Decreased thymopoietic capacity contributes to the severe and clinically significant immune deficiency seen after bone marrow transplantation (BMT). One mechanism for thymopoietic failure is damage to the interleukin 7 (IL-7)–producing thymic epithelial cells (TECs) by irradiation and chemotherapy, which can be partially treated by IL-7 administration. Pretreatment of BMT recipients with keratinocyte growth factor (KGF, or Fgf7), an epithelial cell–specific growth factor, protects mucosal, cutaneous, and pulmonary epithelial cells from cytotoxic therapy-induced damage in experimental murine models. Like other epithelial cells, TECs specifically express KGF receptors. Because KGF specifically protects KGF receptor–bearing epithelial cells and post-BMT immune deficiency is caused by loss of TECs, we hypothesized that KGF pretreatment would improve post-BMT thymic function. To test the hypothesis, BMT recipient mice were given KGF or placebo prior to congenic or allogeneic BMT. Administration of KGF before murine BMT significantly increased the capacity of the thymus to generate donor-derived thymocytes. KGF pretreatment also normalized the proportion of thymic subpopulations, increased the number of naive T cells in the periphery, and improved the response to neoantigen immunization. KGF treatment caused increased production of intrathymic IL-7, and the thymopoietic effects of KGF required an intact IL-7 signaling pathway. These results demonstrate that KGF may have immunomodulatory effects by a unique mechanism of protection of TECs. Furthermore, thymic injury and prolonged posttransplantation immune deficiency in BMT recipients can be prevented by KGF administration.
Introduction
Post–bone marrow transplantation (BMT) immune deficiency causes significant morbidity and mortality in BMT recipients.1-3 Despite hematopoietic stem cell (HSC) engraftment and recovery of marrow function, recipients have prolonged defects in generation of functional T lymphocytes.4-6Although infections related to neutropenia predominate in the immediate posttransplantation period, infections occurring after myeloid engraftment are generally due to bacterial, fungal, and viral pathogens that are controlled by the adaptive immune system.7 The clinical significance of this problem is demonstrated by the high incidence of infectious complications in patients who received higher doses of pre-BMT cytotoxic therapy, donor HSC sources depleted of mature T lymphocytes, and HSCs from HLA-mismatched donors. Factors contributing to post-BMT immune deficiency are recipient age, radiation and other pretransplantation cytotoxic therapies, and graft-versus-host disease (GVHD). Normal thymopoiesis depends on the interaction of the thymic stroma–derived cytokine interleukin 7 (IL-7) with its receptor.8,9 IL-7 production by a subset of thymic epithelial cells (TECs) is required for normal thymic differentiation, proliferation, and survival.9-12 Damage to TECs is one mechanism by which pre-BMT conditioning or GVHD may impair the ability to generate mature T lymphocytes after BMT.13
Specific cytokines have been shown to regulate the development and proliferation of epithelial cells. Keratinocyte growth factor (KGF), a member of the acidic fibroblast growth factor (Fgf) family, is produced by fibroblasts and many types of mesenchymal cells.14 KGF, which is also known as Fgf7, binds exclusively to an epithelial cell–specific splice variant of the fibroblast growth factor receptor-2 family (FgfR2-IIIb or KGFR).14,15 Mice that are deficient in FgfR2-IIIb have significant defects in thymopoiesis with decreased thymic cellularity and evidence of a maturational block in TEC development.16 In addition to KGF, FgfR2-IIIb binds to Fgf1, Fgf3, and Fgf10.15 17-22 The ligands for the FgfR2-IIIb are generally produced by mesenchymal tissues.
Besides its proliferative effects on epithelial cells, KGF protects epithelial cells from injury induced by chemotherapy, radiation, and oxidative stress in murine models.22-26 In murine GVHD models, KGF pretreatment decreased damage to epithelial cells such as skin and gastrointestinal mucosa, resulting in significantly reduced morbidity and mortality.27-29 We hypothesized that KGF pretreatment would prevent TEC damage after BMT, leading to increased IL-7 production and improved thymopoiesis. Here, we demonstrate the protective effect of pre-BMT administration of KGF on thymopoiesis after BMT.
Materials and methods
Animals
The 6- to 8-week-old BALB/c (H2d), C57BL/6J (H2b)(CD45.2), and B6.SJL (H2b)(CD45.1) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The mice were rested for at least 5 days after receipt before transplantation. IL-7−/− mice on a C57BL/6J background (gift from Dr R. Murray) were bred at Childrens Hospital Los Angeles and maintained there and at the University of Minnesota. All of the protocols were approved by the Animal Care Committee of Childrens Hospital Los Angeles and the University of Minnesota.
BMT procedure
Recombinant human KGF (Amgen, Thousand Oaks, CA) was administered to recipients subcutaneously for 3 consecutive days (5 mg/kg per day) before cytotoxic treatment. Congenic BMT from B6.SJL donors to C57BL6/J mice was performed after 650, 1000, and 1400 cGy irradiation as previously described.13,30 Allogeneic BMT studies were performed using pan–T cell depleted bone marrow obtained from BALB/c donors, which was administered to C57BL/6 recipients who had received either 900 cGy irradiation alone or cyclophosphamide (120 mg/kg per day for 2 days) followed by 750 cGy irradiation, as previously described.27 In this model and in the present experiments, the allogeneic recipients did not develop GVHD clinically or histologically.
Fluorescence-activated cell sorter analyses
Single-cell suspensions of thymocytes and splenocytes were prepared by collagenase digestion. Samples of 1 × 106cells were washed with ice-cold phosphate-buffered saline (PBS) and incubated with optimal concentrations of monoclonal antibodies directed against CD3, CD4, CD8, CD45.1, CD45.2, Thy1.2, CD44, CD45RB, and I-E/I-A (B-D Pharmingen, San Diego, CA) for 30 minutes at 4°C, as described previously.13,27 30 Antibody against CD45.1 was used to detect the congenic marker expressed by the donor B6.SJL cells, and CD45.2 was used to detect recipient-derived C57BL/6J cells. A total of 10 000 gated events/sample were acquired on a FACScalibur flow cytometer (B-D Immunocytometry Systems, San Jose, CA) and analyzed with CellQuest software.
