In vitro studies suggest that resistance to chemotherapy-induced apoptosis might explain poor response to therapy in fatal cases. Actual execution of apoptosis depends on proper functioning of effector caspases, particularly caspase 3, and on the expression levels of apoptosis-regulating proteins, including Bcl-2 and the recently identified granzyme B– specific protease inhibitor 9 (PI9). Thus, high levels of caspase 3 activation should reflect proper functioning of the apoptosis pathways, resulting in chemotherapy-sensitive neoplastic cells and a favorable prognosis. We tested this hypothesis by quantifying numbers of tumor cells positive for active caspase 3, Bcl-2, and PI9, respectively, in pretreatment biopsies of systemic anaplastic large cell lymphoma (ALCL) patients and by comparing these numbers with clinical outcome. Activation of caspase 3 in more than 5% of the tumor cells was strongly correlated with a highly favorable outcome. High numbers of Bcl-2– and PI9-positive tumor cells were found to predict unfavorable prognosis. This prognostic effect was strongly related to anaplastic lymphoma kinase (ALK) status: ALK-positive ALCL had significantly higher levels of active caspase 3, while high expression of the antiapoptotic proteins Bcl-2 and PI9 was almost completely restricted to ALK-negative cases. In conclusion, high numbers of active caspase 3–positive tumor cells predict a highly favorable prognosis in systemic ALCL patients. Poor prognosis is strongly related to high numbers of Bcl-2– and PI9-positive neoplastic cells. These data support the notion that a favorable response to chemotherapy depends on an intact apoptosis cascade. Moreover, these data indicate that differences in prognosis between ALK-positive and ALK-negative ALCL might be explained by differences in expression of apoptosis-inhibiting proteins.
Introduction
Anaplastic large cell lymphoma (ALCL) is a non-Hodgkin lymphoma characterized by CD30+ cells with abundant cytoplasm and pleomorphic nuclei.1 Prognosis of patients with systemic ALCL is variable and depends strongly on expression of a fusion protein containing the anaplastic lymphoma kinase (ALK) oncogene.2-4 Although different clinical risk factors have been identified, including age and stage at first presentation, the prognosis of these patients remains unpredictable in individual cases.
In vitro data indicate that a poor response to chemotherapy in fatal cases may be caused by inhibition of the apoptosis cascade.5-8 Apoptosis, or programmed cell death, is characterized by distinct, recognizable morphologic phases. It can be triggered by a variety of stimuli, including cytotoxic T-lymphocyte (CTL)–mediated killing via either the CD95 or granzyme B/perforin-mediated pathway but also by ionizing radiation and many cytostatic drugs.9 Upon induction of apoptosis, a cascade of proteases called caspases (cysteine-containing aspartic acid–specific proteases) is activated through cleavage of the proenzymes.9 These enzymes, once activated, dismantle the cell by selectively cleaving key proteins.
In vitro studies have elucidated 2 major apoptosis pathways: (1) a stress-induced pathway leading, via mitochondrial cytochrome C release, to activation of caspase 910-12 and (2) a cell surface death receptor signaling pathway leading to activation of caspase 8.13 Both pathways induce apoptosis via activation of caspase 3 and possibly other effector caspases (6 and 7) that execute cell death through degradation of vital proteins, including substrates involved in cell structure, signaling, cell cycle control, and DNA repair.9,14 A typical substrate of effector caspases is poly-ADP ribose polymerase-1 (PARP-1), a DNA repair enzyme that is cleaved into p89 by activated effector caspases.15,16Granzyme B has been shown to activate the stress-induced pathway via Bid or by direct cleavage of caspase 3.17-19 Thus, all major pathways depend upon activation of effector caspases, particularly caspase 3, for the final execution of apoptosis.
Using a novel method to detect activation of caspase 3 on formalin-fixed paraffin-embedded tumor biopsies, we previously found that, in vivo, apoptosis in B-cell lymphomas and reactive tissues indeed always involves activation of caspase 3.20Therefore, it might be expected that high levels of active caspase 3 reflect proper functioning of one or both major apoptosis pathways, resulting in chemotherapy-sensitive neoplastic cells and a favorable response to chemotherapy.
