Allogeneic hematopoietic stem cell transplantation (HSCT) corrects the Wiskott-Aldrich syndrome (WAS) phenotype. However, the toxicity and mortality frequently associated with this approach warrant the exploration of new therapeutic strategies. Transplantation studies of a murine model of WAS deficiency have been limited by the occurrence of a radiation-induced fatal exacerbation of a pre-existing colitis in the peritransplantation period. Here we demonstrate that when crossed to a C57/B6 background, WAS-deficient males show little if any colitis and reliably survive HSCT. We show that HSCT corrects the hematologic and functional deficiencies of WAS knockout mice. These results strengthen the analogy between murine and human WAS and provide a basis for the use of WAS-deficient mice to explore novel approaches for correction of the disease phenotype.
Introduction
Wiskott-Aldrich syndrome (WAS) is an X-linked immunodeficiency of variable penetrance, presenting classically with the triad of immunodeficiency, thrombocytopenia, and eczema.1,2 Severely affected males who receive symptomatic care alone rarely survive beyond the second decade of life. In contrast, hematopoietic stem cell transplantation (HSCT) from matched sibling donors is curative, showing excellent long-term survival.3-5 However, many patients lack such a donor, and the use of alternate donors has shown less promising results. Hence, the identification of an animal WAS model that recapitulates the human phenotype would provide an opportunity to test novel therapeutic strategies, such as transplantation with nonmyeloablative conditioning or gene therapy. However, exacerbation of a pre-existingHelicobacter-associated colitis in WAS-deficient 129/SvEv mice after transplant conditioning results in mortality in the majority of animals studied.6 Our studies were designed to determine whether transfer of the WAS genotype to the C57/BL6J strain would provide mice to test novel therapies.
Study design
129/SvEv-Wasptm1Sbs, C57/BL6J, and C57/BL6J Ly5.1 (B6.SJL-Ptprca Pep3b/BoyJ) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). 129/SvEv-Wasptm1Sbs mice were bred with the C57/BL6J mice for 2 to 4 generations (see figure legends). Genetic screening of progeny was performed by polymerase chain reaction amplification of the neo insert and/or by Southern blotting.6 Donor marrow cells were obtained from the hind limbs of C57/BL6J Ly5.1 mice (Figure 2B-D) or from littermates of the recipients. Recipients were lethally irradiated (1100 cGy) and injected with 1.0 to 7.5 × 106cells into the tail vein. Flow cytometry of peripheral leukocytes, gating lymphocytes on forward versus side scatter and subsequently on fluorescently tagged cell-bound anti-Ly5.1 (CD45.1), CD90.2 (Thy-1.2), or CD45R/B220 (BD Pharmingen, San Diego, CA) was used to monitor engraftment.
Whole spleens were crushed and the cells were filtered through 70-μm mesh. Following red cell lysis and removal of B lymphocytes by adsorption to antibody-coated flasks (goat anti-mouse IgG + IgM; Jackson Immunoresearch, West Grove, PA), T cells were treated with anti-CD3ε antibody (BD Pharmingen) that had been bound overnight (4°C) to 96-well dishes, or with human interleukin-2 (IL-2; Chiron, Emeryville, CA). A total of 1.0 μCi/well (0.037 MBq/well)3H-thymidine was added at 48 hours, and incorporation was measured at 72 hours.
Hematologic values were measured on a HemaVet modified Coulter counter (CDC Technologies, Seymour, CT). Statistical analysis was performed using the exact Wilcoxon rank test. Significance values noted in Figure 2 were assessed atP < .05/3 using the exact Wilcoxon rank test with Bonferroni correction.
Results and discussion
C57/BL6J WAS-null mice show no significant colitis
The utility of the C57/BL6J mouse strain for study of HSCT is well established.7-9 We crossed 129/SvEv WAS-null mice (129−WAS−) for 2 to 4 generations onto the C57/BL6J background. Necropsy studies of C57/BL6J WAS− (C57−WAS−) males revealed normal colons without the inflammatory infiltrates and crypt abscesses previously reported on the 129/SvEv background.6A slightly increased number of mononuclear cells in the lamina propria was found in WAS-null animals compared with wild-type (WT) littermates, an abnormality slightly exacerbated by irradiation (Figure1). Necropsy studies of 129−WAS− males revealed only acute and chronic inflammatory cells in the colonic lamina propria in 1 of 3 animals.