Reverse transcription–polymerase chain reaction
RNA from each sample was isolated by using RNagent (Promega, Madison, WI). The primer sequences and polymerase chain reaction (PCR) conditions were as follows: IL-7 (A), 5′-GTGGGCCGCTCTAGGCACCAA-3′; IL-7 (B), 5′-GGACATTGAATTCTTCACTG-3′; stem cell factor (SCF) (A), 5′-AATCTCCGAAGAGGCCAGAA-3′; SCF (B), 5′-CCATGGCTGTCCATTGTAGG-3′; FgfR2-IIIb (A), 5′-AACGGTCACCACACCGGC-3′; KGFR (B), 5′-AGGCAGACTGGTTGGCCTG-3′; KGF (A), 5′-ATCCTGCCAACTCTGCTCTACAGA-3′; KGF (B), 5′-CTTCCCTTTGACAGGAATCCCCTT-3′; β-actin (A), 5′-GTGGGCCGCTCTAGGCACCAA-3′; and β-actin (B), 5′-GGACATTGAATTCTTCACTG-3′. Each reaction was done at 94°C for 1 minute for denaturation, at optimal annealing temperature (IL-7, 52°C; SCF, 55°C; FgfR2-IIIb, 56°C; KGF, 57°C; and β-actin, 52°C) for 1 minute and at 72°C for 1 minute for elongation. PCR products were analyzed by ethidium bromide staining of 1.5% agarose gels.
Sheep red blood cell immunization
Sheep red blood cell (SRBC) immunization was performed as previously described.30 Briefly, between days 28 and 34 after BMT and again 14 days later, SRBCs (Colorado Serum, Denver, CO) were administered to the BMT recipients by intraperitoneal injection. Blood was obtained at 14 days after the primary and secondary immunization. The average anti-SRBC antibody titer in the serum was determined by serial dilution of the samples and duplicate measurement of agglutination of SRBCs in 96-well V-bottom microplates.
In situ hybridization
Cryosections (5 μm) were hybridized with a digoxigenin-labeled antisense mouse IL-7 RNA probe, representing nucleotides 608-1103. The probe was generated by reverse transcription (RT)–PCR of RNA from murine marrow cells, using upstream 5′-GCCTGTCACATCATCTGAGTGCC-3′ and downstream 5′-CAGGAGGCATCCAGGAACTTCTG-3′ primers. The product was ligated into the pCRII plasmid (Invitrogen, Carlsbad, CA) by TA cloning, and the orientation was confirmed by restriction mapping. The antisense riboprobe was transcribed with Sp6 RNA polymerase from plasmid linearized with XbaI, labeled with digoxigenin-uridine triphosphate, and hybridized as described.31 Immunologic detection of RNA duplexes was accomplished with alkaline phosphatase-conjugated antidigoxigenin antibody and NBT/BCIP substrate (Boehringer Mannheim, Indianapolis, IN).
Statistical analysis
Differences between groups were analyzed by 2-tailedt test with unequal distributions.
Results
Expression of FgfR2-IIIB by TECs
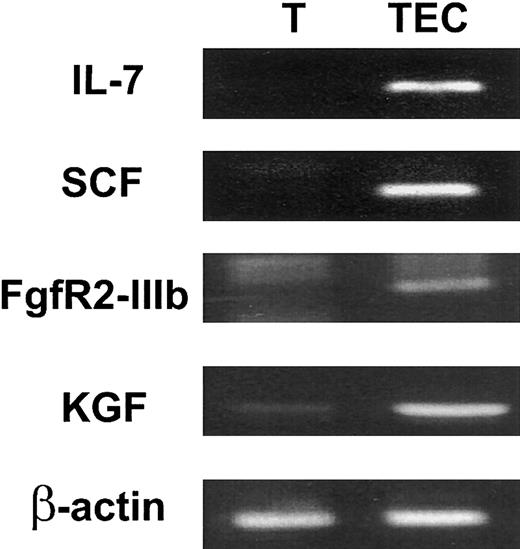
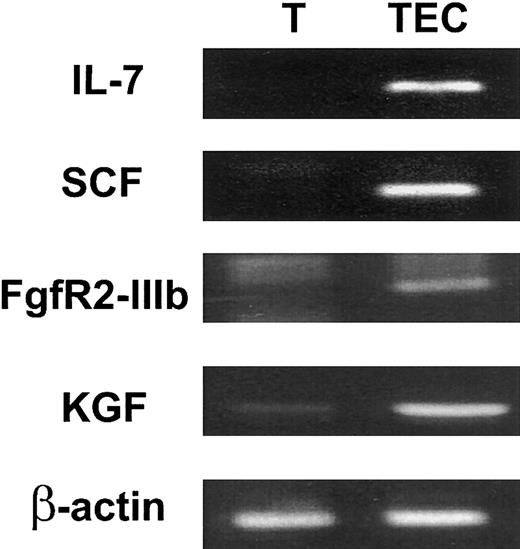
To determine whether KGF might be biologically relevant for thymopoiesis, the expression of KGF and FgfR2-IIIB by intrathymic subpopulations was examined. TECs were enriched by FACS sorting of CD45− major histocompatibility complex (MHC) class II+ stromal cells. Expression of the 2 TEC-derived cytokines IL-7 and SCF confirmed that the relevant TEC population was present in the sorted cells (Figure 1). FgfR2-IIIb was specifically expressed by CD45− MHC II+ TECs but not by CD45+ thymocytes (Figure1). In contrast, KGF expression was detected in both stroma and thymocyte populations. KGF expression was found in thymocytes at all stages of differentiation, including CD3−CD4−CD8− (triple-negative, TN), CD4+CD8+ (double-positive, DP), and CD4+ or CD8+ single-positive (SP) cells (data not shown). The expression of KGF by thymocytes and of FgfR2-IIIb by TECs indicates that an intrathymic KGF signaling pathway between thymocytes and epithelial cells might exist.
KGF and FgfR2-IIIb are expressed intrathymically.
CD45+ thymocytes (T) and CD45− MHC class II+ stromal cells (TECs) were FACS isolated. The CD45− MHC class II+ cells included TECs and fibroblasts. Expression of the TEC-derived cytokines, IL-7 and SCF, and FgfR2-IIIb and KGF by intrathymic subpopulations was analyzed by RT-PCR. β-actin was used as a control for loading of RNA.
KGF and FgfR2-IIIb are expressed intrathymically.
CD45+ thymocytes (T) and CD45− MHC class II+ stromal cells (TECs) were FACS isolated. The CD45− MHC class II+ cells included TECs and fibroblasts. Expression of the TEC-derived cytokines, IL-7 and SCF, and FgfR2-IIIb and KGF by intrathymic subpopulations was analyzed by RT-PCR. β-actin was used as a control for loading of RNA.