Inhibition of the apoptosis cascade may occur at several levels. One of the proteins implicated in causing resistance to apoptosis is Bcl-2, which specifically inhibits the stress-induced pathway9,21and has been demonstrated in several lymphomas to be related to poor clinical outcome.22,23 Recently, a novel human intracellular serine protease inhibitor (serpin) called protease inhibitor 9 (PI9) was identified as an inhibitor of granzyme B–induced apoptosis. This serpin efficiently inhibits the function of granzyme B in vitro and in vivo and protects cells transfected with PI9 against granzyme B–mediated apoptosis.24,25 We recently found PI9 to be expressed in the neoplastic cells of several lymphoma types, including systemic ALCL.26
In the present study we investigated whether activation of caspase 3 and cleavage of PARP-1 in pretreatment biopsies of systemic ALCL patients is related to clinical outcome. Furthermore, we investigated whether differences in clinical outcome are related to differences in expression levels of Bcl-2 and the recently identified granzyme B antagonist PI9. Differences in expression of active caspase 3, Bcl-2, and PI9 were also investigated in relation to expression of ALK.
Patients, materials, and methods
Patient selection and clinical characteristics
Formalin-fixed, paraffin-embedded tumor biopsies of 64 systemic nodal ALCLs were retrieved from the files of the Comprehensive Cancer Center Amsterdam (diagnosed between 1980 and 1999). Of all patients, the primary diagnostic lymph node biopsy taken prior to the start of chemotherapy was investigated. Cases were classified according to the World Health Organization classification,1 and diagnostic immunophenotyping included CD30, CD15, ALK1, T-cell markers (CD3, CD4, CD8, TIA-1, and granzyme B), and B-cell markers (CD20 and CD79a). There were no ALCLs of B-cell phenotype or secondary ALCLs included in this study; nor were there ALCLs arising in immunocompromised patients. Paraffin-embedded hyperplastic tonsils were used as a control for immunohistochemical detection of the apoptosis-related proteins.
For each patient, the following characteristics were noted from the medical records: age at diagnosis, sex, Ann Arbor stage at presentation, therapy, achievement of complete remission, the occurrence of relapses, and cause of death. The International Prognostic Index was determined as described previously.27Patient characteristics stratified according to ALK status are summarized in Table 1. Four of 11 patients with stage I disease were treated with radiotherapy alone; all other patients received polychemotherapy consisting of CHOP regimens (cyclophosphamide, doxorubicin, vincristine, prednisone) with or without involved field radiation. ALK expression was found in 25 (39%) of 64 cases and was significantly related to younger age at presentation, lower stages of disease, and a more favorable clinical outcome (Table 1). Clinical data of most patients were published previously2 and are in line with other studies.3 4 The study was approved by the institutional review board of the VU Medical Center. Informed consent was provided according to the Declaration of Helsinki.
Detection of procaspase and active caspase 3, cleaved PARP-1/p89, Bcl-2, and PI9
Sections 4 μm thick from the paraffin-embedded biopsies were stained using a standard 3-step streptavidin–biotin complex method with 3,3′-diaminobenzidine or 3-amino-9-ethylcarbazole as chromogen. The following monoclonal antibodies were used: anti–procaspase 3 (clone 19, Coulter, Hialeah, FL), monoclonal rabbit anti–active caspase 3 (Pharmingen, San Diego, CA), anti–Bcl-2 (Dako, Glostrup, Denmark), and PI9-17, which was developed in our laboratory.28 The polyclonal rabbit anti–PARP-1/p89 antibody (Promega, Madison, WI) was used to detect the cleaved form of PARP-1. All antibodies required antigen retrieval by microwave irradiation for 10 minutes in a citrate buffer (10 mM/L, pH 6.0, at 700 W), after which sections were incubated for 1 hour at room temperature (most antibodies) or incubated overnight (Bcl-2). For all antibodies, staining intensity was enhanced using the catalyzed reporter deposition method (Dako).
Staining for active caspase 3 and PI9 could not be performed in 12 and 19 cases, respectively, due to paraffin material not being available anymore.
