYQZQfcLVr4myxENhKqovBlQH-M8qVNxUtoAGuQ~7Q1csu4dBYf1tZOoCLZ7NKUDLKtzEOdthfgDW-IVmPhpa9mRyGrnE5SbFQqKrIkWS-6UoI1UMuBl4LJFa40VjQcKMwaPo89XLKMHbPz3SIRD9IR0M6hPwOXt-L4ahlsLhX4winH9lP4q2VegoaiTa9P8OLQseNPHsFigt6FpaW9rQg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)