Effects of KGF on thymic reconstitution in BMT recipients
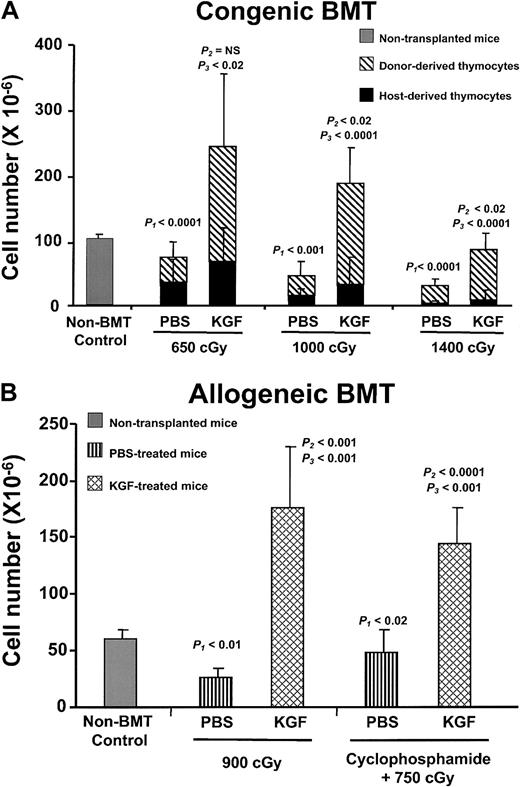
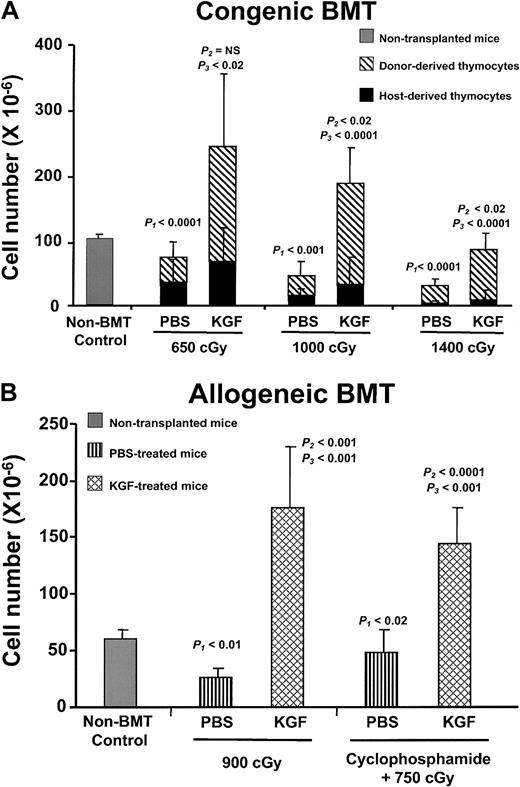
To examine the effect of KGF on thymopoietic capacity, graded doses of irradiation (650, 1000, and 1400 cGy) were given to congenic BMT recipients. Recipient mice received either daily KGF or placebo (PBS) injections for 3 days before irradiation. The number of total thymocytes decreased in a radiation dose-dependent manner in both the PBS- and KGF-treated groups (Figure 2A). Compared with the PBS controls, the KGF-treated groups had significantly increased thymic cellularity at all radiation doses, when examined at day 28 after BMT. KGF-treated mice had at least 3-fold more thymocytes than PBS controls. Importantly, thymic cellularity from KGF-treated mice after 650 or 1000 cGy was greater than that of non-BMT controls. Even after 1400 cGy, the thymic cellularity of KGF-treated mice was almost 70% that of non-BMT controls by day 28 after BMT. In contrast, the thymic cellularity of PBS-treated BMT controls was only 3% to 60% of non-BMT controls. The differences in thymic cellularity were due to increased numbers of donor-derived thymocytes, not preservation of radioresistant recipient-derived thymocytes (Figure 2A).
KGF pretreatment increases post-BMT thymopoiesis.
(A) Mean absolute thymocyte numbers ± SD from non-BMT control (n = 5) and either PBS- or KGF-treated congenic BMT recipients (n = 9-10 per group) conditioned with 3 doses of radiation (650, 1000, and 1400 cGy) were analyzed at day 28 after BMT. Gray bar, non-BMT control; diagonally hatched bar, donor-derived thymocytes; black bar, host-derived thymocytes.P1 = non-BMT control versus PBS;P2 = non-BMT control versus KGF;P3 = PBS versus KGF. (B) Mean absolute thymocyte numbers ± SD from either PBS- (vertical hatched bar) or KGF-treated (cross-hatched bar) allogeneic BMT recipients (n = 8 per group), conditioned with either 900 cGy irradiation alone or cyclophosphamide plus 750 cGy, were analyzed at day 28 after BMT.
KGF pretreatment increases post-BMT thymopoiesis.
(A) Mean absolute thymocyte numbers ± SD from non-BMT control (n = 5) and either PBS- or KGF-treated congenic BMT recipients (n = 9-10 per group) conditioned with 3 doses of radiation (650, 1000, and 1400 cGy) were analyzed at day 28 after BMT. Gray bar, non-BMT control; diagonally hatched bar, donor-derived thymocytes; black bar, host-derived thymocytes.P1 = non-BMT control versus PBS;P2 = non-BMT control versus KGF;P3 = PBS versus KGF. (B) Mean absolute thymocyte numbers ± SD from either PBS- (vertical hatched bar) or KGF-treated (cross-hatched bar) allogeneic BMT recipients (n = 8 per group), conditioned with either 900 cGy irradiation alone or cyclophosphamide plus 750 cGy, were analyzed at day 28 after BMT.
One manifestation of the thymopoietic defect in BMT recipients is abnormal progression from the TN to DP stages of thymocyte differentiation, resulting in an increased proportion of TN cells and decreased proportion of DP cells.13,30 Normally, immature TN cells are a minor subpopulation, whereas DP cells represent the majority of thymocytes. As had been observed in previous studies, the PBS-treated congenic recipients had increased frequency of TN cells at day 28 after BMT, compared with the non-BMT controls (Table1).13 30 In contrast, KGF administration appeared to release the block from the TN to DP stage. The absolute number of all donor-derived thymic subpopulations (TN, DP, CD4+ SP, and CD8+ SP) was significantly increased in the KGF groups (Table 1 and data not shown). Thus, KGF administration pre-BMT enhanced the development of thymocytes from immature progenitors after BMT.
After clinical BMT, the thymopoietic capacity, peripheral T-lymphocyte numbers, and immune function are significantly worse in recipients of allogeneic HSCs than in recipients of autologous HSCs. To test the effects of KGF on thymopoietic capacity after allogeneic BMT, the clinical situation was simulated by a fully allogeneic BMT model by using T-cell–depleted marrow. To further simulate clinical BMT, some groups of mice received radiation and cyclophosphamide, which is frequently used as pre-BMT immunosuppression. Recipient C57BL/6J mice were conditioned with irradiation only or cyclophosphamide plus irradiation, followed by BMT of MHC-mismatched marrow from BALB/c donors. Similar to the results in congenic BMT recipients, KGF pretreatment increased thymic cellularity by 3- to 6-fold to nearly normal levels at day 28 after BMT, depending on the conditioning regimen tested (Figure 2B). Post-BMT thymic cellularity was severely impaired in the control allogeneic recipients. Furthermore, thymic differentiation and generation of all thymic subpopulations (TN, DP, CD4+ SP, and CD8+ SP) in the allogeneic recipients were also increased by KGF administration (Table 1). These data indicate that KGF prevented loss of thymopoietic capacity caused by irradiation alone or in combination with chemotherapy in a fully allogeneic model of BMT.