Phenotypic analysis of active caspase 3–positive cells
Double stainings were performed for active caspase 3 and CD68 or CD30. Primary antibodies were incubated simultaneously, active caspase 3 being detected with a biotinylated polyclonal donkey antirabbit antibody and visualized with 3,3′-diaminobenzidine. Thereafter, CD68 or CD30 (KP1 and BerH2, respectively, Dako) were detected with an alkaline phosphatase–conjugated rabbit antimouse antibody, and alkaline phosphatase was visualized with new fuchsin/naphthol AS biphosphate. Negative controls included simultaneously processed sections with omission of the active caspase 3–, CD68-, and CD30-specific antibodies, respectively.
Quantification of neoplastic cells expressing apoptosis-related proteins
Percentages of procaspase 3–, active caspase 3–, PARP-1/p89–, Bcl-2–, and PI9-positive neoplastic cells were quantified using a commercially available interactive video overlay-based measuring system (Q-PRODIT, Leica, Cambridge, United Kingdom) as described previously.2 Briefly, numbers of positively staining tumor cells were expressed as percentages of all tumor cells present in a tissue section as judged by morphology. To avoid counting of active caspase 3–positive reactive lymphocytes, only unequivocally neoplastic cells with large nuclei (more than 3 times the size of a lymphocyte) were counted. Moreover, only cells with nuclear staining were counted to avoid counting macrophages with phagocytosed apoptotic debris (Figure 1A-D).
Detection of active caspase 3, PARP-1/p89, Bcl-2, and PI9 in tumor cells of systemic ALCL.
(A) Biopsy specimen of an ALCL patient with more than 5% active caspase 3–positive tumor cells (brown nuclear staining). (B) Biopsy specimen of the same ALCL patient with more than 5% PARP-1/p89–positive tumor cells (brown nuclear staining). Original magnification for panels A and B is × 200. (C) Biopsy specimen of an ALCL patient with more than 5% active caspase 3–positive tumor cells (brown nuclear staining) in combination with cytoplasmic staining of nuclear debris in cells with a macrophagelike morphology (arrows). (D) Double staining of active caspase 3 (brown) and CD68 (red) in a case of ALCL showing cytoplasmic staining of active caspase 3–positive debris. Apart from 2 CD68-negative cells with large, active caspase 3–positive nuclei, 3 CD68–positive cells are observed with active caspase 3–positive cytoplasmic debris (arrows). (E) Biopsy specimen of an ALCL patient with 50% or more Bcl-2–positive tumor cells (brown cytoplasmic staining). Reactive lymphocytes serve as an internal positive control. (F) Red cytoplasmic staining of PI9 in tumor cells of an ALCL case, with dendritic cells serving as an internal positive control. Original magnification for panels C-F is × 400.
Detection of active caspase 3, PARP-1/p89, Bcl-2, and PI9 in tumor cells of systemic ALCL.
(A) Biopsy specimen of an ALCL patient with more than 5% active caspase 3–positive tumor cells (brown nuclear staining). (B) Biopsy specimen of the same ALCL patient with more than 5% PARP-1/p89–positive tumor cells (brown nuclear staining). Original magnification for panels A and B is × 200. (C) Biopsy specimen of an ALCL patient with more than 5% active caspase 3–positive tumor cells (brown nuclear staining) in combination with cytoplasmic staining of nuclear debris in cells with a macrophagelike morphology (arrows). (D) Double staining of active caspase 3 (brown) and CD68 (red) in a case of ALCL showing cytoplasmic staining of active caspase 3–positive debris. Apart from 2 CD68-negative cells with large, active caspase 3–positive nuclei, 3 CD68–positive cells are observed with active caspase 3–positive cytoplasmic debris (arrows). (E) Biopsy specimen of an ALCL patient with 50% or more Bcl-2–positive tumor cells (brown cytoplasmic staining). Reactive lymphocytes serve as an internal positive control. (F) Red cytoplasmic staining of PI9 in tumor cells of an ALCL case, with dendritic cells serving as an internal positive control. Original magnification for panels C-F is × 400.