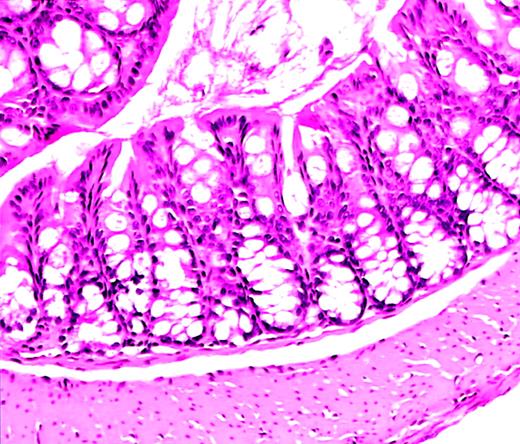
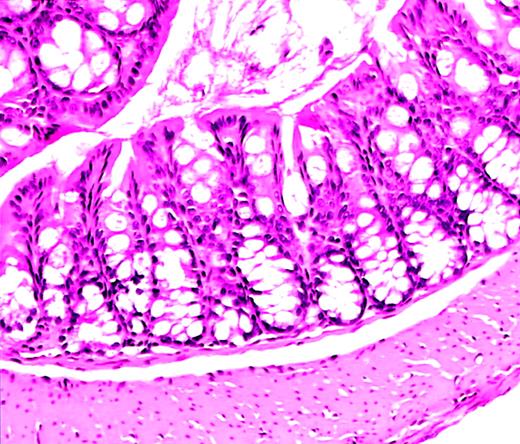
Normal colon histology in C57−WAS− mouse after transplantation.
Hematoxylin and eosin–stained section from the colon of a C57/BL6J WAS-null mouse (age 10 weeks, H hepaticuspositive) that became acutely ill and was killed 10 days after lethal irradiation and bone marrow transplantation. A severe hemorrhagic cystitis was found at necropsy. Colon histology shows rare acute and chronic inflammatory cells, but is essentially normal. Magnification, × 50.
Normal colon histology in C57−WAS− mouse after transplantation.
Hematoxylin and eosin–stained section from the colon of a C57/BL6J WAS-null mouse (age 10 weeks, H hepaticuspositive) that became acutely ill and was killed 10 days after lethal irradiation and bone marrow transplantation. A severe hemorrhagic cystitis was found at necropsy. Colon histology shows rare acute and chronic inflammatory cells, but is essentially normal. Magnification, × 50.
More than 60 mice have received lethal irradiation and bone marrow rescue, with a survival rate (longer than 6 weeks) of greater than 80% (Figure 2A). Donor marrow was derived from WT littermates or from C57/BL6J Ly5.1 animals (see below). No consistent differences in morbidity or mortality were observed between these donor sources, nor was there a correlation between posttransplantation mortality and the number of back-crosses (Figure2A).
Transplantation of normal hematopoietic cells corrects the WAS-null phenotype.
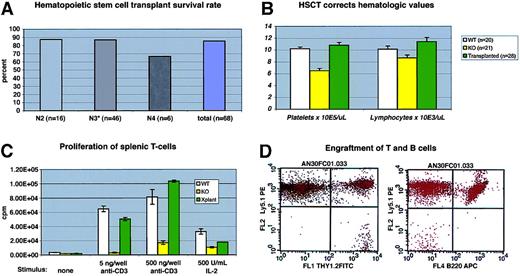
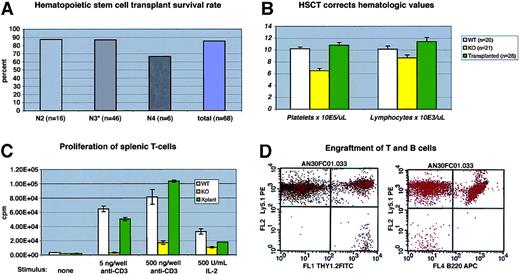
(A) Survival of C57/BL6J WAS-null mice after bone marrow transplantation. Transplant procedures were as described in “Study design.” N3 recipients included 3 “F1” N3 mice (progeny of N3×N3 matings). (B) Mean hematologic values in C57/BL6J WAS-null males (“KO”), WT littermates, and WAS−/C57/BL6J males 12 to 14 weeks after transplantation with WT (Ly5.1) marrow. The difference between WT and KO lymphocyte counts was significant atP = .03; all other differences between KO and either WT or transplantation groups were significant to P < .01. Error bars = 1 standard error. (C) Proliferation of primary splenic T-cell cultures after stimulation with immobilized anti-CD3 antibody or soluble human IL-2. Each assay was performed in triplicate. Error bars = 1 standard error. (D) Representative flow cytometry data from recipient peripheral blood lymphocytes at 14 weeks after transplantation, showing complete B-cell (B220+) Ly5.1 donor engraftment and a small amount (2.4% in this case) of residual Ly5.2 host-derived T cells (Thy 1.2+).