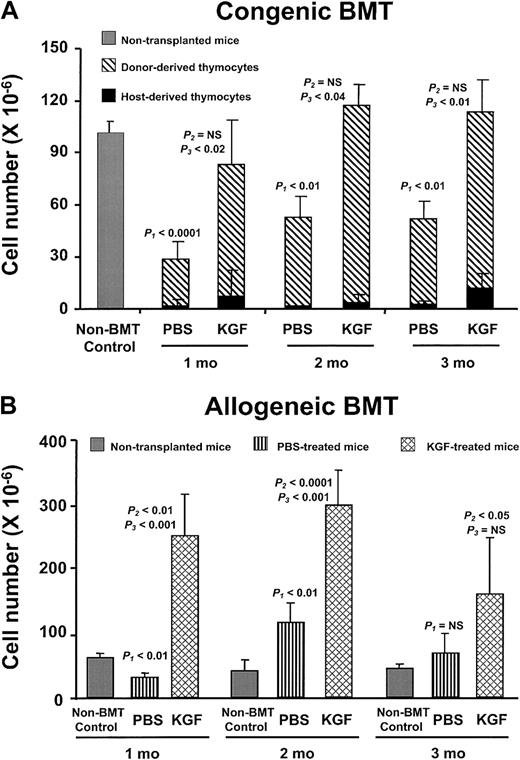
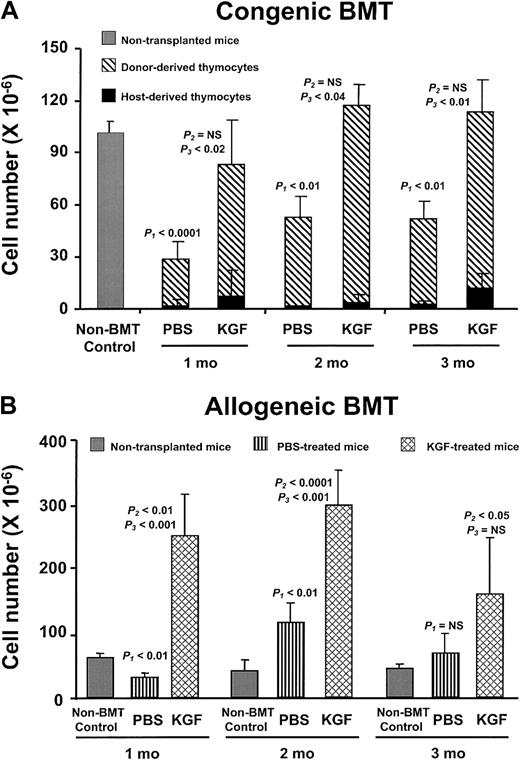
Administration of other cytokines, eg, IL-7, after BMT increases thymopoietic capacity immediately after transplantation, but thymic cellularity decreases after termination of the treatment (K.I.W., unpublished observations, December 2001).30 In contrast, the thymic cellularity after KGF pretreatment continued to rise and was sustained for at least 3 months after BMT in both the congenic and allogeneic recipients (Figure 3A,B). The number of thymocytes in the allogeneic PBS recipients also rebounded to greater than the non-BMT controls, but the KGF recipients always had significantly greater thymic cellularity than either the PBS-treated or non-BMT control mice. The prolonged alterations in thymopoietic capacity are remarkable, because only a single brief course of KGF treatment had been given prior to BMT.
KGF durably improves post-BMT thymopoiesis.
(A) PBS- or KGF-treated congenic BMT recipients after 1400 cGy conditioning analyzed over the 3 months following BMT. Shown are mean absolute thymocyte numbers ± SD. Gray bar, non-BMT control; diagonally hatched bar, donor-derived thymocytes; black bar, host-derived thymocytes. (B) PBS- (vertically hatched bar) or KGF-treated (cross-hatched bar) allogeneic BMT recipients after 900 cGy conditioning analyzed over the 3 months following BMT. Shown are mean absolute thymocyte numbers ± SD.
KGF durably improves post-BMT thymopoiesis.
(A) PBS- or KGF-treated congenic BMT recipients after 1400 cGy conditioning analyzed over the 3 months following BMT. Shown are mean absolute thymocyte numbers ± SD. Gray bar, non-BMT control; diagonally hatched bar, donor-derived thymocytes; black bar, host-derived thymocytes. (B) PBS- (vertically hatched bar) or KGF-treated (cross-hatched bar) allogeneic BMT recipients after 900 cGy conditioning analyzed over the 3 months following BMT. Shown are mean absolute thymocyte numbers ± SD.