Analysis of clinical data
Survival time was measured from time of initial diagnosis until death due to ALCL or until end of follow-up. Patients who died of causes unrelated to the disease were censored at the time of death. Survival curves were constructed with the Kaplan Meier method, and differences between the curves were analyzed using the log-rank test. Univariate and multivariate analyses were performed with the Cox proportional hazard regression model (enter and remove limits 0.1). Qualitative variables were analyzed by Pearson χ2 test or by the Fisher exact test, when appropriate. All P values are based on 2-tail statistical analysis, and P values below .05 were considered significant. All analyses were performed using the SPSS statistical software package (version 9.0, SPSS, Chicago, IL). The optimal cutoff values for the percentage of active caspase 3– or Bcl-2–positive cells were determined via 2 methods: (1) using the log-rank test, by testing the prognostic value for each possible cutoff point (1%, 2%, 3%, et cetera, for active caspase 3 and 10%, 20%, 30%, et cetera, for Bcl-2); and (2) using Cox regression analysis, including all possible cutoff points as categorical variables. Both methods gave identical results.
Results
Caspase 3 activation occurs in less than 1% to 12% of tumor cells and results in cleavage of PARP-1
In all 52 ALCLs tested for active caspase 3, this effector caspase was detected as primarily nuclear staining of tumor cells and small reactive lymphocytes, whereas granular cytoplasmic staining in macrophages probably represented phagocytosed apoptotic debris (Figure 1A-C). Double stainings confirmed that exclusive cytoplasmic staining of active caspase 3 was restricted to CD68+ macrophages (Figure 1D). Double staining for active caspase 3 and CD30 did not reveal any double-positive cells. Apparently, CD30 expression is lost early in apoptosis, before activation of caspase 3. Therefore, discrimination between active caspase 3–positive neoplastic and reactive lymphoid cells was done only by the size of the nucleus.
The percentage of active caspase 3–positive tumor cells ranged from less than 1% to 12%, with a mean of 3.8%. In most cases, the number of active caspase 3–positive tumor cells correlated strongly with the number of PARP-1/p89–positive tumor cells, indicating the proper functioning of active caspase 3 (Figure 1B). The proenzyme form of caspase 3 was detected in most (50%-100%) tumor cells in all systemic ALCL cases, except 6 cases in which expression of procaspase 3 was restricted to less than 10% of tumor cells. These cases all showed low numbers (5% or less) of active caspase 3–positive tumor cells.
Expression of Bcl-2 and PI9 in neoplastic cells
Bcl-2 was detected as cytoplasmic staining in tumor cells in 48 of 64 ALCL cases. In all 64 cases, Bcl-2–positive reactive lymphocytes were found interspersed between tumor cells, serving as an internal positive control (Figure 1E). When positive, the percentage of Bcl-2–positive tumor cells ranged from 2% to 95%, with a mean of 40%.
PI9-expressing tumor cells were detected in 6 of 45 ALCL cases; staining was cytoplasmic (Figure 1F). Dendritic cells, which were previously shown to express PI9,26 served as an internal positive control. Cases were considered positive if clear staining was observed in at least 5% of the tumor cells. In positive cases, percentages of PI9-positive tumor cells ranged from 5% to 75%.
Percentages of active caspase 3–, Bcl-2–, and PI9-positive tumor cells are related to clinical outcome
Using Cox regression analysis, the influence on overall survival time of percentage of active caspase 3–positive tumor cells was estimated. Prognosis was found to be more favorable in cases with higher percentages of active caspase 3–positive tumor cells (entered as a continuous variable) (P = .02). When patients were subdivided into a group with 5% or less, or more than 5% active caspase 3–positive tumor cells (the threshold with strongest discriminative power), the presence of more than 5% active caspase 3–positive tumor cells identified a group of patients with a highly favorable prognosis. None of 13 ALCL patients with more than 5% active caspase 3–positive tumor cells died, compared with 17 of 39 patients with 5% or less active caspase 3–positive tumor cells (Table2; Figure2A).
Comparison of overall survival time in systemic nodal ALCL.
Comparison is according to the percentage of active caspase 3–positive tumor cells (A), the percentage of Bcl-2–positive tumor cells (B), and expression of PI9 by tumor cells (C).