Transplantation of normal hematopoietic cells corrects the WAS-null phenotype.
(A) Survival of C57/BL6J WAS-null mice after bone marrow transplantation. Transplant procedures were as described in “Study design.” N3 recipients included 3 “F1” N3 mice (progeny of N3×N3 matings). (B) Mean hematologic values in C57/BL6J WAS-null males (“KO”), WT littermates, and WAS−/C57/BL6J males 12 to 14 weeks after transplantation with WT (Ly5.1) marrow. The difference between WT and KO lymphocyte counts was significant atP = .03; all other differences between KO and either WT or transplantation groups were significant to P < .01. Error bars = 1 standard error. (C) Proliferation of primary splenic T-cell cultures after stimulation with immobilized anti-CD3 antibody or soluble human IL-2. Each assay was performed in triplicate. Error bars = 1 standard error. (D) Representative flow cytometry data from recipient peripheral blood lymphocytes at 14 weeks after transplantation, showing complete B-cell (B220+) Ly5.1 donor engraftment and a small amount (2.4% in this case) of residual Ly5.2 host-derived T cells (Thy 1.2+).
Of 29 transplant recipients for which fecal specimens were tested prior to lethal irradiation, 24 were positive for Helicobacter hepaticus and 5 for other Helicobacter species. All but 2 mice in the first group, and all of the second group, have survived for more than 6 weeks after transplantation. Necropsy of the nonsurviving mouse positive for H hepaticusrevealed no significant colitis (Figure 1).
Transplantation corrects hematologic and functional deficiencies
Hematologic and immunologic parameters were measured in several cohorts of animals and compared with WT and C57−WAS− littermate controls. We found that C57−WAS− males (age range, 8-34 weeks) showed a significant thrombocytopenia and lymphocytopenia in comparison with age-matched WT littermates (Figure 2B). Age did not significantly discriminate between the genotypes in this study. As is the case for human WAS, T cells from C57−WAS− proliferated poorly in response to T-cell receptor stimulation, but responded to IL-2 stimulation (Figure2C). HSCT corrected the thrombocytopenia and lymphocytopenia of C57−WAS− mice by 12 to 14 weeks after transplantation (Figure 2B). The inability of WAS-derived splenic T cells to proliferate normally following stimulation with immobilized anti-CD3 antibody was also corrected (Figure 2C), demonstrating that T cells derived from donor marrow had repopulated the spleen normally.
To follow the rate and extent of donor engraftment accurately, we subjected a cohort of 6 C57−WAS− males (which express the Ly5.2 epitope of CD45 on their leukocytes) to transplantation with marrow derived from Ly 5.1 (B6.SJL-Ptprca Pep3b/BoyJ) mice. At 6 weeks after transplantation, all lineages except peripheral blood T cells were derived entirely from the donor (Figure 2D and data not shown). Consistent with past reports of radiation-resistant T-cell subsets,10 we found that a fraction (12%) of peripheral T cells were host derived at 7 weeks after transplantation, but no host T cells were found at 24 weeks (data not shown). Spleen-derived T cells from the transplant recipients were also 100% donor derived at late times after transplantation (data not shown).
The lack of colitis in our WAS knockout mice at less than 4 months contrasts with prior results in older mice, and we found that the presence of Helicobacter species in WAS− mice is not sufficient to induce colitis. Given the variable penetrance of WAS mutations clinically, the phenotypic difference we have seen between these strains of WAS-null mice could reflect a similarly variable penetrance in this species.
Our results demonstrate that C57−WAS− mice can survive HSCT, which corrects several key aspects of the murine WAS− phenotype. These observations strengthen the similarity between these mice and their human counterparts and will allow us to study whether murine WAS can be phenotypically corrected by alternative transplantation methods such as gene therapy.
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2001-12-0319.
Supported by the National Heart, Lung, and Blood Institute Program Project Grant P01 HL 53749, Cancer Center Support CORE Grant P30 CA 21765, and American Lebanese Syrian Associated Charities (ALSAC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Arthur W. Nienhuis, Division of Experimental Hematology, St Jude Children's Research Hospital, 332 N Lauderdale, Memphis, TN 38105; e-mail: arthur.nienhuis@stjude.org.