Increased mature T lymphocytes in the KGF-treated BMT recipients
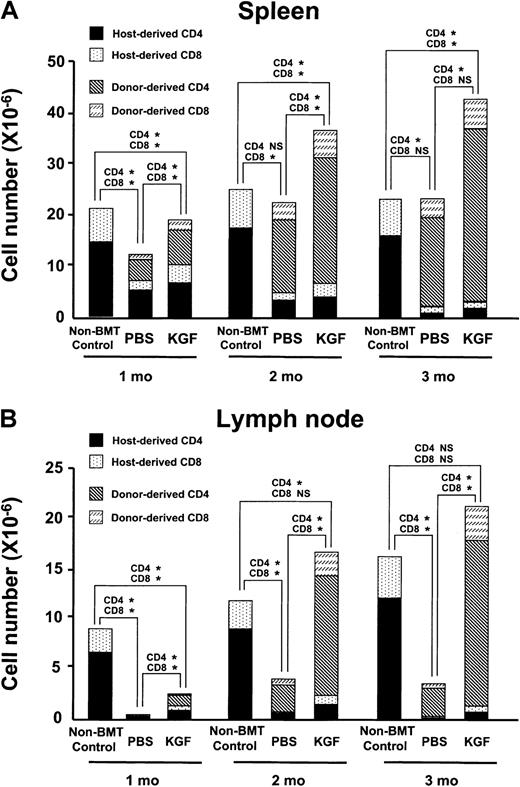
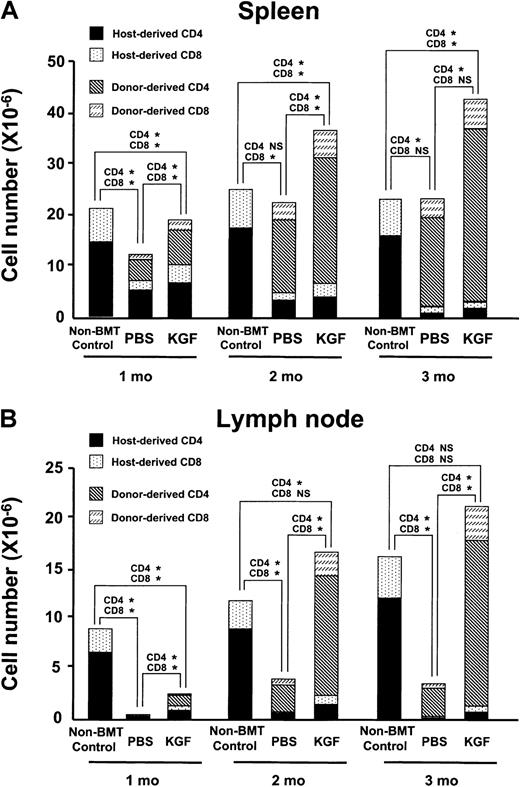
To determine whether increased thymopoiesis induced by KGF could lead to greater reconstitution of peripheral T-lymphocyte numbers and function, the number of splenic and lymph node (LN) T lymphocytes was measured in both the congenic and allogeneic recipients over the first 3 months after BMT.32 33 In the congenic recipients, the absolute number of donor-derived splenic T lymphocytes did not recover until 2 to 3 months after BMT, and the KGF group was not significantly greater than the PBS controls (data not shown). In contrast, the number of donor-derived T lymphocytes in the spleens of the allogeneic recipients was significantly greater in the KGF group than in the PBS controls at all time points examined (Figure4A). Because allogeneic recipients typically experience profound defects in peripheral T-cell reconstitution that can be readily identified by examining the LN compartment, the effect of KGF pretreatment on LN cellularity and phenotype was examined (Figure 4B and data not shown). Remarkably, by 2 months after BMT, the KGF group had normalized their LN compartment. The absolute number of CD4+ T cells, CD8+ T cells, and B cells was 4, 5, and 3 times higher, respectively, in the KGF group than in the PBS control.
KGF pretreatment improves reconstitution of peripheral T-lymphocyte populations.
(A) The mean absolute number of donor-derived and host-derived mature T lymphocytes in the spleens of allogeneic BMT recipients (n = 4-5 in each group) conditioned with 900 cGy radiation determined over 3 months after BMT. P values of comparisons of donor-derived CD4 and CD8 cells are shown above bars: *P < .02; NS, P > .05. (B) The mean absolute cell number of donor-derived and host-derived T lymphocytes in the lymph node of allogeneic BMT recipients conditioned with 900 cGy radiation determined over 3 months after BMT (n = 4-5 in each group).P values of comparisons of donor-derived CD4 and CD8 cells are shown above bars: *P < .04; NS,P > .05.
KGF pretreatment improves reconstitution of peripheral T-lymphocyte populations.
(A) The mean absolute number of donor-derived and host-derived mature T lymphocytes in the spleens of allogeneic BMT recipients (n = 4-5 in each group) conditioned with 900 cGy radiation determined over 3 months after BMT. P values of comparisons of donor-derived CD4 and CD8 cells are shown above bars: *P < .02; NS, P > .05. (B) The mean absolute cell number of donor-derived and host-derived T lymphocytes in the lymph node of allogeneic BMT recipients conditioned with 900 cGy radiation determined over 3 months after BMT (n = 4-5 in each group).P values of comparisons of donor-derived CD4 and CD8 cells are shown above bars: *P < .04; NS,P > .05.
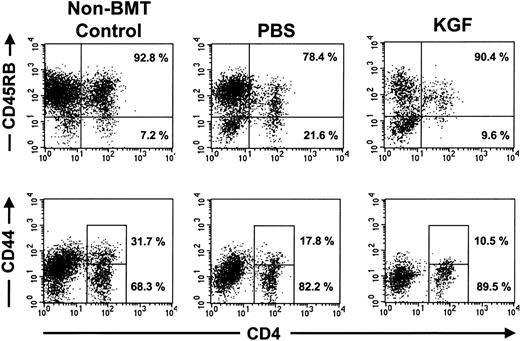
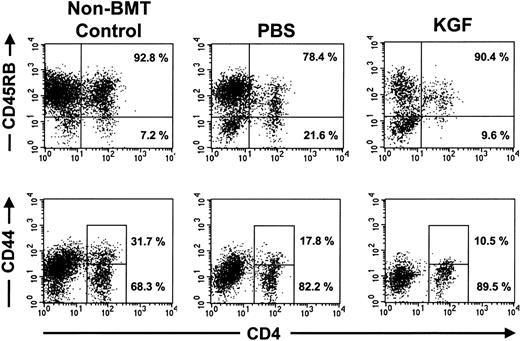
The increased numbers of T lymphocytes observed in the KGF recipients could be due to either increased generation of naive T lymphocytes or peripheral expansion of mature T lymphocytes. Naive CD4+ T lymphocytes are CD45RBhigh and CD44low.33 Analyses of the donor-derived CD4+ T lymphocytes in the congenic recipients after 1000 or 1400 cGy demonstrated that the CD4+ T lymphocytes resulting from KGF pretreatment were predominantly CD45RBhigh and CD44low naive cells (Figure5). The presence of naive T lymphocytes and the kinetics of peripheral lymphoid reconstitution indicate that most of the effects of KGF were mediated by increased production of naive T lymphocytes, not peripheral expansion.
Peripheral CD4 T lymphocytes in KGF-treated mice were mostly CD45RB+ CD44 low naive T lymphocytes.
FACS analyses of splenocytes from representative nontransplanted mice and congenic BMT recipients that were conditioned with 1400 cGy, examined at day 28 after BMT. Shown are the analyses on gated CD45.1+ donor cells. The percentage of the CD4 T lymphocytes that were CD45RBhigh and CD44loware shown. Similar results were observed in 4 to 5 mice from each treatment arm.
Peripheral CD4 T lymphocytes in KGF-treated mice were mostly CD45RB+ CD44 low naive T lymphocytes.
FACS analyses of splenocytes from representative nontransplanted mice and congenic BMT recipients that were conditioned with 1400 cGy, examined at day 28 after BMT. Shown are the analyses on gated CD45.1+ donor cells. The percentage of the CD4 T lymphocytes that were CD45RBhigh and CD44loware shown. Similar results were observed in 4 to 5 mice from each treatment arm.