Comparison of overall survival time in systemic nodal ALCL.
Comparison is according to the percentage of active caspase 3–positive tumor cells (A), the percentage of Bcl-2–positive tumor cells (B), and expression of PI9 by tumor cells (C).
When percentages of Bcl-2– and PI9-positive tumor cells were analyzed in a similar way, the prognosis was relatively poor in cases with high percentages of positive tumor cells (P = .002 andP = .003, respectively).
When patients were subdivided according to the percentage of Bcl-2–positive tumor cells (using a 50% cutoff value), a group with a favorable prognosis was identified (Table 2; Figure 2B). Only 11 of 47 ALCL patients with less than 50% Bcl-2–positive tumor cells died, compared with 11 of 17 patients with 50% or more Bcl-2–positive tumor cells. When patients were divided into groups with and without PI9 expression in tumor cells, only 11 of 39 patients with PI9-negative ALCL died, compared with all PI9-positive ALCL patients (Table 2; Figure 2C).
No significant relation between either Bcl-2 or PI9 expression and caspase 3 activation was observed, although expression of PI9 was observed only in cases with 5% or less active caspase 3–positive cells (Table 3).
Expression levels of active caspase 3, Bcl-2, and PI9 are related to ALK status
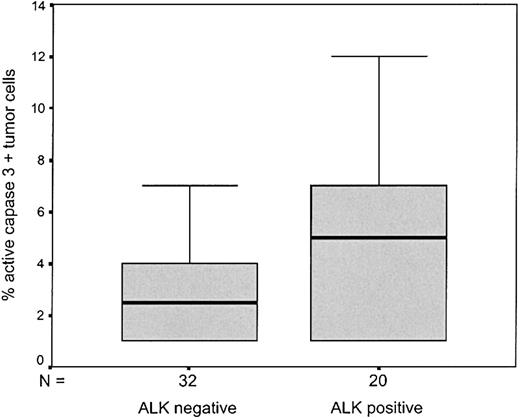
Expression levels of Bcl-2 and active caspase 3 were strongly related to ALK status (Table 4). ALK-positive ALCL contained more than 5% active caspase 3–positive tumor cells more frequently than ALK-negative cases (P = .002) (Figure 3). In contrast, cases with 50% or more Bcl-2–positive tumor cells were nearly completely restricted to ALK-negative cases (P = .001). Although the relationship was not significant, probably due to the small number of cases tested for PI9, expression of PI9 was entirely restricted to ALK-negative cases (Table 4).
Box plot depicting percentage of active caspase 3–positive cells in ALK-negative and ALK-positive ALCL.
The boxes are defined by the lower and upper quartiles; the median is marked by a subdivision of the box.
Box plot depicting percentage of active caspase 3–positive cells in ALK-negative and ALK-positive ALCL.
The boxes are defined by the lower and upper quartiles; the median is marked by a subdivision of the box.