T-cell–dependent antibody responses
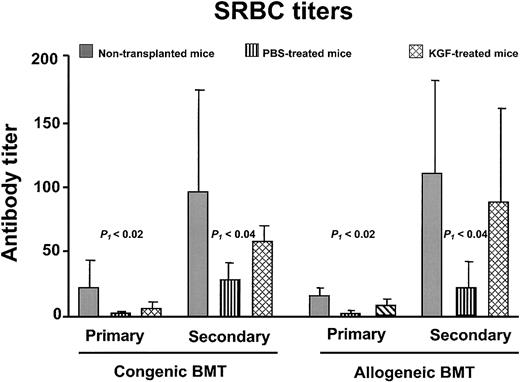
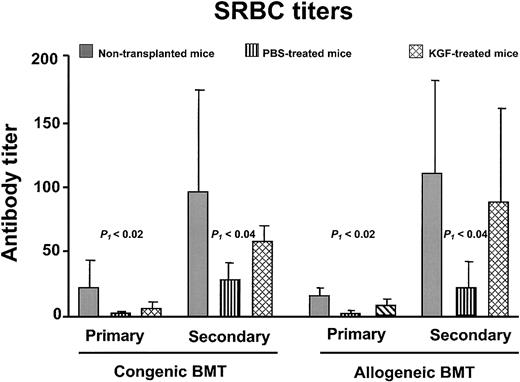
Failure of T-cell–dependent antigen-specific responses is commonly observed in BMT recipients.4,30,34 35 To determine whether the increased numbers of T lymphocytes resulted in improved immune function, congenic and allogeneic BMT recipients were challenged with a neoantigen (SRBCs). SRBC hemagglutinin is a T-cell–dependent antigen to which neither the donor nor recipient mice had been previously exposed. Mice received primary immunization between days 28 to 34 and secondary immunization 14 days later. Both the primary and secondary anti-SRBC titers of PBS-treated BMT recipients were significantly less than normal (P < .05), whereas the KGF recipients had comparable titers to normal nontransplanted control mice (Figure 6). Besides having an increased anti-SRBC response, the KGF recipients underwent normal immunoglobulin class-switching, as the secondary response is composed of more than 50% immunoglobulin G (data not shown). Thus, the increased thymopoiesis and naive T-cell generation induced by KGF resulted in improved immune responses to a T-cell–dependent neoantigen.
KGF pretreatment improves anti-SRBC titers after BMT.
Primary immunization was performed at day 28 and secondary immunization at day 42 after BMT. The congenic BMT recipients received conditioning with 1000 cGy, and the allogeneic recipients received 900 cGy (n = 4-5 in each group). Gray bar, nontransplanted controls; vertical hatched bar, PBS-treated; cross-hatched bar, KGF-treated. P1 indicates non-BMT control versus PBS; the non-BMT control and KGF groups were not significantly different.
KGF pretreatment improves anti-SRBC titers after BMT.
Primary immunization was performed at day 28 and secondary immunization at day 42 after BMT. The congenic BMT recipients received conditioning with 1000 cGy, and the allogeneic recipients received 900 cGy (n = 4-5 in each group). Gray bar, nontransplanted controls; vertical hatched bar, PBS-treated; cross-hatched bar, KGF-treated. P1 indicates non-BMT control versus PBS; the non-BMT control and KGF groups were not significantly different.
Augmented intrathymic IL-7 expression induced by KGF pretreatment
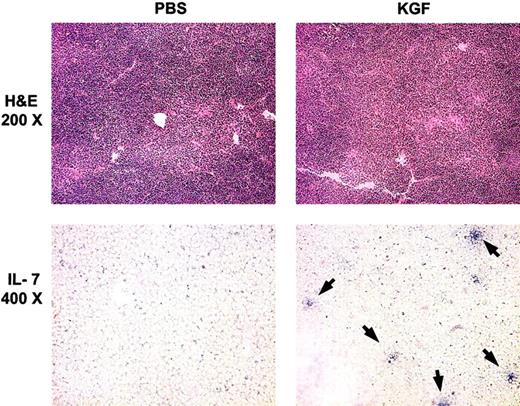
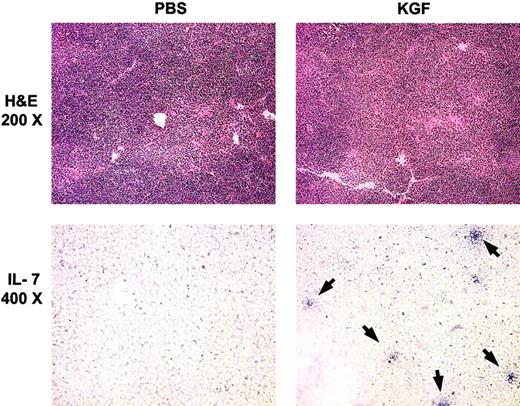
Previous studies have demonstrated that pre-BMT radiation treatments caused decreased intrathymic IL-7 transcripts and that administration of IL-7 post-BMT enhanced thymopoiesis in the recipients.13 30 A potential mechanism for the action of KGF in post-BMT immune reconstitution is increased intrathymic production of IL-7 by TECs. To investigate the relationship between KGF administration and IL-7 expression in TECs, cryopreserved thymic tissue sections from BMT recipients were analyzed by in situ hybridization for IL-7 transcripts. Although PBS-treated recipients had almost no detectable IL-7+ mRNA cells, KGF-treated mice had increased numbers of IL-7+ mRNA cells at day 28 after BMT (Figure7 and data not shown). Increased numbers of IL-7+ cells were seen in KGF-treated mice at all radiation doses in both congenic recipients and allogeneic recipients.
KGF pretreatment increases the number of IL-7–producing TECs.
Top panels, hematoxylin-eosin (H & E) staining of thymic sections from congenic BMT recipients (1400 cGy) at day 28 after BMT. Lower panels, in situ hybridization for intrathymic IL-7 transcripts from the same mice as for H & E stains. Arrows indicate IL-7+ cells.
KGF pretreatment increases the number of IL-7–producing TECs.
Top panels, hematoxylin-eosin (H & E) staining of thymic sections from congenic BMT recipients (1400 cGy) at day 28 after BMT. Lower panels, in situ hybridization for intrathymic IL-7 transcripts from the same mice as for H & E stains. Arrows indicate IL-7+ cells.
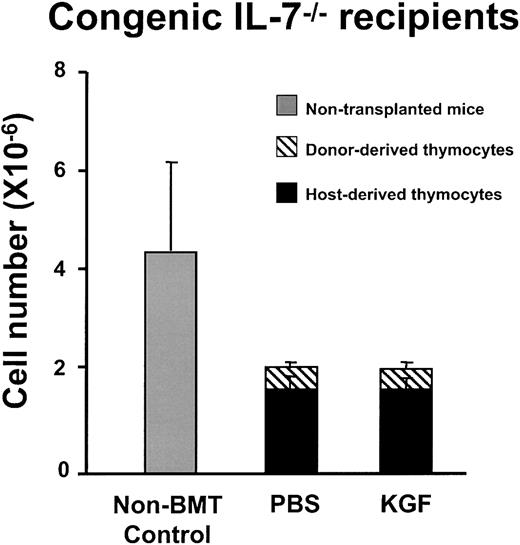
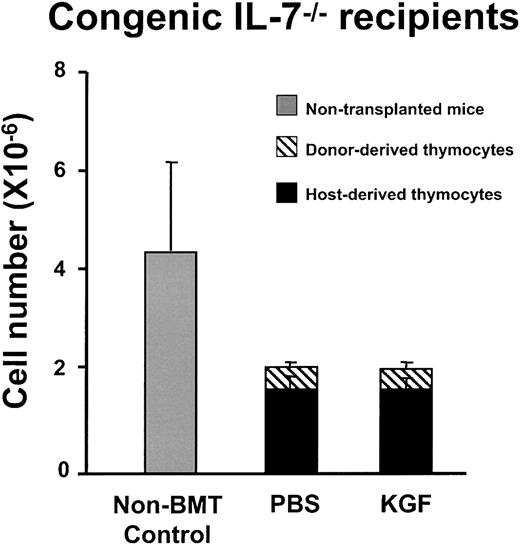
The increased frequency of intrathymic cells expressing IL-7 transcripts in KGF-treated mice are consistent with the hypothesis that KGF enhanced thymopoiesis by increasing the number of IL-7–producing TECs, rather than by a direct effect on thymocyte progenitors. To more directly analyze this hypothesis, IL-7−/− mice were transplanted after either PBS- or KGF-pretreatment. If KGF enhances thymopoiesis by stimulating IL-7 production by TEC, IL-7−/− recipients would not be expected to have increased thymopoiesis after KGF administration. There was no difference in thymic size and cellularity between KGF and PBS-treated IL-7−/− recipients of either congenic or allogeneic BMT (Figure 8 and data not shown). The thymuses of either PBS- or KGF-treated IL-7−/− mice were hypocellular and consisted of only 20% donor-derived thymocytes. There was no difference in the numbers of each thymic subpopulation (TN, DP, CD4 SP, and CD8 SP; data not shown). Therefore, KGF administration did not affect thymopoiesis of IL-7−/− mice after BMT. The results strongly indicate that IL-7 production by TECs is required for the observed effects of KGF on post-BMT thymopoiesis and immune reconstitution.
KGF does not increase post-BMT thymopoiesis in IL-7
−/−mice. C57BL/6J IL-7−/− mice were treated with PBS or KGF followed by 1000 cGy irradiation and BMT from congenic CD45.1 donors (n = 4 in each group). Thymic cellularity was analyzed at day 28 after BMT. The thymic cellularity of the PBS- and KGF-treated IL-7−/− mice is significantly less than that of the nontransplanted IL-7−/− mice (P < .02), but there is no difference between the PBS and KGF groups.
KGF does not increase post-BMT thymopoiesis in IL-7
−/−mice. C57BL/6J IL-7−/− mice were treated with PBS or KGF followed by 1000 cGy irradiation and BMT from congenic CD45.1 donors (n = 4 in each group). Thymic cellularity was analyzed at day 28 after BMT. The thymic cellularity of the PBS- and KGF-treated IL-7−/− mice is significantly less than that of the nontransplanted IL-7−/− mice (P < .02), but there is no difference between the PBS and KGF groups.
Discussion
Poor immune function after BMT has emerged as a major clinical problem, which significantly contributes to the morbidity and mortality of a potentially life-saving treatment.2-6 Although the mechanisms are complex, a common theme that emerges from many studies is that impaired thymopoietic capacity limits the ability of BMT recipients to generate new T lymphocytes and a functional immune repertoire. Pre-BMT conditioning induces damage to the thymic microenvironment, hampering the ability to generate new T lymphocytes derived from transplanted donor hematopoietic progenitors.13 The present report represents a novel approach to the problem of poor immune reconstitution in which an epithelial cell–specific growth factor (KGF) was used to improve the function of the microenvironment after BMT, resulting in improved immune development and function. The KGF-treated recipients showed enhanced thymopoiesis and increased numbers of functional T lymphocytes in the periphery. KGF pretreatment increased thymopoietic capacity of mice after either congenic or allogeneic BMT and after any of 5 different conditioning regimens that differed in radiation dose or adjunctive cytotoxic chemotherapy (Figure 2). The kinetics of thymic regeneration, the lag in repopulation of the spleen and lymph nodes, and the increased numbers of CD45RBhigh, CD44low naive CD4+ T lymphocytes in the KGF recipients are most consistent with the peripheral T-lymphocyte populations being the progeny of the thymocytes (Figures 3, 4, 5). KGF pretreatment also had improved humoral responses to the SRBC hemagglutinin, which is a T-lymphocyte–dependent antigen. Regardless of whether the mechanism of the improved anti-SRBC titers and class-switching was increased T-cell or B-cell responsiveness, the data indicate that the newly generated T lymphocytes in the KGF-treated animals were functional. Thymopoietic capacity was increased for several months after KGF pretreatment and BMT to levels that exceeded those of nontransplanted mice. The long-lasting increase in the ability to produce new thymocytes and T lymphocytes indicate that KGF induced profound durable changes in the thymic microenvironment.
The most likely targets of KGF-mediated enhancement of immune function after BMT were IL-7–producing TECs. FgfR2-IIIb was expressed by TECs, not thymocytes (Figure 1). The in situ hybridization analyses clearly demonstrated that the frequency of intrathymic cells expressing IL-7 transcripts was higher in KGF recipients (Figure 7). The results of the experiments with IL-7−/− BMT recipients indicate that IL-7 production was required for the effects of KGF on thymic reconstitution (Figure 8). Although the lack of effect of KGF in the IL-7−/− recipients could be explained by another KGF-induced pathway being inoperative in the absence of IL-7 or KGF being unable to overcome the dominant effects of IL-7 deficiency, the most likely reason was that KGF acted directly on IL-7–producing TECs. Besides IL-7, TEC production of SCF is also necessary for normal thymopoiesis. However, the defects observed in mice with defects in expression of either SCF or its receptor, c-kit, are much less profound than those seen in IL-7−/− mice.9,11 36Although the effects of KGF on SCF production were not tested, it is unlikely that increased production of SCF alone can account for the profound increases in thymopoietic capacity observed in the KGF-treated mice.