Prognostic value of active caspase 3–, Bcl-2–, and PI9-positive tumor cells is retained in ALK-negative ALCL
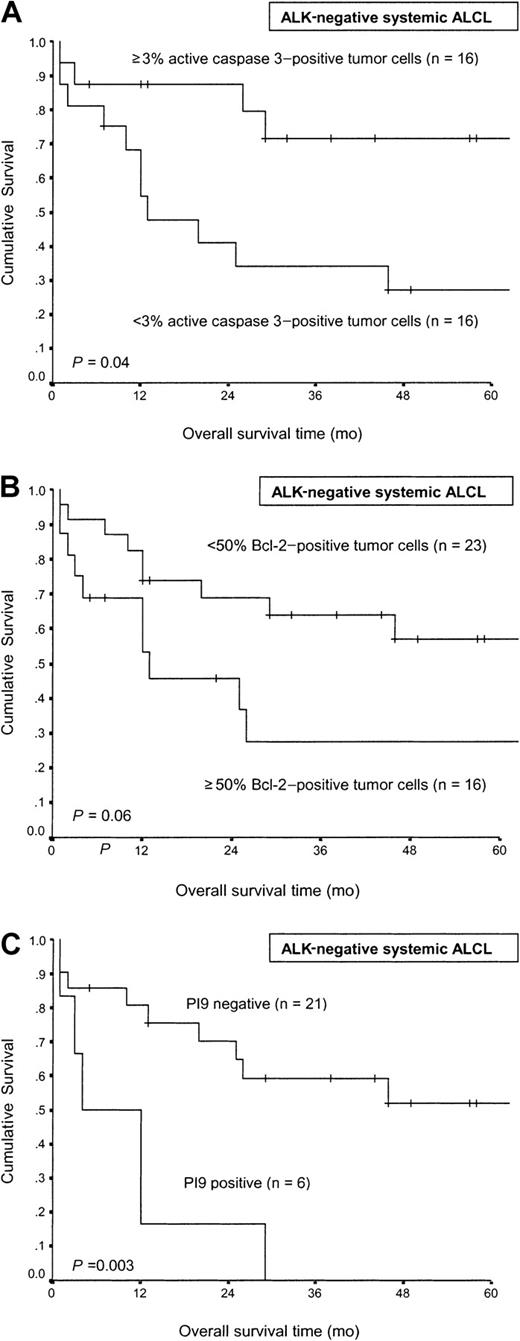
In ALK-negative cases the median number of caspase 3–positive cells was 3% (Figure 3). When 3% was taken as cutoff value, the number of active caspase 3–positive tumor cells retained its prognostic value also in ALK-negative ALCL patients (P = .04, Figure 4A).
Comparison of overall survival time in ALK-negative systemic nodal ALCL.
Comparison is according to the percentage of active caspase 3–positive tumor cells (A), the percentage of Bcl-2–positive tumor cells (B), and expression of PI9 by tumor cells (C).
Comparison of overall survival time in ALK-negative systemic nodal ALCL.
Comparison is according to the percentage of active caspase 3–positive tumor cells (A), the percentage of Bcl-2–positive tumor cells (B), and expression of PI9 by tumor cells (C).
In multivariate survival analysis ALK status and Bcl-2 are independent prognostic markers
In Cox regression analysis of overall survival time, including all 64 ALCL cases, percentages of Bcl-2–positive tumor cells (entered as a continuous variable, P = .001) and ALK status (P = .001) remained independent prognostic markers, while percentages of active caspase 3– and PI9-positive tumor cells gave no additional information. When only ALK-negative ALCLs were included in the analysis, the percentage of Bcl-2–positive tumor cells appeared to be the strongest prognostic marker (P = .014). Again, percentages of active caspase 3– and PI9-positive tumor cells gave no additional information.
Discussion
In this study, we have shown that in systemic ALCL expression of apoptosis-regulating proteins Bcl-2 and PI9 and activation of caspase 3 are strongly related to ALK status. In addition, we found that numbers of active caspase 3–, Bcl-2–, and PI9–positive tumor cells predict clinical outcome in systemic ALCL cases. Moreover, the 3 markers retained their prognostic value when only ALK-negative ALCLs were investigated. Proper functioning of active caspase 3, with execution of apoptosis, was demonstrated by the concomitant detection of its cleaved substrate PARP-1/p89 in comparable numbers of tumor cells.
Our results indicate that the difference in clinical outcome between ALK-positive and ALK-negative cases may well be explained by differences in levels of apoptosis inhibition. These results are in line with a recent paper by Rassidakis et al.29 Using a modified terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling assay, these authors demonstrated that levels of apoptosis are relatively high in ALK-positive cases and are related to expression levels of members of the Bcl-2 protein family. Although most ALK-positive cases run a highly favorable course,2-4 the individual prognosis of patients with ALK-negative ALCL remains highly variable.2 Our study clearly shows that caspase 3 activation and, in particular, expression of Bcl-2 and PI9 can also be used to predict clinical outcome in patients with ALK-negative ALCL.
In vitro data indicate that a poor response to therapy in lymphomas may be caused by inhibition of the apoptosis cascade.5-8 The strong relationship between high numbers of active caspase 3–positive tumor cells and excellent prognosis supports this view and suggests that a favorable response to chemotherapy depends, at least partially, on activation of downstream effector caspases. However, functional studies on isolated lymphoma cells are necessary to determine whether ALCL cases with many active caspase 3–positive tumor cells are indeed more sensitive to the apoptosis-inducing effect of chemotherapy than cases harboring low numbers of active caspase 3–positive tumor cells. Cell lines appear to be less suitable to test this hypothesis because cell lines are strongly selected for survival in culture and may acquire additional lesions that positively or negatively affect treatment sensitivity, as recently demonstrated by Schmitt et al.30
The apoptosis-inhibiting function of Bcl-2 has been shown to involve inhibition of the stress-induced apoptosis pathway by interfering with either mitochondrial cytochrome C release or by directly inhibiting the function of caspase 9.9 Because the stress-induced apoptosis pathway is primarily involved in chemotherapy-induced cell death, the strong relation between high numbers of Bcl-2–positive tumor cells and poor prognosis can well be explained.31The same prognostic effect of Bcl-2 expression has been demonstrated extensively in other aggressive lymphoma types.22 23
Why expression of PI9 in tumor cells should result in poor prognosis is less understandable, because PI9 appears to be a specific granzyme B inhibitor. Thus, although PI9 might inhibit granzyme B–induced cell death, its expression does not explain why tumor cells respond poorly to chemotherapy. A possible explanation is that PI9 not only inhibits granzyme B but also some caspases,32 33 which may render PI9-positive tumor cells less susceptible to chemotherapy.
Recently, it was shown in vitro that ALK expression activates the phosphatidylinositol 3 kinase/Akt pathway.34 Akt is able to phosphorylate the proapoptotic Bad (a member of the Bcl-2 family), thereby preventing the protein from binding to and inhibiting the antiapoptotic members Bcl-2 and Bcl-XL.35 36Although the number of active caspase 3–positive cells is relatively high in ALK-positive cases, some level of apoptosis inhibition may well exist in these cases. However, this apparently has relatively little influence on sensitivity to chemotherapy, as indicated by the favorable prognosis of ALK-positive cases.
High levels of Bcl-2 and/or PI9 expression could not explain low levels of caspase 3 activation and/or a fatal outcome in all cases. Alternative apoptosis-inhibiting proteins that might be involved are members of the inhibitors of apoptosis family,37 other antiapoptotic members of the Bcl-2 family, and FLIP (FLICE or caspase 8–inhibiting protein).38 In addition, resistance to apoptosis may also be conferred by loss of function of caspases or other proteins involved in the apoptosis cascade. In 6 cases with low levels of caspase 3 activation, we found expression of procaspase 3 in less than 10% of the tumor cells, suggesting that down-regulation of procaspase 3 or defects in its encoding genes may cause defective caspase 3 activation. Loss of APAF-1 function, crucial for caspase 9 activation, may also cause defective caspase 3 activation, as was recently demonstrated in melanoma cells.39
We have previously shown that a high percentage of activated (granzyme B–positive) CTLs in the reactive infiltrate of ALCL is strongly related to poor prognosis2 and hypothesized that in cases with poor clinical outcome a selection for apoptosis-resistant tumor cells has taken place. Indeed, it appeared that cases with many granzyme B–positive infiltrating CTLs always demonstrate low numbers of active caspase 3–positive tumor cells (data not shown). The presence of an immunogenic pressure caused by high numbers of CTLs is further supported by the fact that expression of PI9 was found primarily in cases harboring many granzyme B–positive infiltrating lymphocytes (data not shown).
We conclude that high numbers of active caspase 3–positive tumor cells predict a highly favorable clinical outcome in systemic ALCL patients and that the presence of high numbers of Bcl-2– and PI9-positive tumor cells are strong predictors of a poor clinical outcome. These data support the notion that a favorable response to chemotherapy depends on an intact apoptosis cascade. Expression levels of active caspase 3, Bcl-2, and PI9 are strongly related to ALK status, underlining the intrinsic differences between ALK-positive and ALK-negative ALCL and providing an explanation for their differences in clinical outcome.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Chris J. L. M. Meijer, VU University Medical Center, Dept of Pathology, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands; e-mail: cjlm.meijer@vumc.nl.