Cytokine therapies that directly modulate lymphocyte maturation and function have been previously used to improve immune function in BMT recipients.30,37,38 Although still not tested in a clinical setting, IL-7 appears to act on immature progenitors to increase the number of donor-derived thymocytes and immune function after BMT.30 IL-7 has also been shown to regulate peripheral homeostatic expansion of mature T lymphocytes.39-42 Normally, intrathymic IL-7 production is derived from TECs, not thymocytes. IL-7–producing TECs have been isolated by immunophenotype and are known to also produce SCF.43,44 Such TECs are essential for the in vitro generation of T lymphocytes from immature hematopoietic progenitors.45 On the basis of defects in thymopoiesis in IL-7−/−, IL-7R−/− mice, or humans with severe combined immune deficiency, intrathymic IL-7 production is also required in vivo for thymopoiesis.9,11,45,46 Previously, we reported that TECs are directly damaged by irradiation, leading to decreased intrathymic production of IL-7.13 The number of TECs and amount of IL-7 production in these studies were inversely related to the dose of pre-BMT irradiation. Thus, administration of IL-7 after BMT may be considered to be a therapy that replaces a defective TEC function, whereas KGF treatment either protects TECs or enhances their recovery after cytotoxic therapy. Previous studies have demonstrated that KGF exerts proliferative and antiapoptotic effects on a wide variety of epithelial cells.22-24 At this time, it is unknown whether KGF increased the survival of the TECs or enhanced recovery of TECs after radiation and/or chemotherapy. It is also possible that KGF directly induced greater production of IL-7 by the TECs.
The potential role of KGF in the normal development and maintenance of the thymic microenvironment is unknown. FgfR2-IIIb−/−mice have profound loss of thymic cellularity at birth.15Because the loss of FfgR2-IIIb signaling results in neonatal death from lack of lung development, it is not known whether thymopoiesis is permanently abrogated or merely delayed by the lack of the FgfR2-IIIb.15 In contrast, KGF−/− mice did not have significant defects in morphogenesis and displayed no obvious abnormalities in epidermal growth or wound healing after mechanical trauma.46 Thymopoiesis and immune function in KGF−/− mice have not been elucidated yet. The gross phenotypic differences between FgfR2-IIIb−/− and KGF−/− mice are most likely due to the redundancy of KGF with Fgf1, Fgf3, and Fgf10, all of which can bind to the FgfR2-IIIb. The RT-PCR analyses of FgfR2-IIIb expression by TECs indicate that intrathymic KGF signaling may be important in the development or maintenance of the TEC population. It is possible that steady-state thymopoiesis in the KGF−/− might be relatively normal but that the ability to recover after cytotoxic injury is abnormal in the absence of KGF. The ability of KGF treatment to rescue thymopoiesis after BMT raises the possibility that the KGF produced by thymocytes is necessary for TEC homeostasis.
Interactions between the thymic microenvironment and thymocytes are important for both thymocyte differentiation and maintenance of TEC organization.47 The development of the thymic cortex is impaired in mice with intrinsic defects in thymocyte development, indicating that thymocyte-derived signals are necessary for the maturation of the thymic microenvironment. The microenvironmental defects can be corrected by transplantation of the mice at an early age with normal hematopoietic progenitors.48 In the thymus, KGF might play a crucial role in reciprocal interactions between TEC and thymocytes. The RT-PCR analyses demonstrated that KGF was produced by thymocytes, which has also been observed by Farr et al.49 The analyses of the FgfR2-IIIb−/− mice indicated that intrathymic KGF was produced by mesenchymal cells, but the radioactive fluorescent in situ hybridization assay used was probably less sensitive and specific than RT-PCR of FACS-isolated thymocytes. In the present studies, the sorted stromal cells also expressed KGF, although some fibroblasts were isolated along with the TECs (data not shown). Therefore, it is likely that KGF is normally produced in the thymus by both thymocytes and nontransplantable stromal cells and/or fibroblasts. The relative contributions of thymocyte and fibroblast-derived KGF production to thymopoiesis need to be determined. The killing of KGF-producing thymocytes by irradiation or chemotherapy may be an important pathophysiologic event in thymic damage. The relative loss of intrathymic KGF production may lead to TEC damage from injuries such as radiation, chemotherapy, and GVHD, which is prevented by the exogenous administration of KGF. Further studies will be necessary to determine whether KGF caused increased radioresistance of existing TECs or whether it induced the differentiation of immature TEC precursors to mature IL-7–secreting TECs.
Thymopoietic capacity is also decreased in other conditions. GVHD has been associated with decreased thymopoietic capacity and immune deficiency, probably by damage to the thymic microenvironment.50,51 KGF has also been shown previously to decrease the incidence and severity of GVHD in murine allogeneic recipients, probably by decreasing the degree of epithelial cell damage that predisposes to GVHD.29 Because the allogeneic model used in the present experiments was one in which overt GVHD does not develop, further experiments will be required to determine whether KGF administration prevents GVHD-induced thymic injury. High-dose chemotherapy for cancer has also been associated with decreased thymopoiesis and loss of mature T lymphocytes and immune function.52 Recently, the loss of thymopoietic capacity in aging has also been shown to be mediated by decreased intrathymic IL-7 production and to be correctable by IL-7 administration.53It is not known whether this effect is due to loss of IL-7–producing TECs or altered regulation of IL-7 production by TECs. If the former mechanism is operative, then KGF treatment might ameliorate age-related thymic atrophy by increasing the number of IL-7–producing TECs.
The experiments described in the present report used one brief course of KGF treatment before BMT; the application of KGF treatment to other conditions will require testing of the effects of different dosage schedules, eg, chronic or repeated administration, for each prospective disease or condition. Although the current study demonstrates that KGF administration protects thymopoietic capacity in BMT models, chronic administration of KGF to healthy animals induces a different effect. Seven consecutive days of KGF administration to healthy mice induced substantial thymic atrophy because of a block in thymopoiesis at the TN stage.54 Hence, the length of KGF administration as well as the condition of the thymus (healthy versus diseased) is apparently critical in determining whether KGF induces thymic protection or thymic atrophy.
KGF is currently being evaluated in clinical trials for its safety and efficacy in preventing mucosal damage from irradiation and BMT. In addition to the predicted effects on skin and oral and intestinal epithelium, the present studies indicate that KGF may have immunomodulatory effects by the unique mechanism of protection of TECs. A potential clinical application of KGF is the amelioration of prolonged post-BMT immune deficiency in BMT recipients.
We thank Dr Richard Murray for his gift of the IL-7−/− mice.
Supported by grants HL54729, HD37598, HL54850, HL7005 from the National Institutes of Health, AmFAR 02594-23-RGI, and by the T.J. Martell Foundation (K.I.W.); and by grants AI34495, HL63452, and HL55209 from the National Institutes of Health (B.R.B.).
B.R.B. and K.I.W. were equal contributors.
Three authors (D.M.D., C.F., and D.L.L.) are employees of Amgen Inc.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth Weinberg, Division of Research Immunology and Bone Marrow Transplantation, MS 62, Childrens Hospital Los Angeles, 4650 Sunset Blvd, Los Angeles, CA 90027; e-mail:kweinberg@chla.usc.edu.